Abstract
Cucumber (Cucumis sativus L.) seeds were pretreated with exogenous abscisic acid (ABA) prior to germination. After germination, seedlings with three leaves were exposed to gradual dehydration. The effects of ABA on photosynthetic rate (Pn), daily water loss (WL) and water utilization efficiency (WUE) during dehydration were investigated, in addition to the variation of carbohydrates in leaves. ABA improved the Pn, WL and WUE of cucumber seedlings during dehydration. After rehydration, the seedlings pretreated with ABA showed a higher recovery in Pn, WL and WUE, as compared to those without an ABA pretreatment. Subsequent to dehydration, concentration of stachyose, raffinose, sucrose, glucose, and fructose increased in seedlings pretreated with ABA. Dehydration altered the proportions of the sugars in the total carbohydrates, and accelerated the accumulation of stachyose, raffinose and sucrose. After rehydration, carbohydrate concentrations of seedlings pretreated with ABA recovered to levels observed prior to dehydration. These results demonstrated that pretreatment of seeds with exogenous ABA enhanced carbohydrate tolerance to dehydration of cucumber seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gradual soil water depletion is the most common situation for drought in natural ecosystems. When plants subjected to dehydration, it usually begins with decreased stomata conductance, which in turn leads to reduced net CO2 assimilation rate, impaired photosynthesis, followed by solute accumulation in cells, and finally, when water availability is very low, leaf senescence is induced and shoots growth ceases (Passioura 1996).
Synthesis, degradation, and transport of soluble sugars are thought to interactively control the endogenous sugar concentration in higher plants in response to environmental conditions (Bachmann et al. 1994; Strand et al. 2003). During water stress, some plants accumulate soluble carbohydrates (Ingram et al. 1997; Ghasempour et al. 1998; Whittaker et al. 2001; Scott 2000; Cooper and Farrant 2002; Norwood et al. 2003) such as the disaccharide, trehalose, and the raffinose family oligosaccharides (RFOs) (Shaun et al. 2007). Those soluble carbohydrates may function as cryoprotectants, antioxidants and osmotic regulators and act as compatible solutes or osmoprotectants to allow osmotic adjustment of plant cells exposed to water deficit stress (Blackman et al. 1992; Joyce et al. 2003). Sugars can also act as regulators of gene expression and signal molecules. Until recently, however, there were few observations of carbohydrate metabolism in cucumber (Miao et al. 2007; Meng et al. 2008).
In the green leaf tissue of variegated coleus (Coleus blumei Benth.), drought stress greatly decreased the levels of the RFOs stachyose and raffinose, as well as other non-structural carbohydrates (galactinol, sucrose, hexoses and starch), and decreased galactinol synthase activity was accompanied by an accumulation of O-methyl-inositol (Wattana and Monica 1999). RFOs have been suggested to act as anti-stress agents in both generative and vegetative tissues (Koster and Leopold 1988; Bachmann et al. 1994), and to play a protective role against desiccation damage (Horbowicz and Obendorf 1994; Black et al. 1996; Joyce et al. 2003). To date, the role of compatible solutes, particularly oligosaccharides such as RFOs, in the dehydration tolerance of cucumber has not been investigated.
Raffinose biosynthesis is regulated by the action of two enzymes, galactinol synthase (GolS), which catalyzes the committed step of the conversion of UDP-galactose and myo-inositol to galactinol, and raffinose synthase. Raffinose degradation proceeds by the action of galactosidase, which catalyzes the hydrolytic cleavage of the terminal-linked moiety from gal-containing oligosaccharides (Joyce et al. 2003). Galactinol is found exclusively in plants and serves solely as the galactosyl donor for RFO biosynthesis (Peterbauer et al. 2002). Galactinol synthase activity is the key regulatory factor in RFO biosynthesis.
ABA concentration increases significantly in xylem sap, leaves and reproductive structures of drought-stressed plants (Setter et al. 2001; Liu et al. 2003). During dehydration in cucumber, ABA may contribute to water loss resistance in the soil through increased Pn, stomata conductance, chlorophyll fluorescence and improved osmoprotection (Wang et al. 2010). ABA can also modify carbohydrate concentrations and the activities of the enzymes involved in carbohydrate biosynthesis (Blöchl et al. 2005; Kobashi et al. 1999; Taji et al. 2002). Accumulation of stachyose and raffinose has been found in ABA treated somatic embryos of alfalfa (Blöchl et al. 2005), and ABA treatment also leads to sugar accumulation in peach fruit and cucumber during low temperatures (Kobashi et al. 1999; Meng et al. 2008). To date, the mechanisms of ABA effects on carbohydrate concentrations in water deficit stressed cucumber are not clearly understood.
Cucumber, one of the most popular greenhouse vegetables in China, is highly sensitive to water deficit stress and the effect of water stress on the growth of cucumber have been well documented (Wang and Zhang 2000; Wang et al. 2010). As ABA is involved in drought-induced root-to-leaf signaling, the objective of this study was to investigate the effects of dehydration, in regard to content and composition in carbohydrates, on cucumber seedlings after germination of ABA pre-treated seeds, and to characterize the plant defense strategies used by cucumber to circumvent the deleterious effects of water stress on seedling growth. This study also provides information on carbohydrates involved in the plant’s response and acclimatization to, and recovery from, a gradual water deficit due to low water availability.
Materials and methods
Cucumber (Cucumis stivus L.) cultivar ‘Zhongnong 12’, purchased from the Institute of Vegetable and Flower Chinese Academy of Agriculture Science, was used for all experiments in this study. Stachyose, raffinose, sucrose, galactose, fructose and glucose were purchased from Sigma-Aldrich Trade Co., Ltd (Shanghai, China).
Seed germination test
Seeds were surface disinfected by submerging them in 1% NaOCl for 20 min, followed by rinsing with sterile distilled water. The disinfected seeds were soaked in aqueous solutions containing 0, 100, 300 or 500 μM (±) ABA for 6 h at room temperature, and then placed in Petri dishes (90 mm diameter) that were lined with two sheets of sterilized filter paper (90 mm diameter, Whatman No. 3) and moistened with 5 ml of sterilized water. The Petri dishes were placed in the dark in an environmental chamber and maintained at 25°C for 3 days. Seeds were watered daily with sterilized water, and the germination status was recorded every 12 h. A total of 50 seeds were assessed, with five replicates for each treatment. As demonstrated in our laboratory previously (Wang et al. 2010), 500 μM ABA impacted seed germination at 12 h, this was used as the final ABA concentration for the dehydration and rehydration studies.
Seedling dehydration test
Seeds were disinfected and subsequently soaked in either water or 500 μM ABA at 25°C in the dark for 6 h. Seeds were then transferred to plastic containers and remained in the dark, at 25°C, to allow for germination. Germinated seeds were sown in plastic containers filled with a mixture of half vermiculite and half peat (v:v), the basic and saturated water contents of the mixtures were evaluated prior to use.
All seedlings were cultivated in a phytotron with a day/night temperature regimen of 25/18°C, an air relative humidity of 60–70% and PPFD of 500 μmol m−2 s−1 for 10 h day−1. The surface of the plastic containers was sealed with aluminum foil to prevent water from evaporating. All seedlings were maintained at normal relative soil water content (85–90% of the field water content) (Wang and Zhang 2000). When water moisture reached 85% of the field water content, plants were watered to the soil moisture of 90% of the field water content according to Wang and Zhang (2000).
Seedlings with three true leaves and of similar sizes were selected for the following treatments: no ABA pretreatment and natural dehydration (ND), ABA pretreatment and natural dehydration (AD) and no ABA pretreatment and no dehydration (control, NAND). When photosynthetic rates of ND seedlings approached near 0.0 μmol m−2 s−1 during dehydration (6 days after onset of dehydration), all seedlings were rehydrated to original water moisture levels (85–90% of the field water content).
Leaf gas exchange
Photosynthetic rates (Pn) and transpiration rates (Tr) were measured using a portable photosynthesis system (LI-6400, LI-COR Co., Lincoln, Nebraska, USA) under the following conditions: CO2 concentration of the in-flow air was 380 ± 10 μmol mol m−2 s−1, the PPFD = 500 μmol photons m−2 s−1, the ambient relative humidity was 60 ± 5% and the leaf temperature was maintained at 25°C. At least six functional leaves from each treatment were measured. Water utilization efficiency (WUE) was determined as WUE = Pn/Tr. Daily water loss (WL) was calculated as
Where, W1 is the weight of the whole plastic container measured at 8:00 a.m. each day and, W2 is the weight of the whole plastic container measured the following day at 8:00 a.m. prior to watering.
Extraction and analysis of carbohydrates
Carbohydrates were analyzed essentially as described by Hu et al. (2009). Cucumber seedling leaves (0.5 g FW), frozen in liquid nitrogen, were homogenized and extracted 3 times, for 30 min each time, in 5 ml 80% ethanol at 80°C. The samples were then centrifuged for 10 min at 17,000 rpm. Supernatants were evaporated to dryness at 50°C in a vacuum; pellets were dissolved in 1 ml of deionized water and then filtered through a 0.22 μm filter. An Agilent 1200 HPLC system (Agilent Technologies, USA) was used to analyze carbohydrates. Fifty microlitres of 20 μM fructose (purchased from Sigma-Aldrich Trade Co., Ltd, Shanghai, China) was used as an internal standard. Carbohydrate compounds were separated on a Waters Sugar Pak column (Agilent Technologies, USA, 6.5 × 300 mm) at 50°C with water as mobile phase at a flow rate of 0.5 ml min−1. Stachyose, raffinose, sucrose, glucose, galactose and fructose were identified by comparison of retention times of known standards purchased from Sigma-Aldrich Trade Co., Ltd (Shanghai, China), and quantified by a refractive index detector (G1362A RID, Agilent Technologies, USA). Total sugar amounts were calculated as the sum of stachyose, raffinose, sucrose, glucose, galactose and fructose.
Statistical analysis
Data were analyzed using the Data Processing System (DPS, China) 7.05 software with Duncan’s newmultiplerange test, as well as Excel2003 (Microsoft office 2003, USA).
Results
Effect of dehydration on the Pn, WL and WUE of cucumber seedlings
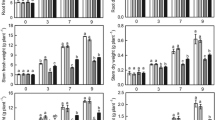
Two days after the onset of dehydration, the photosynthetic rate (Pn) of dehydrated seedlings (ND and AD) decreased quickly, and was only half of that observed for NAND seedlings (Fig. 1). The lowest Pn, for all the groups, was observed 6 days after dehydration in ND seedlings, which was 1.7 μmol CO2 m−2 s−1, almost one seventh of that seen in NAND seedlings. After rehydration, the Pn of dehydrated seedlings in the AD and ND treatments increased quickly, especially in AD where recovered to 8.1 μmol CO2 m−2 s−1. During both dehydration and rehydration, the Pn of the AD group was always greater than that of the ND group (Fig. 1). The results demonstrated that a reduction in Pn under dehydration was significantly alleviated by ABA pretreatment.
Effect of dehydration and rehydration on the photosynthetic rate (Pn), daily water loss (WL) and water utilization efficiency (WUE) of cucumber seedlings. NAND control, no ABA pretreatment and no dehydration, ND no ABA pretreatment and natural dehydration, AD ABA pretreatment and natural dehydration. Error bars represent standard errors
Dehydrated plants in AD and ND had a consistently lower WL than that seen in NAND plants at all experimental time points (Fig. 1). The lowest WL overall was observed in AD seedlings. After rehydration, the ND group showed only a 65% recovery in WL of pre-dehydration levels, which was higher than that observed in the AD group upon rehydration. These results indicate that ABA can reduce the WL of cucumber plant during water stress.
Unlike Pn and WL, the WUE of AD seedlings steadily increased from the initial stage before dehydration until the fourth day after dehydration, at which it was almost 2 times that seen in the NAND seedlings during both dehydration and rehydration. The lowest WUE, 2.1 μmol CO2 mmol−1 H2O, appeared in the ND seedlings at the end of the dehydration, (Fig. 1), and the ND plants appeared wilting at the end of dehydration. Two days after rehydration, the WUE of AD seedlings declined, however it was still higher than that observed in ND seedlings, but was lower than that seen in the NAND seedlings at the end of treatment (Fig. 1). This result demonstrated that ABA pretreatment allowed for a higher WUE in cucumber during dehydration.
Effect of dehydration on soluble sugar concentration
During dehydration, the ratios of nonstructural carbohydrate concentrations were altered in leaf tissues. Particularly, stachyose, raffinose, sucrose, and fructose concentrations increased gradually in ND and AD seedlings under dehydration, and the highest concentration was observed at the end of dehydration in ND seedlings (Fig. 2). The increase in stachyose, raffinose and sucrose in AD and ND seedlings was higher than that of other carbohydrates measured, such as glucose, fructose, galactose and galactinol. Compared with the ND group, carbohydrate concentrations in AD seedlings varied little during all of dehydrated period. NAND seedlings had relatively constant carbohydrate concentrations during the entire sampling period.
Upon rehydration, all of the carbohydrate concentrations were reduced in both AD and ND seedlings, as compared to pre-dehydration measurements. Carbohydrate concentrations in ND seedlings were higher than those observed in the AD seedlings, and showed a 20–30% recovery of the initial measurements seen before dehydration. The elevated stachyose and raffinose concentrations observed in AD and ND seedlings recovered to 85–90% of pre-dehydration levels, while sucrose, glucose and fructose showed only a 35–50% recovery after rehydration. These results imply that stachyose and raffinose are more sensitive to water stress in cucumber leaves under dehydration, than glucose, sucrose and fructose.
Discussion
Plant response to dehydration
The general pattern of photosynthetic activity throughout the day period was similar in the NAND and ND treatment groups, in that photosynthetic activity decreased from the first day of dehydration until the sixth day, while dehydrated ND plants had a consistently lower Pn than NAND plants during the experiment (Fig. 1). The WL of the ND plants was also somewhat reduced compared with NAND plants, and thus followed the same general daytime Pn pattern.
In response to water deficit, an increased concentration of endogenous ABA rapidly limits water loss from transpiration by reducing stomata conductance, which results in a decrease in Tr (Wang et al. 2010). Our experiments demonstrated that, under dehydration, WL decreased and ABA could improve water loss tolerance in cucumber. Water stress significantly decreased Pn, whereas, under dehydration, ABA pretreated cucumber seedlings had higher Pn than those without pretreatment. Maintenance of a higher Pn and stomata conductance in ABA pretreated seedlings confirmed the higher stability of the photosynthetic apparatus compared to the non-pretreated seedlings (Wang et al. 2010).
Rehydration resulted in a recovery in Pn of AD and ND seedlings, suggesting a reversible inhibition of the photosynthetic apparatus in the seedlings. The slow recovery of Pn in ND seedlings, upon rehydration, suggested that the basic mechanisms of photosynthesis might be impaired due to water stress. In the present study, the leaves of 5–6 day old plants were selected for measurement, and it is possible the leaf age contributed to the observed decrease in Pn in the NAND seedlings. In addition, the Pn of the AD and ND seedlings was lower than that seen in the NAND seedlings, and the Pn of the ND seedlings was lower than that of the AD group, which indicated that water loss accelerated with leaf senescence (Bogeat-triboulot et al. 2007) and that ABA may mitigate the rate of senescence caused by dehydration.
Dehydration and carbohydrates
It is well established that dehydration can alter the composition of carbohydrates in many plants (Wattana and Monica 1999; Kerepesi et al. 1996; Kerepesi and Galiba 2000). Carbohydrates have been generally proposed to act as compatible solutes or osmoprotectants to allow osmotic adjustment of plant cells exposed to environment stress. Oligosaccharides have been considered as factors involved in glass formation in cells and as a means of prevention against deterioration of macromolecular structures during desiccation (Horbowicz and Obendorf 1994). With incremental soil water loss, sucrose concentration in soybean nodules increased, as well as did carbohydrates in roots and stems (Robert et al. 1987). RFOs were the predominant fraction (75–77%) of all soluble carbohydrates measured in drought stressed lupine seeds (Zalewski et al. 2001) and raffinose showed significant increases during water deficit in Populus euphratica and Xerophyta viscosa under water stress (Bogeat-triboulot et al. 2007; Shaun et al. 2007). Carbohydrates induced by drought stress may serve not only as an osmo-protectant and free radical scavenger, as was previously thought, but also as a phloem-mobile anti-senescence metabolite (Wattana and Monica 1999).
In this study, stachyose, raffinose, sucrose, glucose, fructose and galactinol concentrations all increased in cucumber leaf during dehydration. Sucrose and hexose accumulation was proposed to play a role in osmotic adjustment in sucrose-transporting plants such as maize (Setter et al. 2001). However, for cucumber seedlings, which are RFO transporting plants, sucrose, stachyose and raffinose were the main carbohydrates (Schaffer et al. 1996), especially during dehydration in the present study. The increased carbohydrate concentrations, along with the elevated protein and proline levels (Wang et al. 2010), all contributed to the osmosis during dehydration.
ABA and carbohydrates
ABA plays important roles in various responses to abiotic stresses such as drought, cold and high salinity. Exogenous ABA in the 50 nM–50 mM range significantly increased the accumulation of galactinol, raffinose and stachyose in alfalfa somatic embryos, and at the highest ABA concentration, the amount of galactinol, raffinose and stachyose accumulated was three-, four- and seven-fold higher, respectively, than that of controls (Blöchl et al. 2005). In our study, the leaf content of raffinose and stachyose in AD seedlings decreased, compared with that of NAND plants during dehydration, demonstrating that cucumber seeds pretreated with ABA could increase the tolerance of cucumber plants to water loss stress. As documented by Meng et al. (2008), exogenous ABA (50 and 150 μM) sprayed cucumber plants accumulated substantial amounts of soluble carbohydrates in the leaves under low temperature, and at higher ABA concentrations, the carbohydrate content decreased. In our previous study, cucumber seeds pretreated with 500 μm ABA showed an increase in the WUE of the plants, and, in addition, demonstrated an increase in scavenging enzyme activity (Wang et al. 2010). In desiccated seeds, RFOs accumulated in the cotyledons or endosperm, and they were also involved in the acquisition of desiccation tolerance (Koster and Leopold 1988; Horbowicz and Obendorf 1994). In our study, the increased raffinose, stachyose and sucrose may have contributed to dehydration tolerance in ND seedlings, and ABA may increase seedling tolerance to dehydration.
Conclusion
The results presented here suggest that all the carbohydrates analyzed increased in cucumber leaf under dehydration, with raffinose, stachyose and sucrose being the largest components in cucumber seedling leaf, contributing to the prevention of harm from dehydration. Cucumber seeds pretreated with 500 μm ABA showed a decrease in the harmful effects of dehydration to seedlings had a change in the concentration of carbohydrates, and an improvement in the WUE of the seedlings.
Abbreviations
- RFOs:
-
Raffinose family oligosaccharides
- ABA:
-
Abscisic acid
- PPFD:
-
Photosynthetic photon flux density
- Pn:
-
Photosynthetic rate
- Tr:
-
Transpiration rate
- WL:
-
Water lost every day
- WUE:
-
Water utilization efficiency
- ND:
-
No ABA pretreatment and natural dehydration
- AD:
-
ABA pretreatment and natural dehydration
- NAND:
-
No ABA pretreatment and no dehydration all the time
- FW:
-
Fresh weight
References
Bachmann M, Matile P, Keller F (1994) Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Cold acclimation, translocation, and sink to source transition: discovery of chain elongation enzyme. Plant Physiol 105:1335–1345
Black M, Corbineau F, Grzesik M, Guy P, Come D (1996) Carbohydrate metabolism in the developing and maturing wheat embryo in relation to its desiccation tolerance. J Exp Bot 47:161–169
Blackman SA, Obendorf RL, Leopold AC (1992) Maturation proteins and sugars in desiccation tolerance of developing soybean seeds. Plant Physiol 100:225–230
Blöchl A, Grenier-de MG, Sourdioux M, Peterbauer T, Richter A (2005) Induction of raffinose oligosaccharide biosynthesis by abscisic acid in somatic embryos of alfalfa (Medicago sativa L.). Plant Sci 168:1075–1082. doi:10.1016/j.plantsci.2004.12.004
Bogeat-triboulot MB, Brosche M, Renaut J, Jouve L, Le TD, Fayyaz P, Vinocur B, Witters E, Laukens K, Teichmann T, Altman A, Hausman J, Polle A, Kangasjarvi J, Dreyer E (2007) Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol 143(2):876–892. doi:10.1104/pp.106.088708
Cooper K, Farrant JM (2002) Recovery of the resurrection plant Craterostigma wilmsii from desiccation: protection versus repair. J Exp Bot 53:1805–1813. doi:10.1093/jxb/erf028
Ghasempour HR, Gaff DF, Williams RPW, Gianello RD (1998) Contents of sugars in leaves of drying desiccation tolerant flowering plants, particularly grasses. Plant Growth Regul 24:185–191. doi:10.1023/A:1005927629018
Horbowicz M, Obendorf RL (1994) Seed desiccation tolerance and storability: dependence of flatulence-producing oligosaccharides and cyclitols. Seed Sci Res 4:385–405. doi:10.1017/S0960258500002440
Hu LP, Meng FZ, Wang SH, Sui XL, Li W, We YX, Sun JL, Zhang ZX (2009) Changes in carbohydrate levels and their metabolic enzymes in leaves, phloem sap and mesocarp during cucumber (Cucumis sativus L.) fruit development. Sci Hortic 121(2):131–137. doi:10.1016/j.scienta.2009.01.023
Ingram J, Chandler JW, Gallagher L, Salamini F, Bartels D (1997) Analysis of cDNA clones encoding sucrose–phosphate synthase in relation to sugar interconversions associated with dehydration in the resurrection plant Craterostigma plantagineum Hochst. Plant Physiol 115:113–121
Joyce CP, Michelle LJ, Cecil S (2003) Down-regulating α-galactosidase enhances freezing tolerance in transgenic petunia. Plant Physiol 133:901–909. doi:10.1104/pp.103.024554
Kerepesi I, Galiba G (2000) Osmotic and salt stress induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci 40:482–487
Kerepesi I, Toth M, Boross L (1996) Water-soluble carbohydrates in dried plant. J Agric Food Chem 10:3235–3239. doi:10.1021/jf960242b
Kobashi K, Gemma H, Iwahori S (1999) Sugar accumulation in peach fruit as affected by abscisic acid (ABA) treatment in relation to some sugar metabolizing enzymes. J Jpn Soc Hortic Sci 68:465–470
Koster KL, Leopold AC (1988) Sugars and desiccation tolerance in seeds. Plant Physiol 96:302–304
Liu F, Andersen MN, Jensen CR (2003) Loss of pod set caused by drought stress associated with waters status and ABA content of reproductive structures in soybean. Funct Plant Biol 30:271–280. doi:10.1071/FP02185
Meng FZ, Hu LP, Wang SH, Sui XL, Li W, Wei YX, Sun JL, Zhang ZX (2008) Effects of exogenous abscisic acid (ABA) on cucumber seedling leaf carbohydrate metabolism under low temperature. Plant Growth Regul 56:233–244. doi:10.1007/s10725-008-9303-6
Miao MM, Xu XF, Chen XH, Xue LB, Cao BS (2007) Cucumber carbohydrate metabolism and translocation under chilling night temperature. J Plant Physiol 164:621–628. doi:10.1016/j.jplph.2006.02.005
Norwood M, Toldi O, Richter A, Scott P (2003) Investigation into the ability of the roots of the poikilohydric plant Craterostigma plantigineum to survive dehydration stress. J Exp Bot 54:2313–2321. doi:10.1093/jxb/erg255
Passioura JB (1996) Drought and drought tolerance. Plant Growth Regul 20:79–83. doi:10.1007/BF00024003
Peterbauer T, Mucha J, Mach L, Richter A (2002) Chain elongation of raffinose in pea seeds-isolation, characterization, and molecular cloning of a multifunctional enzyme catalyzing the synthesis of stachyose and verbascose. J Biol Chem 277:194–200. doi:10.1074/jbc.M109734200
Robert JF, Robert PP, David RC Jr, Dorothy H (1987) Nodule activity and allocation of photosynthate of soybean during recovery from water stress. Plant Physiol 84:456–460
Schaffer AA, Pharr DM, Madore MA (1996) Cucurbits. In: Zamski E, Schaffer AA (eds) Photoassimilate distribution in plants and crops. Marcel Dekker, New York, pp 729–757
Scott P (2000) Resurrection plants and the secrets of eternal leaf. Ann Bot 85:159–166. doi:10.1006/anbo.1999.1006
Setter TL, Flannigan BA, Melkonian J (2001) Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisicacid, and cytokinins. Crop Sci 41:1530–1540
Shaun P, Sagadevan GM, Thomson JA, Jill M (2007) Farrant and felix keller protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. J Exp Bot 58(8):1947–1956. doi:10.1093/jxb/erm056
Strand A, Foyer CH, Gustafsson P, Gardestrom P, Hurry V (2003) Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant Cell Environ 26:523–535. doi:10.1046/j.1365-3040.2003.00983.x
Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29:417–426
Wang SH, Zhang FM (2000) Effect of different soil water content treatment on physiological characters of cucumber in solar greenhouse. China Veg (s): 26–29
Wang SH, Sui XL, Hu LP, Sun JL, Wei YX, Zhang ZX (2010) Effects of exogenous abscisic acid treatment of cucumber seeds on seedling growth and water-stress tolerance. N Z J Crop Hortic Sci 38(1):8–21
Wattana P, Monica AM (1999) Water deficit effects on raffinose family oligosaccharide metabolism in coleus. Plant Physiol 121:987–993
Whittaker A, Bochicchio A, Vazzana C, Lindsey G, Farrant J (2001) Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation-tolerant species Sporobolus stapfianus and Xerophyta viscosa. J Exp Bot 52(358):961–969
Zalewski K, Lahuta LB, Horbowicz M (2001) Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation-tolerant species icale kernels. Acta Physiol Plant 23(1):73–78
Acknowledgments
This work was supported by the National Key Technologies Research and Development (R & D) Program of China (2006BAD07B04, 2007BAD69B06), the Key Program of Agricultural Ministry in China (nyhyzx07-007) and the earmarked fund for Modern Agro-industry Technology Research System. The authors are indebted to Yang Liu for her work during the experiment. The anonymous reviewing editors and reviewers are gratefully thanked for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Hu, L., Sun, J. et al. Effects of exogenous abscisic acid on leaf carbohydrate metabolism during cucumber seedling dehydration. Plant Growth Regul 66, 87–93 (2012). https://doi.org/10.1007/s10725-011-9632-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9632-8