Abstract
Large-scale propagation of oil palm (Elaeis guineensis, Jacq.) is difficult due to its single apical meristem. Thus, obtaining plants is mainly through seed germination, and a long growing period is required before oil production is possible. An alternative to large-scale seedling production is indirect somatic embryogenesis. The aim of this study was to analyze the somatic embryogenesis process in oil palm (E. guineensis Jacq.) with amino acids and low concentrations of auxins. The Tenera hybrid was analyzed by cytochemical and ultrastructural methods and was used to regenerate oil palm plants. First, calli were induced in MS culture media supplemented with 2,4-D and picloram. Two types of calli were obtained, characterized by beige or translucent color. Beige calli had embryogenic characteristics, such as large nuclei with prominent nucleoli, and they were multiplied for 8 months in MM culture (half strength MS, 1 mg L−1 2,4-D, 2 mg L−1 2iP, 1 mg L−1 IBA, 250 mg L−1 citric acid, 10 mg L−1 cysteine, 100 mg L−1 inositol, 1 mg L−1 thiamine, 1 mg L−1 pyridoxine, 1 mg L−1 nicotinic acid, 1 mg L−1 glycine, 200 mg L−1 malt extract, and 100 mg L−1 casein hydrolysate). After multiplication, the MCB culture medium (half strength MS, supplemented with 0.25 mg L−1 NAA, 2 mg L−1 BAP, MM vitamins and 200 mg L−1 malt extract, and 100 mg L−1 casein hydrolysate) was the most efficient for embryo formation, showing meristematic centers with totipotent cells in histochemical analyses. The somatic embryos were developed and germinated in MG medium (half strength MS, 0.45 mg L−1 IAA, 0.25 mg L−1 BAP, and MM vitamins), transplanted into polyethylene tubes containing pine bark substrates, and acclimatized in a greenhouse, achieving a 97% survival rate. The use of picloram for callus induction and somatic embryogenesis is advantageous and multiplication in MM medium is an important step for increasing cell mass. The calli with light beige color and nodular structures have meristematic cells with dense cytoplasm and totipotential features that later give rise to protoderm, procambium, and ground meristem during the globular, cordiform, and torpedo embryogenesis phases. In MCB medium, the concentration of vitamins and amino acids are crucial for somatic embryogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil palm (Elaeis guineensis, Jacq.) is of great economic interest due to high oil production by its fruit, approximately 6 t ha−1 (Anuário 2014), and its use as nutritional food and in the cosmetics, pharmaceuticals, and biofuel industry (Boari 2008). However, the current oil yield of plantations could theoretical be increased to more than 10 t ha−1 (Murphy 2014).
Expansion of oil palm into new markets requires breeding efforts (Sambanthamurthi et al. 2009) and the development of several tools, including biochemical studies, somatic embryogenesis, and genetic transformation. In this respect, the propagation and multiplication of plant material is a culturing issue that remains to be solved.
Seed propagation is further hampered by the long time required for germination (approximately 3 years) and the low germination rate of 30% of seeds sown (Luis et al. 2010; Martine et al. 2009). In addition to time and production rate limitations, production of oil palm seedlings from seeds has the drawback that genetic segregation of relevant traits is required, because parent plants are allogamous, with high heterozygosity (Chanprasert et al. 2012; Martine et al. 2009; Myint et al. 2010). Moreover, propagation by conventional methods, such as through cuttings, is impossible because oil palm has only one shoot apical meristem and, thus, it is only propagated by seed germination (Jouannic et al. 2011).
Oil palm propagation by in vitro techniques is a commercially viable way to supply the more than one hundred million plantlets from tissue culture that are needed per year. Several authors have described production of oil palm plantlets by somatic embryogenesis (Balzon et al. 2013; Duval et al. 1988; Konan et al., 2006; Rival et al. 1997; Scherwinski-Pereira et al. s; Teixeira et al. 1993; Thuzar et al. 2011).
However, oil palm somatic embryogenesis is indirect, requiring induction of embryogenic calli and subsequent regeneration of plants. Oil palm calli are generally heterogeneous, and only a limited number of cells exhibit embryogenic potential. This potential is related to genotype, tissue origin, and growth regulators added to a culture medium (Ammirato 1983; Brown and Atanassov 1985; Carvalho et al. 2013).
Several authors have described oil palm somatic embryogenesis through the use of growth regulators, e.g., high concentrations of auxin, 100 mg L−1 of auxin and picloram (Thuzar et al. 2011; Balzon et al. 2013; Silva et al. 2014b). These authors used zygotic embryos to induce 97.5% embryogenic calli (15 somatic embryos/calli) and the regeneration of 10 plants/callus (Balzon et al. 2013). Scherwinski-Pereira et al. (2010) evaluated the growth regulators 2,4-D, picloram (54 and 108 mg L−1), and amino acid glutamine (500 mg L−1) and observed a reduction in calli induction at lower concentrations of growth regulator.
However, this methodology presents some obstacles, such as the high concentration of growth regulators, which can promote somaclonal variations (Silva et al. 2012; Balzon et al. 2013), initial explants, and zygotic embryos that cannot be mother plant clones. Somaclonal variation observed in oil palm, specifically in flower form and fruit mantling, has limited large-scale commercial production of oil palm through tissue culture. Five percent of oil palm plants derived from somatic embryos show abnormalities in floral development (Armstrong and Phillips 1988; Rival 2000; Corley and Tinker 2003; Mgbeze and Iserhienrhien 2014). These abnormalities have been associated with somaclonal high 2,4-D (Duval et al. 1988; Sogeke 1998) as the cells, especially in the callus stage, remain in culture medium for a long time, increasing their chromosomal instability (Rohani et al. 2003).
Thus, it is necessary to improve the oil palm somatic embryogenesis protocol using other explants and low concentrations of growth regulators. Auxins, such as picloram and 2,4-D (Silva et al. 2012; Balzon et al. 2013), are the substances most widely used for somatic embryogenesis.
Therefore, it is necessary to optimize the process of using low concentrations of growth regulators, and microscopic analyses can help understand callus and somatic embryo development (Bairu et al. 2011). Cells with high embryogenic potential have dense cytoplasms with many organelles and large nuclei. The large quantities of mitochondria indicate a high metabolic rate, suggesting that they are undergoing constant cell division (Aslam et al. 2011; Steinmacher et al. 2011). The absence of cytoplasmic organelles and vacuoles indicates cell death (Filonova et al. 2000; Steiner et al. 2005; Pádua et al. 2014a). Furthermore, microscopy analysis during somatic embryo development allows identification of possible failures in meristematic tissue formation and the lack of synchronization required for embryo development (Benelli et al. 2010; Bar et al. 2014).
This study contributed to elucidate the process of oil palm somatic embryogenesis using low concentrations of growth regulators and amino acids in the culture medium. In addition, embryogenic callus development was histologically characterized. Knowledge regarding histodifferentiation of oil palm somatic embryos would allow for efficient production of clonal plants and an understanding of the cellular features and morphological development associated with distinct embryo stages. Therefore, this study was carried out to develop an efficient system for inducing somatic embryos in the inflorescence of the Tenera hybrid oil palm using low concentrations of auxin and to describe the histodifferentiation of the somatic embryo during plantlet regeneration and development.
Materials and methods
Plant material and disinfection
To carry out this study, we used inflorescences of the Tenera hybrid of E. guineensis Jacq. from the DENPASA company in the state of Pará, Brazil.
The inflorescences were washed in tap water and the first bract was removed. Then they were placed in a laminar flow hood under constant shaking for 20 min in a sodium hypochlorite solution at 1.25% plus 5 drops of Tween per liter. Subsequently, the inflorescences were washed three times in autoclaved distilled water, and the second bract was removed to expose the inflorescences.
Callus induction and multiplication
For callus induction, the explants were inoculated in Petri dishes containing MS medium (Murashige and Skoog 1962) plus the auxin picloram or 2,4-D at concentrations of 0, 5.0, 10.0, or 50.0 mg L−1. All culture media were supplemented with 0.1% activated charcoal.
After inoculation, the explants were kept in a growth room in the dark at a temperature of 27 ± 2 °C. Eight replicates were performed per treatment, with 10 explants each.
Microscopic evaluation began after the appearance of corms, which were monitored every 30 days.
For multiplication of the embryogenic callus, a culture medium (MM) was used, consisting of half strength MS, 1.1 mg L−1 2,4-D, 2 mg L−1 2iP, 1 mg L −1 IBA, 250 mg L−1 citric acid, 10 mg L−1 cysteine, 100 mg L−1 inositol, 1 mg L−1 thiamine, 1 mg L−1 pyridoxine, 1 mg L−1 nicotinic acid, 1 mg L−1 glycine, 200 mg L−1 malt extract, and 100 mg L−1 casein hydrolysate.
The experiment consisted of 20 replications with approximately 50 mg of callus, totaling 1000 mg of callus. The culture medium was replaced every 30 days and the callus was kept in a growth room, in the dark, at a temperature of 27 ± 2 °C.
After 8 months of culturing, the formations of two regions were visualized with masses of distinct colors (light beige and dark beige) that were collected for cytochemical analyses (item 3.1).
Formation and germination of somatic embryos
The light beige calli were transferred to two different culture media. The first, referred to as MCA, had MS half salts and was supplemented with concentrations of 0.25 mg L−1 α-naphthaleneacetic acid (NAA), 2 mg L−1 BAP, 400 mg L−1 malt extract, 100 mg L−1 casein hydrolysate, and 100 mg L−1 inositol. The second medium, called MCB, was composed of MS half salts and was supplemented with 0.25 mg L−1 NAA, 2 mg L−1 BAP, 100 mg L−1 inositol, 1 mg L−1 thiamine, 1 mg L−1 pyridoxine, 1 mg L−1 nicotinic acid, 1 mg L−1 glycine, 400 mg L−1 malt extract, and 100 mg L−1 casein hydrolysate. Calli were kept in a growth chamber under a 16-h photoperiod at a light intensity of 45 μmol m−2 s−1 and a temperature of 26 ± 2 °C.
After 3 months of culturing, inoculated calli showed visual differences in both media. The regeneration in MCA and MCB was analyzed by cytochemical tests and scanning electron microscopy (items 3.1 and 3.2).
The embryos from the previous experiment were transferred to a germination medium (MG), consisting of half strength MS plus 0.45 mg L−1 IAA, 0.25 mg L−1 BAP, 100 mg L−1 inositol, 1 mg L−1 thiamine, 1 mg L−1 pyridoxine, 1 mg L−1 nicotinic acid, and 1 mg L−1 glycine.
All culture media used in this study were supplemented with 3% sucrose and 0.6% agar (Sigma) and had their pH adjusted to 5.7 ± 0.1 before autoclaving at 121 °C, 1.16 atm, for 20 min.
The embryos were kept in a growth chamber under light, at a temperature of 27 ± 2 °C.
The culture medium was replaced every 30 days, and after 90 days, the development of embryos was analyzed by cytochemical tests (Section “Cytochemical analysis”).
Acclimatization
For acclimatization, 50 plants with roots and 50 plants without roots were used. Plants with roots were washed with tap water to remove any residue of culture medium, and they were transplanted in 56 cm polyethylene tubes containing commercial pine bark substrate. After planting, the tubes were transferred to a greenhouse with controlled temperature and humidity.
After 60 days, the seedling survival percentage was evaluated, and the seedlings that survived were considered acclimatized.
The acclimatized plants were transported to the nursery in the Denpasa company in Para, and after 120 days, we counted the number of acclimatized plants.
Microscopic analysis
Cytochemical analysis
Calli and embryos were fixed in FAA (formaldehyde, acetic acid, and ethanol) for 72 h and transferred to 70% ethanol. After fixation, they were placed in a solution of 50% alcohol with Leica resin (7022 18 500) for 12 h and then transferred to pure resin for 48 h. Subsequently, they were soaked in Leica resin, according to the manufacturer’s protocol. The samples were sectioned at a thickness of 5 μm using a rotary microtome and stained with 0.05% solution of toluidine blue and Lugol’s solution. The stained sections were mounted on slides and observed with a Zeiss Scope A1 photonic microscope with a camera attached.
The cell diameters and nuclei were measured using the Axion Vision program.
Scanning electron microscopy
Callus samples were fixed in Karnovsky’s solution (2.5% glutaraldehyde and 2.5% paraformaldehyde) in 0.05 M cacodylate buffer, pH 7.0, for 24 h at 4 °C. The calli were placed in 30% glycerol for 30 min and then washed three times (10 min) in 0.05 M cacodylate buffer and post-fixed in 1% osmium tetroxide for 2 h.
Subsequently, the samples were dehydrated in an increasing gradient of acetone (25, 50, 75, and 90%) for 10 min each and in 100% acetone twice for 10 min. The samples were then dried completely in a critical point drying apparatus using liquid CO2. The samples were subsequently mounted on aluminum brackets (stubs) coated with gold using a 050 SDC gold evaporator, and samples were observed using a LEO EVO 40 XVP scanning electron microscope.
Transmission electronic microscopy
For analysis in a transmission electron microscope (Zeiss EM 109), samples of callus and somatic embryos were immersed in fixative (modified Karnovsky, 2.5% glutaraldehyde, 2.0% paraformaldehyde, cacodylate buffer 0.05 M, pH 7.2) for 24 h and prepared according to the protocol described by Bossola and Russell (1999).
Semithin (0.85 μm) and ultrathin (<100 nm) sections of the samples were cut using a Reichrt-jung (Ultracut) ultramicrotome and stained with uranyl acetate and lead citrate (Bossola and Russell 1999). Examinations and photography were performed using a TE microscope Zeiss EM 109 at 80 kV coupled to a Megaview digital camera and captured with iTEM software (Olympus Software Imaging Solutions).
Results and discussion
Callus formation and multiplication
Callus formation of inflorescences was observed approximately 16 days after explant inoculation in the culture medium with both growth regulators. The highest calli formation was observed in 50 mg L−1 of picloram (92%) or 2,4-D (90%), followed by 10 mg L−1 (88% for picloram and 84% for 2,4-D) and 5 mg L−1 (82% for picloram and 79% for 2,4-D) (Table 1). All calli were watery and translucent (Fig. 1a), formed of a cluster of small isodiametric cells surrounded by elongated cells that had been scattered (Fig. 1b). The calli cells were composed of vacuolated cells with few cytoplasmic organelles, and the cell walls were broken, probably due to an apoptotic process (Fig. 1c). Pádua et al. 2013 reported that oil palm calli arising from leaf explants and cultured in 2,4-D and picloram have elongated, vacuolated, and dispersed cells. Large vacuoles may be related to the degradation of cell contents because the vacuole plays a critical role in programmed cell death (Lam et al. 2000).
Callus induction on the Tenera hybrid oil palm. a Translucent calli. b Photomicrograph of translucent callus cells stained with toluidine blue showing elongated and dispersed cells (arrows). c Transmission electromyography of translucent calli showing cells with large vacuoles (Va) and large gap between cells (black arrows)
In many cases, somatic embryos appear to originate directly from single somatic cells that are induced by either high doses of auxin or stress-related treatments (Halperin 1995; Verdeil et al. 2007). For many species, including oil palm, the most successful studies of vegetative reproduction have used media containing auxin 2,4-D to induce somatic embryogenesis (Konan et al. 2006; Thuzar et al. 2011; Balzon et al. 2013). The standardized media for somatic embryo induction from immature inflorescence is Y3 media supplemented with 2,4-D (9.64 mg L−1), NAA (7.44 mg L−1), 2,4,5-trichlorophenoxyacetic acid (2.55 mg L−1), thiadiazuron (2.20 mg L−1), and 6-benzyladenine (2.25 mg L−1) (Jayanthi et al. 2011). The highest frequency of embryogenic callus induction (89%) was obtained by Balzon et al. (2013), culturing mature zygotic embryos in callus induction medium with 108 mg L−1 picloram and 2.5 g L−1 activated charcoal.
Jayanthi et al. (2015) tested four different auxin treatments, 2,4-D (66.6 mg L−1), picloram (72.4 mg L−1), combinations of 2,4-D + picloram (33.3 and 36.2 mg L−1 each), and NAA (55.8 mg L−1), for callus induction in Dura and Tenera oil palm varieties, and overall callus induction percentage ranged from 54.67 to 82.44% in Dura and 45.67 to 80% in Tenera palm. In our study, we obtained a good percentage of callus formation with a concentration of approximately one tenth the amount of picloram.
Activated charcoal is the main antioxidant that is used for absorption of phenolic compounds in palm tissue (Steinmacher et al. 2007), but the activated charcoal absorbs auxin and this effect must be coupled with the use of high concentrations of 2,4-D. In this study, just 0.1% was effective in controlling oxidation, allowing a decrease in the auxin concentration.
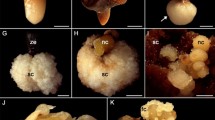
Approximately 10 months later, 6% of the explants maintained in the culture medium supplemented with 10 and 50 mg L−1 of picloram showed new calli, with nodular features and beige color (Fig. 2a). These calli had cells isodiametrically arranged in clusters (Fig. 2b), with large nuclei with prominent nucleoli, mitochondria, a Golgi apparatus, endoplasmic reticulum, and a large amount of starch grain (Fig. 2c). The calli maintained in the culture medium supplemented with 10 and 50 mg L−1 of 2,4-D did not exhibit this type of callus, but remained translucent.
a Beige and nodular calli of Tenera hybrid oil palm. b Photomicrography of embryogenic characteristics of cells stained with toluidine blue. c Transmission electron microscopy of calli showing nucleus (N) with prominent nucleolus (Nu). d Amyloplast (arrow) (A). e Mitochondrial division (arrows). f Golgi complex (GC)
Similar results were observed by Moura et al. (2009) in macaúba using a culture medium supplemented with picloram. These authors observed the induction of two types of calli, a white and spongy callus and a nodular and yellow callus, with embryogenic characteristics. In oil palm, calli induced in zygotic embryos was observed the presence of isodiametric small cells with dense cytoplasm, nuclei, and nucleoli, these cells were characterized as meristematic, and they subsequently regenerated into plants (Silva et al. 2014; Bar and Dawayat 2014).
In calli obtained from inflorescences of palm trees cultured in a medium with picloram (similar to the conditions used in this study), the formation of somatic embryos on meristematic regions with extensive cells proliferation was observed (Scherwinski-Pereira et al. 2010). Nodular calli with embryogenic characteristics were also observed in oil palm obtained from zygotic embryos cultured in a medium supplemented with 2,4-D growth regulator, and these embryos were then developed into pro-embryos and subsequently into globular embryos (Ângelo et al. 2009). Jayanthi et al. (2015) characterized oil palm embryogenic callus induction by the appearance of white to yellowish globular or nodular structures with a suspensor region, but the embryogenic calli began to multiply and proliferate rapidly only after the fourth subculture (every 4 months).
Therefore, considering histological analyses, the calli from inflorescences cultured in 10 and 50 mg L−1 of picloram, with beige color and nodular morphology, were subcultured in MM culture medium for multiplication. After 8 months in MM medium, the calli exhibited growth of approximately five times the initial volume, and some parts of calli remained beige and others began to turn dark. In the beige part, light beige and dark beige regions could be distinguished; these parts were analyzed by light microscopy and showed cellular differences.
Dark beige parts were composed of large, elongated cells (55.0 × 43.0 μm), and they did not form cell clusters or have embryogenic cell characteristics. The light beige calli had regions with small cells (1.0 μm width × 14.0 μm length). Generally, embryogenic cells are small and isodiametric, a feature that allows intense cell division and formation of clusters that give rise to somatic embryos (Ângelo et al. 2013; Silva et al. 2014b).
In MM medium, the auxin concentration was decreased to 1 mg L−1 of 2,4-D, and 1 mg L−1 IBA and added 2 mg L−1 2iP. Auxins and cytokinins are plant growth regulators involved in plant cell differentiation, and the ratio between them is crucial for specification of cell identity (Jiménez 2005). For maintenance and differentiation of shoot and root apical meristems, cytokinin is required (Perilli et al. 2010). The anatomical characteristics observed in the light beige calli suggested their potential to proliferate and differentiate somatic embryos, according to the signaling exerted by the specific hormonal concentration ratio.
Embryo regeneration and germination
The light beige calli were transferred to two culture media (MCA and MCB) for somatic embryo conversion. The calli cultured in MCA regeneration medium showed dispersed, elongated, and plasmolyzed cells, characterized as nonviable cells, which did not regenerate plants (Fig. 3a). The process of callus cell conversion in somatic embryos occurs with the cessation of repetitive cycles of cell division, associated with physiological and biochemical conditions and with environmental stimuli necessary for cellular differentiation and the subsequent maturation of somatic embryos. These must have high quality and the ability for conversion to plants (Guerra et al. 1999). Calli cultured in MCA medium lost their embryogenic potential and did not trigger somatic embryo conversion. In contrast, 80% of calli cultured in MCB medium showed the formation of somatic embryos. Isodiametric cells (Fig. 3b) and the onset of meristematic centers forming somatic embryos with phenolic compounds (reaction with toluidine) (Fig. 3c) were observed in anatomical analysis. The meristematic centers have totipotent cells and can regenerate a complete somatic embryo (Verdeil et al. 2007). Totipotent cells can give rise to all the cell types constituting the plant body (Verdeil et al. 2007). The characteristics of the pro-embryos observed in this study were also visualized in acai pro-embryos, in a culture medium containing the growth regulators NAA and 2iP, as cells with prominent nuclei and dense cytoplasms (Scherwinski-Pereira et al. 2012).
Tenera hybrid oil palm calli cultured in a medium for conversion into somatic embryos. a Scanning electron micrograph of callus in MCA culture medium, exhibiting elongated and irregularly shaped cells. 20 μm bars. b Scanning electron micrograph of callus in MCB culture medium, exhibiting isodiametric cells. c Photomicrography of somatic embryo histochemical analysis in MCB culture medium. Phenolic compounds (arrows). 100 μm bars
Thus, two cycles occur during the process of somatic embryo formation. In the first cycle, auxins are required for induction and development of embryogenic cells, as observed in culture media with 2,4-D and picloram. The second cycle reduces the concentration or eliminates auxins in the culture medium for the development of pro-embryos (Feher et al. 2003). The auxins were eliminated in MCA and MCB media. However, this tissue culture process is not only affected by the type and concentration of growth regulators, but also by numerous other culture conditions, including the composition of the culture medium and genotype (Feher et al. 2003). The MCA and MCB media differ in vitamin and aminoacyl composition. The MCB medium has 1 mg L−1 thiamine, 1 mg L−1 pyridoxine, 1 mg L−1 nicotinic acid, and 1 mg L−1 glycine. The amino acid-free medium generated fewer somatic embryos than cultures grown in the amino acid-treated medium; the addition of an organic nitrogen form has a positive effect on somatic embryogenesis (Zouine, Hadrami 2007).
For somatic embryo conversion, some amino acids may be required to increase the regeneration rate. Moreover, the introduction of more than one amino acid in the culture medium leads to better results than their individual use (Asad et al. 2009). Studies have demonstrated that during embryogenesis, total protein content increases because of the synthesis of storage proteins, accumulation of stress-related proteins and metabolic proteins (such as those related to glycolysis), Krebs cycle, biosynthesis of carbohydrates, and metabolism of amino acids (Aberlenc-Bertossi et al. 2008). In somatic embryogenesis of African oil palm, there was an accentuated synthesis of amino acids, an increase of more than 10 times, from 30 to 90 days of culturing in the developing explant tissues, and this rapid rise in the levels of total free amino acids in the culture tissues may also be related to the high concentrations of compounds in the nutrient medium (Gomes et al. 2014). The authors found that arginine, glutamine, asparagine, alanine, threonine, glycine, serine, proline, leucine, and histidine were the most relevant amino acids that composed the total free amino acids in somatic embryogenesis (Gomes et al. 2014).
The glycine-rich proteins are expression modulated by phytohormones and accumulate upon auxin depletion, demonstrating the negative regulation exerted by this phytoregulator (Reddy, Poovaiah 1987). A glycine-rich domain is required for stabilization and flexibility of the molecular interaction in different multimolecular complexes (Alberts 1998) because the multiple molecular machines play distinct roles in cell physiology (Alberts 1998). Studies conducted on glycine-rich proteins in plant cells have provided new and interesting insights into molecular and cell biology, and complex regulated promoters and distinct mechanisms of gene expression regulation have been shown (Sachetto-Martins, Franco 2000). Corroborating this data, the presence of glycine in the MCB culture medium is important for the development of oil palm somatic embryos.
Regarding the concentration of vitamins in the MCB medium, vitamins are known to be “essential” for plants, where they generally play their essential roles in metabolism, plus roles characteristic of plant metabolic pathways (Miret and Munné-Bosch 2014). For example, embryo or early-seedling lethal phenotypes are observed in plants with complete loss of the function of enzymes required for de novo synthesis of the vitamins pyridoxine, thiamine, and nicotinic acid, and other B complex vitamins (Hanson and Gregory 2011). The high supply of vitamins in the MCB medium compared to the MCA medium was essential for somatic embryogenesis development.
Pyridoxine is a coenzyme in transamination reactions; nicotinic acid can be component of the coenzymes nicotinamide adeninedinucleotide (NAD) and nicotinamide adeninedinucleotide phosphate (NADP); and phosphorylated thiamine acts as a coenzyme in numerous physiological processes, including glycolysis, the pentose phosphate pathway, and the synthesis of nucleic acids and the niacin-containing coenzyme NADPH (Ahn 2005).
Moreover, nicotinic acid is the precursor of pyridine alkaloids, notably trigonelline, an N-methyl conjugate of nicotinic acid, accumulated upon stress as an osmoprotectant and involved in the control of the cell cycle (Matsui 2007). Nicotinic acid can be involved in control of water uptake and correct cell divisions during oil palm somatic embryogenesis development, as observed in MCB medium. Thiamine and pyridoxine are also potent antioxidants in vivo (Havaux et al. 2009; Boubakri et al. 2016), important for controlling the oxidation that difficulties the in vitro culture, decreasing somatic embryogenesis.
Somatic embryos obtained in the MCB culture medium were transferred to the MG culture medium (half strength MS, 0.45 mg L−1 IAA, 0.25 mg L−1 BAP, and MM vitamins), which develop by passing through the following embryogenic stages: globular, heart-shaped, and torpedo (Fig. 4).
Photomicrography of embryogenic stages of Tenera hybrid oil palm somatic embryos stained with toluidine blue. a Callus mass at the onset of formation of several somatic embryos. b Individual somatic embryo in the globular stage. c Somatic embryo in the cordiform stage. d Somatic embryo in the torpedo stage. Promeristem (arrows). Pt = protoderm. Mf = fundamental ground meristem. Bars = 100 μM
The sequence of embryo induction in oil palm can be divided into four phases: (i) callus induction (ii) proembryogeny, (iii) early embryogeny, (iv) late embryogeny and intensive histogenesis, including establishment of the root and shoot meristems.
In numerous nodular structures in the early formation of somatic embryos, prior to individualization of the cell, meristematic cells and the beginning of procambium formation were observed (Fig. 4a, black arrows). Later, in the heart-shaped stage, the formation of three meristematic tissues, protoderm, ground meristem, and procambium, with a closed vascular system, were observed.
The protoderm, ground meristem, and procambium were clearly identified by Silva (2014b). Somatic embryos in the more advanced torpedo stage, surrounded by a protoderm and with procambial cell organization, were also observed.
These were observed through the heart-shaped, torpedo, and cotyledon stages, which implies normal development of these embryos.
We also observed asynchrony in embryo development. The embryogenic calli multiplied continuously in the MG culture. Asynchronous development of somatic embryos in palms is commonly reported in the literature (Silva et al. 2014b). Asynchrony during the formation of somatic embryos can limit their production on a commercial scale (Pedroso and Parent 1995). One hypothesis regarding asynchrony in the formation of somatic embryos is related to culturing on solid medium (Sumaryono et al. 2008). This may be due to reduced nutrient turnover in relation to the water and increased contact with nutrients. In somatic embryogenesis in oil palm, asynchronous formation occurs (Bar et al. 2014). The lack of synchrony of somatic embryos may cause difficulties during the in vitro culture process, such as rooting, and acclimatization.
After 8 months of culturing in MG medium, the conversion from embryos to plants was assessed. On average, 100 mg of MG somatic embryo calli regenerated 13 plants (Fig. 5).
Root development was observed on 13% of plants cultured in MG medium (Fig. 6). Histological analyses showed development of the stem apex with eight differentiated leaf primordia.
Plant acclimatization
All plants obtained in vitro passed through the acclimatization process in a greenhouse for approximately 150 days. The plants previously rooted in vitro, with roots longer than 1 cm, acclimatized, whereas the plants without roots were more susceptible to acclimatization.
The plants rooted in vitro had an 85% survival rate during acclimatization, and the plants without roots had a 40% survival rate during acclimatization (Pádua et al. 2014b). Ninety-seven percent of the total amount of acclimatized plants survived in the nursery. Gomes et al. (2015) reported that 74.6% of rooted plantlets survived the acclimatization process, significantly higher than the percentage observed for plantlets devoid of roots (47.2%). Thuzar et al. (2011) observed that after acclimatization for 4 weeks in a plastic chamber, more than 85% of transferred plants developed successfully in the soil. Steinmacher et al. (2007) observed that for peach palm the existence of roots in acclimatized plantlets was essential for survival.
Conclusion
In conclusion, this study shows alternative induction of somatic embryogenesis of oil palm with a low concentration of picloram auxins. The induced calli, beige in color and with nodular morphology, exhibited meristematic cells with totipotent features.
Beige calli, after multiplication for 8 months in MM medium, formed light beige clusters that showed anatomical and ultrastructural characteristics with embryogenic potential, and they were successfully used in regeneration of plants. The calli were transferred to MCB medium, where they developed somatic embryos. The somatic embryos were regenerated in MG medium, and the plants rooted and acclimatized. The concentration of thiamine, pyridoxine, nicotinic acid, and glycine in the MCB medium during culturing was essential for obtaining somatic embryos. Therefore, we showed the effect of culture medium, with different concentrations of vitamins and amino acids, on somatic embryo development. The somatic embryos showed the cellular events similar to zygotic embryogenesis pathway. The significant finding in this study is that we were able to obtain a high percentage of primary callus induction from inflorescences with a lower concentration of auxins. The use of picloram for callus induction embryogenesis was found to be advantageous. The strategy for mass multiplication in MM medium is an important step for increasing cell mass. The calli of light beige color and nodular structures were histologically shown to have meristematic cells with a dense cytoplasm and totipotential features.
These calli can be multiplied in MM and converted on embryos on MCB medium, where the vitamin and amino acid concentrations are crucial for somatic embryogenesis. The somatic embryos have protoderm, procambial strands, and a ground meristem, and they developed plants that were rooted and acclimatized.
References
Aberlenc-Bertossi F, Chabrillange N, Duval Y, Tregear J (2008) Contrasting globulin and cysteine proteinase gene expression patterns reveal fundamental developmental differences between zygotic and somatic embryos of oil palm. Tree Physiol 28:1157–1167
Ahn IP, Kim S, Lee YH (2005) Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol 138:1505–1515. doi:10.1104/pp.104.058693
Alberts B. (1998). The cell as a collection overview of protein machines: preparing the next generation of molecular biologists. Cell. 92:291-294. Doi: org/10.1016/S0092-8674(00)80922-8
Ammirato PV (1983) Embryogenesis. In: Evans DA, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of plant cell culture. Mac Millan, New York, pp 82–123
da Ângelo CS, Steinmacher DA, Lopes R, da Cunha RNV, Guerra MP (2013) Histological analysis and transcription profiles somatic embryogenesis in interspecific hybrids of Elaeis guineenses x Elaeis oleifera. Agric Sci 4:1–11. doi:10.4236/as.2013.411A001
da Angelo PCS, Lopes SR, Moraes LAC, da Cunha RNV (2009) Embryogenic calli induced in interspecific (Elaeis guineensis x E. oleifera) hybrid zygotic embryos. Crop Breed Appl Biotechnol 9:274–277. doi: org/10.4236/as.2013.411A001
Armstrong CL, Phillips RL (1988) Genetic and cytogenetic variation in plants regenerated from organogenic and friable, embryogenic tissue cultures of maize. Crop Sci 28:363–369. doi:10.2135/cropsci1988.0011183X002800020038x
Asad S, Arshad M, Mansoor S, Zafar Y (2009) Effect of various amino acids on shoot regeneration of sugarcane (Saccharum officinarum L.). Afr J Biotechnol 8:1214–1218. doi: 10.5897/AJB
Aslam J, Khan SA, Cheruth AJ, Mujiba SMP, Srivastava PS (2011) Somatic embryogenesis, scanning electron microscopy, histology and biochemical analysis at different developing stages of embryogenesis in six date palm (Phoenix dactylifera L.) cultivars. Saudi J Biol Sci 18:369–380. doi:10.1016/j.sjbs.2011.06.002
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173. doi:10.1007/s10725-010-9554-x
Balzon TA, Luis ZG, Scherwinski-Pereira JE (2013) New approaches to improvethe efficiency of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.) from mature zygotic embryos. In vitro Cell Dev Biol Plant 49:41–50. doi:10.1007/s11627-012-9479-3
Bar OAE, Dawayat MME (2014) Histological changes on regeneration in vitro culture of date palm (Phoenix dactylifera) leaf explants. Aust J Crop Sci 8:848–855. doi:10.21475/ajcs.2016.10.12
Benelli C, Germana MA, Camino T, Beghe D, Fabbri A (2010) Morphological and anatomical observations of abnormal somatic embryos from anther cultures of Citrus reticulata. Biol Plant 54:224–230. doi:10.1007/s10535-010-0040-0
de Boari A J (2008) Estudos realizados sobre o amarelecimento fatal do dendezeiro (Elaeis guineensis Jacq.). Belém: EMBRAPA Amazônia Oriental.59 p. (Documentos, 348)
Bossola JJ, Russell LD (1998) Electron microscopy, 2nd. edn. Jones and Bartlett, Boston, p 670
Boubakri H, Gargouri M, Mliki A, Brini F, Chong J, Jbara M (2016) Vitamins for enhancing plant resistance. Planta 244:529–543. doi:10.1007/s00425-016-2552-0
Brown DCW, Atanassov A (1985) Role of genetic background in somatic embryogenesis in Medicago. Plant Cell Tissue Organ Cult 4:111–122. doi:10.1007/BF00042269
de Carvalho MA, Paiva R, Alves E, Nogueira RC, Stein VC, Castro EM (2013) Morphogenetic potential of native passion fruit (Passiflora gibertii N. E. Brown.) calli. Braz J Bot 36:141–151. doi:10.1007/s40415-013-0015-4
Chanprasert W, Myint T, Srikul S, Wongsri O (2012) Effects of neonicotinoid and method of breaking dormancy on seed germination and seedling vigour of oil palm (Elaeis guineensis Jacq.) J. Oil Palm Res 24:1227–1234
Corley RHV, Tinker PB (2003) The oil palm. Wiley, Oxford. doi:10.1002/9780470750971
Duval Y, Durand-Gasselin T, Konan KC (1988) In vitro vegetative propagation of oil palm (Elaeis guineensis Jacq.) Oleagineux 43:145–147
Feher A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228. doi:10.1023/A:1024033216561
Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, Von Arnold S (2000) Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm Norway spruce. J Cell Sci 113:4399–4411
Gomes HT, Bartos PMC, Scherwinski-Pereira JE (2015) In vitro cell. Dev.Biol.-Plant 51:111. doi:10.1007/s11627-015-9669-x
Gomes HT, Bartos PMC, Silva CO, do Amaral LIV, Scherwinski-Pereira JE (2014) Comparative biochemical profiling during the stages of acquisition and development of somatic embryogenesis in African oil palm (Elaeis guineensis Jacq.) Plant Growth Regul 74:199. doi:10.1007/s10725-014-9901-4
Guerra PG, Torres AC, Teixeira JB (1999) Embriogênese somática e sementes sintéticas. In: Torres AC, Caldas LS, Buso IA (Ed.). Cultura de tecidos e transformação genética de plantas. Braília: EMBRAPA-CNPH 2:533–568
Halperin W (1995) In vitro embryogenesis: some historial issues and unresolved problems. In: In vitro embryogenesis in plants (Thorpe, T.A., ed.): 1–16, Kluwer Academic Publishers
Hanson AD, Gregory JF (2011) Folate biosynthesis, turnover, and transport in plants. Annu Rev Plant Biol 62:105–125. doi:10.1146/annurev-arplant-042110-103819
Havaux M, Ksas B, Szewczyk A, Rumeau D, Franck F, Caffarri S, Triantaphylides C (2009) Vitamin B6 deficient plants display increased sensitivity to high light and photooxidative stress. BMC Plant Biol 9:130. doi:10.1186/1471-2229- 9-130
Jayanthi M, Susanthi B, Mohan NMM, Manda PK (2015) In vitro somatic embryogenesis and plantlet regeneration from immature male inflorescence of adult Dura and Tenera palms of Elaeis guineensis (Jacq.) SpringerPlus 4:256. doi:10.1186/s40064-015-1025-4
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. J Plant Growth Reg 47:91–110. doi:10.1007/s10725-005-3478-x
Jouannic S, Lartaud M, Herve J, Collin M, Orieux Y, Verdeil J-L, Tregear JW (2011) The shoot apical meristem of oil palm (Elaeis guineensis; Arecaceae): developmental progression and dynamics. Ann Bot 108:1477–1487. doi:10.1093/aob/mcr019
Konan EE, Durand-Gasselin T, Kouadio JY, Flori A, Riva A (2006) A modeling approach of the in vitro conversion of oil palm (Elaeis guineensis) somatic embryos. Plant Cell Tissue Organ Cult 84:99–112. doi:10.1007/s11240-005-9010-1
Lam E, Fukuda H, Greenberg J (2000) Programmed cell death of tracheary elements as a paradigm in plants. Plant Mol Biol 44:245–253. doi:10.1023/A:1026532223173
Luis ZG, Bezerra KMG, Scherwinski-Pereira JE (2010) Adaptability and leaf anatomical features in oil palm seedlings produced by embryo rescue and pre-germinated seeds. Braz J Plant Physiol. 22:209-215. Doi: org/10.1590/s1677-04202010000300008
MAPA. Anuário Estatístico de Agroenergia 2014; Statistical Yearbook of Agroenergy 2014
Martine BM, Laurent KK, Pierre BJ, Eugene KK, Hilaire KT, Justin KY (2009) Effect of storage and heat treatments on the germination of oil palm (Elaeis guineensis Jacq.) seed. Afr JAgric Res 4:931–937. doi:10.1590/S2317-15372013000300004
Matsui A, Yin Y, Yamanaka K, Iwasaki M, Ashihara H (2007) Metabolic fate of nicotinamide in higher plants. Physiol Plant 131:191–200. doi:10.1111/j.1399-3054.2007.00959.x
Mgbeze GC, Iserhienrhien A (2014) Somaclonal variation associated with oil palm (Elaeis guineensis Jacq.) clonal propagation: a review. Afr J Biotechnol 13:989–997. doi:10.5897/AJBX12.011
Miret JA, Munné-Bosch S (2014) Plant amino acid-derived vitamins: biosynthesis and function. Amino Acids 46:809. doi:10.1007/s00726-013-1653-3
Moura EF, Motoike SY (2009) Induction of somatic embryogenesis in immature seeds of guava tree cv. Paluma Rev Bras Frutic 31:507–511. doi:10.1590/S0100-29452009000200027
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Murphy DJ (2014) The future of oil palm as a major global crop: opportunities and challenges. J Oil Palm Res 26:1–24
Myint T, Chanprasert W, Srikul S (2010) Germination of seed of oil palm (Elaeis guineensis Jacq.) as affected by different mechanical scarification methods. Seed Sci. Technol 38:635–645. doi:10.15258/sst.2010.38.3.11
Pádua MS, Paiva LV, Labory CRG, Alves E, Stein VC (2013) Induction and characterization of oil palm (Elaeis guineensis Jacq.) pro-embryogenic masses. An Acad Bras de Ciênc 85:1545–1556. doi:10.1590/0001-37652013107912
Pádua MS, Paiva LV, da Silva LC, do Livramento KG, Alves E, Castro AHF (2014a) Morphological characteristics and cell viability of coffee plants calli. Ciênc Rural 44:660–665. doi:10.1590/S0103-84782014000400014
Pádua MS, Paiva LV, da Silva LGT, Silva LC, Stein VC (2014b) In vitro development and acclimatization of dendezeiro (Elaeis guineenisis). Rev Árvore 38:1095–1102. doi:10.1590/S0100-67622014000600014
Pedroso CM, Pais MS (1995) Factors controlling somatic embryogenesis. Palnt Cell Tiss Org Cult 43:147–154. doi:10.1007/BF00052170
Perilli S, Moubayidin L, Sabatini S (2010) The molecular basis of cytokinin function. Curr Opin Plant Biol 13:21–26. doi:10.1016/ j.pbi.2009.09.018
Reddy ASN, Poovaiah BW (1987) Biochem Biophys Res Commun 147:885–891
Rival A (2000). Somatic embryogenesis in oil palm. In: somatic embryogenesis in woody plants. Jain, S.M., Guptal, T. K and Newton, R.I (eds). Kluwer Academic Publishers, Netherlands 6:249–290
Rival A, Beule T, Barre B, Hamon S, Duval Y, Noirot M (1997) Comparative flow cytometric estimation of nuclear DNA content in oil palm (Elaeis guineensis Jacq) tissue cultures and seed-derived plants. Plant Cell Rep 16:884–887. doi:10.1007/s002990050339
Rohani O, Zamzuri I, Tarmizi AH (2003) Oil palm cloning: MPOB protocol. MPOB Technology No. 26. MPOB, Bangi
Sachetto-Martins G, de Franco LODD (2000) Plant glycine-rich proteins: a family or just proteins with a common motif. Biochim Biophys Acta (BBA) 21:1–14
Sambanthamurthi R, Singh R, Kadir APG, Abdullah MO, Kushairi A (2009) Opportunities for the oil palm via breeding and biotechnology. In: Mohan Jain S, Priyadarshan PM (eds) Breeding Plantation Tree Crops: Tropical Species. Springer, New York, pp 377–421. doi:10.1007/978-0-387-71201-7
Scherwinski-Pereira JE, da Guedes RS, Fermino PCP Jr, Silva TL, Costa FHS (2010) Somatic embryogenesis and plant regeneration in oil palm using the thin cell layer technique. In Vitro Cell Dev Biol Plant 46:378–385. doi:10.1007/s11627-010-9279-6
Scherwinski-Pereira JE, da Guedes RS, da Silva RA, Fermino PCP Jr, Luis ZG, de Freitas EO (2012) Somatic embryogenesis and plant regeneration in açaí palm (Euterpe oleracea). Plant Cell Tissue Organ Cult 109:501–508. doi:10.1007/s11240-012-0115-z
de Silva RC, Carmo LS, Luis ZG, Silva LP, Scherwinsk-Pereira JE, Mehta A (2014a) Proteomic identification of differentially expressed proteins during the acquisition of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.) J Proteomics 104:112–127b. doi:10.1016/j.jprot.2014.03.013
de Silva RC, Luis ZG, Scherwinski-Pereira E (2014b) The histodifferentiation events involved during the acquisition and development of somatic embryogenesis in oil palm (Elaeis guineenses Jacq.) Plant Growth Regulation 72:67–80a. doi:10.1007/s10725-013-9837-0
de Silva RC, Luis ZG, Scherwinski-Pereira JE (2012) Differential responses to somatic embryogenesis of different genotypes of Brazilian oil palm (Elaeis guineensis Jacq.) Plant Cell Tissue Organ Cult 111:59–67. doi:10.1007/s11240-012-0170-5
Sogeke AK (1998) Stages in the vegetative propagation of oil palm (Elaeis guineensis Jacq.) through tissue culture. J Oil Palm Res 10:1–9
Steiner N, do Vieira FN, Maldonado S, Guerra MP (2005) Effect of charcoal source on morphology and histodiffentiation of Araucaria angustifolia embryogenic cultures. Braz Arch Biol Technol 48:895–903. doi:10.1590/S1516-89132005000800005
Steinmacher DA, Clement CR, Guerra MP (2007) Somatic embryogenesis from immature peach palm inflorescence explants: towards development of an efficient protocol. Plant Cell Tissue Org Cult 89:15–22. doi:10.1007/s11240-007-9207-6
Steinmacher DA, Guerra MP, Saaresurminsk K, Lieberei RA (2011) A temporary immersion system improves in vitro regeneration of peach palm through secondary somatic embryogenesis. Ann Bot 108:1463–1475. doi:10.1093/aob/mcr019
Sumaryono RI, Kasi PD, Ginting G (2008) Growth and differentiation of embryogenic callus and somatic embryos of oil palm (Elaeis guineensis Jacq.) in temporary immersion system. Indones J Agric 1:109–114
Teixeira JB, Sondahl MR, Kirby EG (1993) Somatic embryogenesis from immature zygotic embryos of oil palm. Plant Cell Tissue Org Cult 34:227–233. doi:10.1007/BF00029711
Thuzar M, Vanavichit A, Tragoonrung S, Jantasurivarat C (2011) Efficient and rapid plant regeneration of oil palm zygotic embryos cv. ‘Tenera’ through somatic embryogenesis. Acta Physiol Plant 33:123–128. doi:10.1007/s11738-010-0526-6
Verdeil J-L, Alemanno L, Niemenak N, Tranbarger TJ (2007) Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci 12:245–252. doi:10.1016/j.tplants.2007.04.002
Zouine J, Hadrami IEI (2007) Effect of 2, 4-D, glutamine and BAP on embryogenic suspension culture of date palm (Phoenix dactylifera L.) Sci Hortic 112:221–226. doi:10.1016/j.scienta.2006.12.041
Acknowledgements
Our thanks to the Laboratory of Electron Microscopy and Ultrastructural Analysis, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Pádua, M.S., Santos, R.S., Labory, C.R.G. et al. Histodifferentiation of oil palm somatic embryo development at low auxin concentration. Protoplasma 255, 285–295 (2018). https://doi.org/10.1007/s00709-017-1143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1143-7