Abstract
Understanding the fate and dynamics of cells during callus formation is essential to understanding totipotency and the somatic embryogenesis (SE) mechanisms. In the present study, the histodifferentiation events involved during the acquisition and development of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.) was investigated. Zygotic embryos were inoculated on SE induction medium, and at 14 days the first divisions of the procambial and perivascular cells were observed. This region progressed to the formation of meristematic masses at 21 days, indicating their procambial and perivascular origin. Primary calli emerged at 45 days of culture, followed by progression to embryogenic calli at 90 days. The formation of proembryos (PE) from the meristematic cells occurred at 135 days of cultivation. The PE were isolated from the tissue of origin by the slight thickening of the cell wall, indicating their unicellular origin. When transferred to the maturation phase, differentiation of the somatic embryos at different developmental stages (globular and torpedo) was observed. The differentiated somatic embryos presented protoderm, procambial strands and plumules. Afterwards, they were transferred to culture medium without growth regulators in which conversion of the somatic embryos from torpedo stage into plants was observed. These results enable a greater understanding of the SE process and plantlet formation in E. guineensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil Palm (Elaeis guineensis Jacq.) is a monocotyledonous plant of the palm family (Arecaceae). It is an economically important plant species and provides the second largest source of edible oil in the world (Jouannic et al. 2005). Brazilian production of this oil is currently around 115 thousand tons/year, but this is <1 % of the total produced by Malaysia, and represents only 0.5 % of the world’s production. The State with the highest production is Para, which accounts for approximately 85 % of the oil produced in the country. In Latin America, Brazil occupies 3rd place in terms of production, after Colombia and Ecuador (Lopes and Steidle Neto 2011).
In this context, breeding programs have been strategically implemented by research institutions, with emphasis on the introduction, selection and genetic improvement of materials with agronomic characteristics of interest. However, being a typical monocotyledonous species, the oil palm has a single growing apex, which prevents its vegetative multiplication by conventional methods, a fact that makes it practically impossible to obtain uniform cultures and perpetuate the characteristics of an individual plant with high selected genetic value. Therefore, further advances in research are essential, to obtain safe, reliable protocols for the clonal propagation of superior materials, whether for large-scale production of plantlets, or to reduce selection cycles and assist genetic improvement programs (Scherwinski-Pereira et al. 2010; Jaligot et al. 2011; Ooi et al. 2012; Silva et al. 2012).
Of the different in vitro cloning techniques, somatic embryogenesis (SE) is, without doubt, the most commonly recommended for clonal propagation of palms (Schwendiman et al. 1988; Karun et al. 2004; Scherwinski-Pereira et al. 2010; Jaligot et al. 2011). This technique enables plants to be regenerated by means of bipolar structures (somatic embryos) from somatic cells, through an ordered series of embryogenic phases, without the occurrence of gamete fusion (Portillo et al. 2007). During the clonal propagation process, the somatic embryos present anatomical characteristics similar to zygotic embryos. Both are characterized by the stem and root apex differentiation, and generally go through the following embryonic development stages: globular, cordiform, torpedo and cotyledonary. However, despite the existence of a vast number of works in the literature, SE is still far from being completely mastered and understood, although it is known that in general, callus induction and the use of auxins are fundamental pre-requisites for obtaining satisfactory results (Verdeil et al. 2001; Corredoira et al. 2006; Karami and Saidi 2010).
The use of histological observations during the SE process has proven to be a useful tool for understanding the embryogenic process. The study of cell and tissue development in the different process stages has helped improve the clonal propagation protocol efficiency, enabling alterations associated with the position and activity of the cells with embryogenic competence to be identified, as well as the whole ontogenic development (Verdeil et al. 2001; Sané et al. 2006; Santos et al. 2006).
The ontogenic development of the somatic embryos in monocotyledons, from the origin of the meristematic cells to their complete differentiation, varies according to species (Davoodi et al. 2002; Sáenz et al. 2006). During SE of the macauba palm (Acrocomia aculeata) from zygotic embryos (ZE), it was observed that the meristematic cells originated from procambial or perivascular cells, which proliferated to produce meristematic masses in induction medium (Moura et al. 2010). Meanwhile, the start of the oil palm (Eleais guineensis) SE development, also from ZE, was revealed to be of subepidermal origin by morpho-anatomical analysis (Kanchanapoom and Domuoas 1999).
Through morpho-anatomical studies it is also possible to identify the phases of embryonic development (Portillo et al. 2007). In Cocos nucifera the formation of proembryos (PE) was observed at 56 days of cultivation (Verdeil et al. 2001). The authors observed changes in the cell wall, such as the closing of the plasmodesmas and callose deposition. These events would eventually lead to the isolation necessary for cellular reprogramming and the start of embryogenic events. Therefore, deeper knowledge of the morpho-anatomical events involved in the embryogenic process could significantly improve the understanding of the different stages involved in SE, as well as help ensure that certain cultivation conditions, such as the composition of culture media, can be better adjusted. Furthermore, it is not difficult to imagine that the precise morpho-anatomical characterization of the different SE stages in a species may constitute a morphological marker of the process (Sáenz et al. 2006).
In this context, the aim of this work was to elucidate the ontogenesis and histochemical events involved during the SE of the oil palm (E. guineensis Jacq.) from ZE.
Materials and methods
Plant material and culture conditions
Three phases were considered during the somatic embryogenesis of E. guineensis: embryogenic calli induction, somatic embryo differentiation and maturation, and plant regeneration. Cultures were established from ZE obtained from mature seeds (approximately 150 days after pollination) of oil palm (E. guineensis Jacq), variety C2328. The mature seeds were kindly supplied by Embrapa Amazonia Ocidental (Station of Rio Urubu), located in Rio Preto da Eva, Amazonas, Brazil.
The SE was achieved through the previously published protocol of Balzon et al. (2013) containing MS salts and vitamins (Murashige and Skoog 1962) supplemented with 20 g L−1 sucrose, 0.5 g L−1 glutamine, 2.5 g L−1 activated charcoal, 450 μM 4-amino-3,5,6-trichloro-2-pyridinecarboxylic acid (picloram), and solidified with 2.5 g L−1 Phytagel (Sigma®). The explants were kept in this phase for up to 150 days, until embryogenic calli were obtained.
For the somatic embryo differentiation and maturation stage, embryogenic calli from the induction phase were used. In this phase, the culture medium tested was DMM (differentiation and maturation medium), made up of MS medium containing 20 g L−1 sucrose, 0.5 g L−1 glutamine, 0.3 g L−1 activated charcoal, 0.6 μM α-naphthalene acetic acid (NAA) and 12.3 μM 2-isopentenyladenine (2-iP). The explants remained in this phase for 90 days. After the maturation stage, the somatic embryos were transferred to modified medium with macro- and micro-nutrients at half strength, 20 g L−1 sucrose, 2.5 g L−1 activated charcoal, and solidified with 2.5 g L−1 Phytagel, for germination and regeneration of plants.
Histological analyses
For the anatomical analysis, the explants were collected at 0, 14, 21, 30, 45, 60, 90, 135 and 150 days after establishment of explants on the SE induction medium. In the differentiation and maturation phase, the explants containing somatic embryos in the globular and torpedo stages were collected after 60 days, followed by collection, at 30 and 90 days, of somatic embryos fully differentiated and regenerated on plant regeneration medium.
The plant material was immersed in 70 % FAA fixative solution (Formaldehyde, acetic acid and 70 % ethanol, at a ratio of 1:1:18 v/v) (Johansen 1940), under vacuum, for 24 h. The samples were then dehydrated in an increasing ethyl alcohol series (70–100 %), infiltrated, and embedded in historresin (Leica®), according to manufacturer recommendations. Seriate transversal and longitudinal sections (7 μm) were obtained in a manual rotary microtome (Leica model RM 2125). The sections were spread out and placed on microscopic slides on a hot plate heated to 40 °C. The sections were then stained with Toluidine blue (O’Brien et al. 1965), and submitted to Lugol’s iodine test to detect starch levels, followed by slide-coverslip mounting with Entellan. For the identification of total lipids, transversal sections of fresh ZE materials were prepared. The sections were submitted to the Sudan IV test (Gerlach 1984). The results were analysed and the images recorded using an optical microscope (Quimis-Motic BA 300, Diadema-SP, Brazil) attached to a digital image capture system (Software Image-Pro Plus 4.5).
Results and discussion
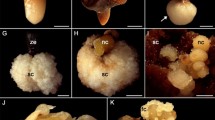
ZE obtained from mature seeds were used as explants for the SE of E. guineensis (Fig. 1a, b). In this stage, the embryo is conical, and small in relation to the total volume of the endosperm, measuring around 3 mm and consisting of a distal and a proximal region (Fig. 1c). The distal region corresponds to the haustorium (cotyledon) and contains three types of cells: those of the fundamental meristem, those of the procambium and those of the protoderm (Fig. 1d). The proximal region presents the embryonic axis, containing a plumule with primordial leaves that surround the apical meristem. The plumule is inside a cavity formed by a ligule (Fig. 1e). However, the two regions can be distinguished by their coloration, where in fresh material, immediately after embryo removal from the seed, the portion with more yellowish coloration corresponds to the proximal region, while the lighter white portion, corresponds to the distal region.
Explant used for induction of somatic embryogenesis of E. guineensis. a Diaspore (bar 3 mm). b Longitudinal section of the diaspore showing the zygotic embryo (arrow) (bar 3 mm). c Zygotic embryo (bar 1 mm). d Longitudinal section of the zygotic embryo (bar 200 μm). e Transversal section of the proximal region, showing the embryonic axis (bar 200 μm). f Lipid accumulation in the zygotic embryo fundamental meristem cells (arrow) (bar 200 μm). ps procambial strands, ed endocarp, ea embryonic axis, en endosperm, cs cotyledon sheath (cotyledon groove), lg ligule, fm fundamental meristem, pd protoderm, lp leaf primordia, pl plumule, dr distal region, pr proximal region, tg tegument
The ZE of E. guineensis is similar to that of the majority of embryos of the Arecaceae family species, such as A. aculeata (Moura et al. 2010), Oenocarpus minor (Oliveira et al. 2010), Euterpe precatoria (Aguiar and Medonça 2003) and C. nucifera (Sugimura and Murakami 1990), presenting a single cotyledon called the haustorium, and the apical meristem covered by the leaf primordia.
The histochemical analysis performed on the ZE demonstrated an accumulation of lipids as reserve material (Fig. 1f), but no starch grains were detected in the explant. The large accumulation of lipids in ZE cells is also common in other species of the Arecaceae family (Demason 1988; Moura et al. 2010). The lipid grains are of extreme importance in the initial stages of germination, and in the establishment of seedlings. In this period, the haustorium plays an active role in the hydrolysis of lipids and mobilization of reserves (Verdeil and Hocher 2002).
The ZE present the first signs of cell proliferation after 14 days on callus induction medium, when it was possible to observe swelling and the formation of bands in the distal region of the explant (Fig. 2a). At this phase, histological observations show that early cell divisions occur in the procambium and in the parenchyma cells adjacent to vascular tissue (Fig. 2b). As a result, there are new added cell layers in the explant, causing the embryos to expand. In palms, the cells adjacent to the vascular tissue apparently have higher morphogenetic capacity (Steinmacher et al. 2007). In studies with E. guineensis (Schwendiman et al. 1988), Euterpe edulis (Guerra and Handro 1998) and Phoenix dactylifera (Sané et al. 2006) showed that the initial events of cell division during somatic embryogenesis were always observed in cells adjacent to the vascular tissue, resulting in primary meristematic or nodular calli similar to those observed in the present study.
Zygotic embryo of E. guineensis at 14 days of cultivation in somatic embryogenesis induction culture medium (450 μM of Picloram). a Swollen zygotic embryo, showing bands in the distal region (bar 5 mm). b Detail of the perivascular cells (arrows) and procambial cells in division (bar 100 μm). c Detail of the tracheal elements with secondary walls shown by the greenish coloration (bar 100 μm). d Distal region of the zygotic embryo, with invaginations of the protoderm (arrow) and formation of procambium bundles (bar 100 μm). e Starch accumulation in the parenchymatic cells adjacent to the region of intense procambium division (bar 100 μm). fm fundamental meristem, pc procambium, pd protoderm, tq tracheal elements
The cell divisions occurred with greater intensity in the region proximal to the explant, demonstrating that this is the region most responsive to stimuli. The formation of isodiametric perivascular cells was also observed, with dense cytoplasm, voluminous nucleus, and clearly evident nucleoli. The procambial cells, meanwhile, are elongated, presenting their longest axis parallel to the longest axis of the embryo. In the vascular cells, differentiation of tracheal elements was observed with secondary wall deposition in a helicoidal pattern, as shown by the greenish coloration (Fig. 2c).
In the distal region of the explant, protoderm invaginations and procambial bundles were observed. The protoderm had only one cell layer, with rectangular cells containing starch grains. It should be emphasized that in the protoderm, the divisions occurred in the periclinical direction, forming only epidermal cells. The subepidermal layers showed smaller cells, while in the interior of the explant, the cells were larger and isodiametric, with less dense cytoplasm (Fig. 2d).
In this phase, an accumulation of starch grains occurred in the parenchymatic tissue, particularly in the cells adjacent to the cell division centers (Fig. 2e). It was verified that the zygotic embryo of E. guineensis does not contain starch as reserve material, indicating that the synthesis of starch occurred during the first 2 weeks of SE induction.
The accumulation of starch in the parenchyma cells adjacent to the regions with intense cell divisions may be related to the acquisition of embryogenic competence. Magnaval et al. (1991) studying the SE of C. nucifera, observed the presence of starch in the cells adjacent to the meristematic cells. Kanchanapoom and Domuoas (1999) also detected the accumulation of starch in calli and in the bipolar embryoids of E. guineensis, indicating that the starch increases with the formation of somatic embryos. However, the accumulation of starch during the SE process is still not clear. The low mitotic activity of the parenchymatic cells that store this reserve material may be the probable hypothesis, as the cells with intense division generally do not present an accumulation of starch. The amount of starch may also change, depending on the embryo growth phase, as cell division and differentiation require energy. From the breakdown of starch, glycolytic intermediates are formed that when submitted to oxidative catalysis, supply the high levels of ATP necessary for cell metabolism (Martin et al. 2000).
At 21 days in induction medium, the explants presented a more swollen appearance, with yellowish coloration, and the protoderm invaginations became more evident (Fig. 3a). In the proximal region, the development of zygotic embryo leaf primordia was observed, in which procambial cell divisions were seen (Fig. 3b). The histological analysis showed that the cells adjacent to the vascular tissue that presented cell division progressed to the formation of meristematic cell masses (Fig. 3c). This meristematic region showed small, isodiametric cells with dense cytoplasm, and evident nucleus and nucleoli (Fig. 3d).
Zygotic embryo of E. guineensis at 21 and 30 days of cultivation in somatic embryogenesis induction culture medium. a Swollen zygotic embryo with bands in the distal region of the explant (arrow) (bar 1 cm). b Proximal region of the explant, with meristematic cell masses around the procambium and development of leaf primordia (bar 100 μm). c Detail of the meristematic cell mass adjacent to the procambial bundle at 21 days (bar 100 μm). d Meristematic region with small, isodiametric cells, dense cytoplasm and evident nucleus (bar 100 μm). e Starch accumulation in the cells adjacent to the meristematic masses at 30 days (bar 100 μm). f Distal region with starch accumulation in the subepidermal cells (arrow) and the procambium (bar 100 μm). mm meristematic mass, pc procambium, pd protoderm, lp leaf primordia
The results obtained in this work are in disagreement with those obtained by Kanchanapoom and Domuoas (1999) who, studying the ontogenesis of SE from ZE of E. guineensis, describe that the meristematic cell masses originate from the subepidermal cells. Moura et al. (2010) also observed that somatic embryos gave rise to meristematic cell masses formed from perivascular cells in ZE of A. aculeata. Although using a different type of plant tissue, Schwendiman et al. (1988) also observed that nodular calli of oil palm, from young leaves, originated from the perivascular cells. This behavior was also observed in the induction of SE from the leaves of E. edulis (Guerra and Handro 1998).
After 30 days of cultivation of the ZE on callus induction medium for SE, it was observed that in the proximal region of the explant, the meristematic cell masses adjacent to the procambial chords became more evident. In the distal region, the procambial bundles located in the protoderm invaginations present intense mitotic activity that led to an increased cell mass in this region of the explant. The accumulation of starch grains on days 21 and 30 of cultivation was concentrated in the cells adjacent to the region of cell division (meristematic cell mass), and in the subepidermal parenchymatic cells of the distal region of the explant, confirming the hypothesis that these accumulations are a strong indicator of tissues with high embryogenic competence (Fig. 3e, f).
At 45 days of cultivation, the formation of primary calli can also be observed, with nodular appearance and yellowish coloration, between the bundles of the procambium and the protoderm (Fig. 4a). Through the histological analyses, it was possible to characterize the nodular calli, which were comprised solely of meristematic cells (Fig. 4b). The active cell division in the meristematic regions led to rupture of the protoderm in the proximal region of the explant, where the embryonic axis was located, exposing the extensions of procambial strands and the meristematic masses (Fig. 4c, d).
Zygotic embryo of E. guineensis at 45 days of cultivation in somatic embryogenesis induction culture medium. a Swollen zygotic embryo with formation of nodular calli with yellowish coloration (arrow) (bar 1 cm). b Meristematic region with small cells (bar 100 μm). c Proximal region of the explant, with meristematic masses around the procambium (bar 100 μm). d Fragmentation of the meristematic masses and formation of nodular calli (bar 100 μm). e Distal region with well-developed tracheal elements (bar 100 μm). f Starch accumulation in the cells adjacent to the meristematic masses (bar 100 μm). nc nodular callus, mm meristematic mass, pc procambium, pd protoderm, tq tracheal elements
Steinmacher et al. (2007) studying the SE of Bactris gasipaes (Kunth), observed in ZE under cultivation, cell divisions throughout the explant, with subsequent formation of primary callus with yellowish coloration after 4 weeks in induction medium. These same authors demonstrated, through histological analyses, that the calli consisted of meristematic cells. Moura et al. (2010) also observed nodular calli in A. aculeata at 50 days of cultivation in induction medium. The calli consisted of small, isodiametric cells with dense cytoplasm and evident nucleus, characterizing meristematic tissue cells.
In this phase, the distal region of the explant presented differentiation of the procambium in tracheal elements (Fig. 4e), and the accumulation of starch in the parenchymatic cells was no longer observed (Fig. 4f). This may be due to the starch breakdown in order to supply the high ATP levels necessary for the procambium divisions and differentiation. However, starch accumulation was observed in the cells adjacent to the meristematic cells of the nodular calli.
The proliferation of primary calli with yellowish coloration was observed after 60 days in induction medium (Fig. 5a). Through the histological analysis, numerous nodular structures were observed, consisting mainly of meristematic cells (Fig. 5b). In this phase, the formation of root primordia was also observed, with fundamental meristem, apical root meristem, and root cap (Fig. 5c). Aberlenc-Bertossi et al. (1999) observed that E. guineensis embryogenic cultures maintained in culture medium supplemented with auxins presented embryos containing only the root apex. On the other hand, these same authors observed that after treatment with cytokinin, the embryos presented differentiation of the two meristematic axes. Buffard-Morel et al. (1992) also observed the formation of root primordia in the induction of C. nucifera explants.
Sections of explant of E. guineensis at 60 (a–c) and 90 (d–g) days of cultivation in somatic embryogenesis induction medium. a Explant with 60 days of cultivation, presenting primary callus (arrow) (bar 0.5 cm). b Callus with meristematic cells, characterized by voluminous nucleus and dense cytoplasm, and with intense cell divisions (bar 100 μm). c General appearance of cell clusters and root primorda in differentiation (bar 100 μm). d Nodular, compact embryogenic calli (arrow) (bar 0.5 cm). e General appearance of embryogenic calli consisting of meristematic cells (bar 100 µm). f Starch accumulation in the cortex of the embryogenic calli (bar 100 μm). g Globular embryo characterized by the start of formation of the protoderm (arrow) (bar 100 μm). ec embryogenic callus, rc root cap, pc primary callus, ge globular embryo, rm root meristem, mm meristematic mass, pd protoderm
At 90 days of cultivation, formation of embryogenic calli was observed (Fig. 5d). In this stage, the formation of calli consisting of meristematic cells was observed (Fig. 5e). In the region of the callus cortex, larger cells were observed, with a low nuclear-cytoplasmic ratio and an accumulation of starch (Fig. 5f). Embryos in the globular stage characterized by the start of formation of the protoderm were also observed in this induction stage (Fig. 5g).
Starch deposition in the cortex in embryogenic calli is observed in various species (Verdeil et al. 2001; Portillo et al. 2007; Moura et al. 2010). These observations suggest that starch mobilized in the cortical region of the callus is used as an energy source for the meristematic cells under intense division. Mangat et al. (1990), working with organogenesis in Begonia rex, suggest that the starch found is mobilized and used as an energy source for the leaf primordia development. In view of the above, the starch metabolism probably helps supply the energy levels necessary for intense cell division and tissue differentiation.
At 135 days of cultivation on callus induction medium, formation of nodular calli was observed (Fig. 6a). These calli were made up of cell groups formed in the middle of the meristematic regions, characterized as PE (Fig. 6b). The PE were isolated by an apparent thickening of the wall, possibly caused by the dissolution of the middle lamella. Verdeil et al. (2001), studying the acquisition of embryogenic competence in C. nucifera, observed isolation of the PE by the thickening of the exterior wall. They observed changes in the cell wall, such as the closing of the plasmodesmas and deposition of callose (glucose polymers), suggesting that these events lead to the isolation necessary for cellular reprogramming and the start of embryogenic events. The thickening of the wall around the PE was also observed by Sané et al. (2006) in the induction of SE in calli of P. dactylifera. In this phase, starch deposition occurred only in the cells adjacent to the PE (Fig. 6c).
Sections of explant of E. guineensis at 135 (a–c) and 150 (d–f) days of cultivation in somatic embryogenesis induction medium. a Explant presenting proembryogenic calli with compact, nodular appearance (arrow) (bar 0.5 cm). b Start of formation of proembryos (arrow) (bar 100 μm). c Detail of starch accumulation (arrow) in the cells adjacent to the proembryos (bar 100 μm). d Embryogenic calli with compact, nodular appearance and yellow coloration (bar 1 cm). e Somatic embryo in the globular stage (bar 100 μm). f Somatic embryo in more advanced stage, surrounded by the protoderm (detail) (bar 100 μm). ge globular embryo, mm meristematic mass, pd protoderm, dr distal region, pr proximal region, pe proembryos
At 150 days of cultivation, the explants presented embryogenic calli with compact consistency, and yellowish coloration (Fig. 6d). The histological analysis in this phase showed regions with formation of embryos in the globular stage, consisting of meristematic cells surrounded by a characteristic protoderm, and not connected to the tissue of origin (Fig. 6e). Isolation of the somatic embryo, demonstrating independence from the tissue of origin, shows a unicellular origin. These results corroborate with those obtained by Kanchanapoom and Domuoas (1999), who describe that the embryogenesis of E. guineensis obtained from ZE is unicellular in origin.
Somatic embryos in a more advanced stage were also observed. These were surrounded by a protoderm and presented the start procambial cell organization. Furthermore, these embryos presented two distinct anatomical structures: an apical region consisting of the haustorium and a basal region which contains the future embryonic axis (Fig. 6f). Santos et al. (2006) observed that the procambium formation onset occurred only when the somatic embryos were in the more advanced globular stage.
The different stages observed at 150 days of cultivation showed non-synchronous development of E. guineensis SE. Asynchronous development of somatic embryos in palms is commonly reported in the literature. Ledo et al. (2002) observed that culture medium supplemented with NAA and 2-iP was effective for the multiplication and maintenance of embryogenic calli of Euterpe oleracea and for promoting progression of the calli in somatic embryos, characterizing a non-synchronous embryogenic development for this species. In peach palm, after the transfer of the embryogenic calli to a regeneration medium, Steinmacher et al. (2007) observed distinct morphological responses, including the non-synchronous development of the somatic embryos.
After 150 days in the induction phase, the embryogenic calli and the PE were transferred to DMM culture medium. After 90 days of cultivation, the passage of the embryogenic calli to the maturation phase showed intense formation of somatic embryos in globular stages and in a more advanced phase, characterized by the well-developed haustorium and the formation of procambial strands (Fig. 7a, b). In this period, a totally differentiated somatic embryo was also observed, with haustorium, apical meristem (plumula) and two leaf sheaths (Fig. 7c, d). The development of isolated somatic embryos from the tissue of origin, in different stages of cell differentiation, was also observed during SE in B. gasipaes (Steinmacher et al. 2007).
Sections of explant of E. guineensis at 60 days of cultivation in somatic embryo differentiation and maturation medium. a Somatic embryos in globular stage (white arrow), and torpedo stage (red arrow) (bar 1 cm). b Somatic embryos (bar 200 μm). c Fully differentiated somatic embryo (bar 100 μm). d Detail of the apical meristem of the stem containing primordium leaf (bar 200 μm). se somatic embryo, am apical meristem, pl plumule, pc procambium, pd protoderm, lp leaf primordium
In this stage of development, no root primordium formation was observed. According to Camillo et al. (2009) the root meristem in E. guineensis only starts to differentiate after the development of the leaf primordia. These results were also observed in embryos of C. nucifera (Mangat et al. 1990) and E. precatoria (Aguiar and Medonça 2003). In the somatic embryos, originating from the differentiation/maturation media tested, no starch accumulation was observed.
Many works have reported that plants regenerated in culture medium supplemented with cytokinin, when cultivated in the field, present floral abnormality (Besse et al. 1992; Jones et al. 1995). However, Aberlenc-Bertossi et al. (1999) observe that cultivation in culture medium with the addition of cytokinin led to an increase in the number of somatic embryos with formation of an apical axis.
After 90 days in maturation medium, the somatic embryos were transferred to the culture medium without growth regulators (regeneration phase). In this stage, the somatic embryos present a rounded, elongated shape, larger in size than the zygotic embryo. The histological analyses showed separation between the distal and proximal regions (Fig. 8a). The protoderm, fundamental meristem and procambium are clearly identified (Fig. 8b). The embryonic axis is fully formed, presenting root pole, apical meristem (plumule) (Fig. 8c) and three leaf primordia (Fig. 8d). Complete differentiation of the somatic embryos was also observed during clonal propagation of P. dactylifera, using zygotic embryos as a source of explant, Sané et al. (2006). These results were also obtained by Scherwinski-Pereira et al. (2010), during SE of E. guineensis, using the TCL (Thin Cell Layer) technique.
Sections of somatic embryo of E. guineensis at 30 (a–d) and 90 (e–g) days in regeneration culture medium. a Longitudinal section of somatic embryo (bar 200 μm). b Detail of the procambial strands (bar 100 μm). c Detail of the embryonic axis (proximal region) (bar 100 μm). d Apical pole of the somatic embryo (bar 50 μm). e Conversion of the somatic embryo into plantlets at 90 days in regeneration medium (bar 1 cm). f Longitudinal section of the plantlets (bar 200 μm). g Detail of the stem apex (bar 50 μm). ls leaf sheath, ea embryonic axis, pc procambium, pd protoderm, lp leaf primordium, pl plumule, rp root primordium, dr distal region, pr proximal region
In this phase, the beginning of the germination process was also observed, characterized by the opening of the cotyledon sheath (Fig. 8d), subsequently enabling protrusion of the leaf primordia. Conversion of the somatic embryos into plantlets was observed at 90 days in regeneration media (Fig. 8e). The histological analyses showed development of the stem apex, which has eight differentiated leaf primordia; the first two remain in the sheath stage involving the primordia that subsequently develop (Fig. 8f, g). However, in this stage, no root protrusion was observed. These data corroborate with those of Camillo et al. (2009) who observed, during the germination of E. guineensis ZE, that the sheaths are the first structures to emerge, and signal the start of germination. These authors observed that the protrusion of the root occurs only after the emergence of the first true leaves, indicating similarity with SE.
Conclusion
The present study anatomically describes the SE process of E. guineensis from ZE. It was demonstrated that meristematic cells are derived either from perivascular cells or vascular tissues. Primary calli, presenting numerous nodular structures with meristematic cells are observed after 60 days of culture, and progresses to EC at 90 days, when small and isodiametric cells, with dense cytoplasm and starch accumulation are observed. From 135 to 150 days of cultivation the EC are made up of groups of cells formed in the middle of the meristematic regions, characterizing PE and embryos in the globular stage, which when transferred to the differentiation and maturation medium, differentiate asynchronically somatic embryos at globular and torpedo stages. Differentiated somatic embryos present all the characteristics of a zygotic embryo with protoderm, procambial strands and plumules, and when they are transferred to fresh medium without growth regulators, protrusion of the leaf primordial is observed, characterizing the onset of somatic embryo germination into plants.
References
Aberlenc-Bertossi F, Noirot M, Duval Y (1999) BA enhances the germination of oil palm somatic embryos derived from embryogenic suspension cultures. Plant Cell, Tissue Organ Cult 56:53–57
Aguiar MO, Medonça MS (2003) Morfo-anatomia da semente de Euterpe precatória (Palmae). Rev Bras Sem 25:37–42
Balzon TA, Luis ZG, Scherwinski-Pereira JE (2013) New approaches to improve the efficiency of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.) from mature zygotic embryos. In Vitro Cell Dev Biol Plant 49:41–50
Besse I, Verdeil JL, Duval Y, Sotta B, Maldiney R, Miginiac E (1992) Oil palm (Elaeis guineensis Jacq.) clonal fidelity: endogenous cytokinins and indoleacetic acid in embryogenic callus cultures. J Exp Bot 43:983–989
Buffard-Morel J, Verdeil JL, Pannetier C (1992) Embryogenese somatique du cocotier (Cocos nucifera L) a partir d’explants foliaires: études histologiques. Can J Bot 70:735–741
Camillo J, Luis ZG, Scherwinski-Pereira JE (2009) Tolerância de sementes de dendezeiro à criopreservação. Pesq Agropec Bras 44:211–215
Corredoira E, Valladares S, Vieitez AM (2006) Morphohistological analysis of the origin and development of somatic embryos from leaves of mature Quercus robur. In Vitro Cell Dev Biol Plant 42:525–533
Davoodi D, Majidi E, Khoshkam A (2002) Some morphological and anatomical aspects of date palm (Phoenix dactylifera L.) somatic embryogenesis in tissue culture. J Agric Sci Technol 4:63–71
Demason DA (1988) Embryo structure and storage reserve histochemistry in the palm Washingtonia filifera. Am J Bot 75:330–337
Gerlach D (1984) Botanische Mikrotechnik. Georg Thieme Verlag, Stuttgart
Guerra MP, Handro W (1998) Somatic embryogenesis and plant regeneration in different organs of Euterpe edulis Mart. (Palmae): control and structural features. J Plant Res 111:65–71
Jaligot E, Adler S, Debladis E, Beule T, Richaud F, Ilbert P, Finnegan EJ, Rival A (2011) Epigenetic imbalance and the floral developmental abnormality of the in vitro-regenerated oil palm Elaeis guineensis. Ann Bot 108(8):1453–1462
Johansen D (1940) Plant micro techniques. McGraw Hill Publication, New York
Jones LH, Hanke DE, Eeuwens CJ (1995) An evaluation of the role of cytokinins in the development of abnormal inflorescences in oil palms (Elaeis guineensis Jacq.) regenerated from tissue culture. J Plant Growth Reg 14:135–142
Jouannic S, Argout X, Lechauve F, Fizames C, Borgel A, Morcillo F, Aberlenc-Bertossi F, Duval Y, Tregear J (2005) Analysis of expressed sequence tags from oil palm (Elaeis guineensis). FEBS Lett 579:2709–2714
Kanchanapoom K, Domuoas P (1999) The origin and development of embryoids in oil palm (Elaeis guineensis Jacq) embryo culture. Sci Asia 25:195–202
Karami O, Saidi EA (2010) The molecular basis for stress-induced acquisition of somatic embryogenesis. Mol Biol Rep 37:2493–2507
Karun A, Siril EA, Radha E, Parthasarathy VA (2004) Somatic embryogenesis and plantlet regeneration from leaf and inflorescence explants of arecanut (Areca catechu L.). Curr Sci 86:1623–1628
Ledo AS, Lameira OA, Benbadi AK, Menezes IC, Oliveira MSP, Filho SM (2002) Somatic embryogenesis from zygotic embryos of Euterpe oleracea Mart. Rev Bras Frut 24:601–603
Lopes DC, Steidle Neto AJ (2011) Potential crops for biodiesel production in Brazil: a review. World J Agric Sci 7(2):206–217
Magnaval C, Noirot M, Verdeil JL, Blattes A, Huet C, Grosdemange F, Buffard-Morel J (1991) Free amino acid composition of coconut (Cocos nucifera L.) calli under somatic embryogenesis induction conditions. J Plant Physiol 146:155–161
Mangat BS, Pelekis MK, Cassells AC (1990) Changes in the starch content during organogenesis in vitro cultured Begonia rex stem explants. Physiol Plant 79:267–274
Martin AB, Cuadrado Y, Guerra H, Gallego P, Hita O, Martin L, Dorado A, Villalobos N (2000) Differences in the contents of total sugars, starch and sucrose in embryogenic and non-embryogenic calli from Medicago arborea L. Plant Sci 154:143–151
Moura EF, Ventrella MC, Motoike SY (2010) Anatomy, histochemistry and ultrastructure of seed and somatic embryo of Acrocomia aculeate (Arecaceae). Sci Agric 67:399–407
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
O’Brien TP, Feder N, Mccully ME (1965) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Oliveira AB, Mendonça MS, Araújo MGP (2010) Aspectos anatômicos do embrião e desenvolvimento inicial de Oenocarpus minor Mart.: uma palmeira da Amazônia. Acta Bot Bras 24:20–24
Ooi SE, Choo CN, Ishak Z, Ong-Abdullah M (2012) A candidate auxin-responsive expression marker gene, EgIAA9, for somatic embryogenesis in oil palm (Elaeis guineensis Jacq.). Plant Cell, Tissue Organ Cult 110:201–212
Portillo L, Santacruz-Ruvalcaba F, Gutiérrez-Mora A, Rodríguez-Garay B (2007) Somatic embryogenesis in Weber cultivar azul. In Vitro Cell Dev Biol Plant 43:569–575
Sáenz L, Azpeitia A, Chuc-Armendariz B, Chan JL, Verdeil JL, Hocher V, Oropeza C (2006) Morphological and histological changes during somatic embryo formation from coconut plumule explants. In Vitro Cell Dev Biol Plant 42:19–25
Sané D, Aberlenc-Bertossi F, Gassama-Dia YK, Sagna M, Trouslot MF, Duval Y, Borgel A (2006) Histocytological analysis of callogenesis and somatic embryogenesis from cell suspensions of date palm (Phoenix dactylifera). Ann Bot 98:301–308
Santos KGB, Mariath JEA, Moço MCC, Bodanese-Zanettini H (2006) Somatic embryogenesis from immature cotyledons of soybean (Glycine max (L.) Merr.): ontogeny of somatic embryos. Braz Arch Biol Technol 49:49–55
Scherwinski-Pereira JE, Guedes RS, Fermino PCP, Silva TL, Costa FHS (2010) Somatic embryogenesis and plant regeneration in oil palm using the thin cell layer technique. In Vitro Cell Dev Biol Plant 46:378–385
Schwendiman J, Pannetier C, Michaux-Ferriere N (1988) Histology of somatic embriogenesis from leaf explants of the oil palm Elaeis guineensis. Ann Bot 62:43–52
Silva RC, Luis ZG, Scherwinski-Pereira JE (2012) Differential responses to somatic embryogenesis of different genotypes of Brazilian oil palm (Elaeis guineensis Jacq.). Plant Cell, Tissue Organ Cult 111:59–67
Steinmacher DA, Cangahuala-Inocente GC, Clement CR, Guerra MP (2007) Somatic embryogenesis from peach palm zygotic embryos. In Vitro Cell Dev Biol Plant 43:124–132
Sugimura Y, Murakami T (1990) Structure and function of the haustorium in germinating Coconut palm seed. Jpn Agric Res Quart 24:1–14
Verdeil JL, Hocher V (2002) Digestion and absorption of food in plants: a plant stomach. Trends Plant Sci 7:280–281
Verdeil JP, Hocher V, Huet C, Grosdemange F, Escoute J, Ferriere N, Nicole M (2001) Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann Bot 88:9–18
Acknowledgments
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Financiadora de Estudos e Projetos (FINEP), Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Carvalho Silva, R., Luis, Z.G. & Scherwinski-Pereira, J.E. The histodifferentiation events involved during the acquisition and development of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.). Plant Growth Regul 72, 67–80 (2014). https://doi.org/10.1007/s10725-013-9837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9837-0