Abstract
Sugarcane is the most important sugar crop in China and the world, which originated in tropical and subtropical areas and is a thermophilic crop. Extreme weather occurred frequently in worldwide that caused serious cold or frost damage in recent years, resulting in enormous losses in sugarcane production. Abscisic acid (ABA) regulates much important plant physiological and biochemical processes, and induces tolerance to different stresses including cold or frost damage. This experiment investigated the interrelationship between low temperature induced ABA biosynthesis and endogenous hormone balance using two sugarcane varieties, i.e. the cold tolerant variety GT 28 and cold susceptible variety YL 6. Plants were sprayed with ABA 12 h before cold treatment as opposed to the control group, where no additional substances were added. When the plants in the control group were exposed to cold stress, plant cell membranes were injured, and the GA3 (Gibberellic acid 3) decreased, while the relative electric conductivity, MDA (Malondialdehyde), ABA, the ratio of ABA/GA3, ratio of ABA/IAA (Indole acetic acid), and the ratio of ABA/ZR (Zeatin Riboside) all increased under the cold stress, and there are genotypic differences in response to the contents of proline, ABA and GA, and the ratio of ABA/GA exists between the sugarcane variety GT 28, cold tolerant and variety YL 6, cold susceptible under cold stress. The contents of proline and ABA and the ratio of ABA/GA are higher and the content of GA is lower in the cold tolerant variety, which is the vital physiological basis that caused two sugarcane varieties with different cold resistance. In the ABA treatment, the cell membrane injury was effectively alleviated and the contents of MDA and GA3 decreased, but the contents of proline, ABA, and the ratio of ABA/GA3 increased. The decreasing contents of MDA and GA3, in contrast with the increasing contents of proline, ABA, and ratio of ABA/GA3 in sugarcane leaves from the ABA treatment groups, were important factors that can effectively increase cold stress tolerance in sugarcane plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum spp.) is the most widespread sugar-producing crop grown in the world. This thermophilic crop originated from tropical and subtropical areas. In China, cultivation of different varieties of sugarcane is especially popular throughout the southern provinces. However, during recent years, serious frost damage has occurred in sugarcane crops, resulting in large losses (Li and Yang 2009). The development of cold stress tolerance in sugarcane is great importance for improving production but it is hindered by lack of more precise physiological knowledge.
One of the efforts in this direction is the use of growth regulators which alters the internal growth regulation system, and the most important phytohormone mediator that responds as alteration of gene expression in plants is abscisic acid (ABA). ABA has been shown to mediate plant responses not only to cold but also to number of environmental stresses like drought, salinity, water logging etc., in addition to the regulation of other growth and developmental processes (Qiu and Yu 2009). The plant hormone ABA, which acts as a stress signal in plant, plays an important role in the regulation of plant responses from the functional level to the cellular level. Previous research results showed that exogenous ABA application before cold stress improves plant cold resistance (Perras and Sarhan 1989; Guy 1990). Exogenous ABA application also improved the content of proline and soluble sugar, enhanced water retention (Deng et al. 2005), reduced membrane lipid peroxidation, protected membrane reliability (Zhou and Guo 2005), and photosynthetic characteristics (He et al. 2008).
However, studies on the effects of exogenous ABA application on cold tolerance of sugarcane are very limited. Therefore, this study was made to determine the effects of ABA on sugarcane cold tolerance.
Materials and Methods
Plant Growth and Treatment
Single-bud setts of sugarcane varieties GT 28 (cold tolerant) and YL 6 (cold susceptible) were initially raised in flats by conventional culture. Then the 45-day old seedlings were transplanted into experimental pots. The soil in the pots contained a mixture of clay soil, organic fertiliser, and sand in 60:30:10 ratios (w/w) with a basic dosage of NPK fertiliser. The cold treatments were set up for different days (0, 1, 3, 7, 10, 14) in cold chambers (0 °C) when the seedlings reached the 5–7 leaf stage with a foliar application of 100 μM ABA and H2O as control. The treatments included: T1 (control), T2 (foliar application of 100 μM ABA). Samples of the top visible dewlap leaf (leaf +1) were taken at 0, 1, 3, 7, 10, and 14 days after treatment (DAT) and were frozen in liquid nitrogen for physiological and biochemical parameter analysis. Six uniform, representative pots were selected for measurements and analysis at each sampling date.
Relative Electric Conductivity (REC)
For determining the relative stability of cell membrane, electrolyte leakage tests were performed according to the method described by Zhang et al. (2009) using a DDS211 conductivity meter. Electrolyte leakage is a key factor for assessing cell membrane stability.
Lipid Peroxidation (Malondialdehyde Concentration)
The level of lipid peroxidation production was estimated by using the method described by Ohkawa et al. (1979). Approximately 0.5 g of frozen leaf sample was homogenized with the addition of 2.5 mL 5 % trichloroacetic acid, and centrifuged at 10,000 r/min for 15 min at room temperature. Equal volume of supernatant and 0.5 % thiobarbituric acid in 20 % trichloroacetic acid were added into a new tube and incubated at 98 °C for 25 min. The tubes were then transferred into an ice bath, and centrifuged again at 8,000 rpm for 5 min. The absorbance of the resulting supernatant was recorded and corrected for non-specific turbidity at 532 nm by subtracting from the absorbance at 600 nm.
Proline Contents
Proline content was measured using the method as described by Bates et al. (1973).
Endogenous Hormone Content
Endogenous hormone concentration was estimated by using the ELISA kit procured from Bei Nong Wei Tian Biological Technology Co. Ltd. (Beijing, China). One gram of fresh leaves was homogenised in chilled pestle and mortar using the extraction buffer, which contained 80 % methanol, and 1 mM 2,6-di-tert-butyl-4-methylphenol. The homogenate was kept at 4 °C for 4 h and centrifuged at 4,000 rpm for 5 min. Then, the supernatant was collected into another tube, and the residue was again mixed with extraction buffer and centrifuged at 4,000 rpm for 5 min. The combined supernatant was lastly used to measure the ABA, GA3, IAA, and ZR concentration following the user manual procedures given in the kit.
Data were subjected to analysis of variance (ANOVA); mean separation was performed using the least significance difference (LSD; P < 0.05) procedure from the SPSS statistical package (SPSS Student version 15.0).
Results
Cold Resistance Index
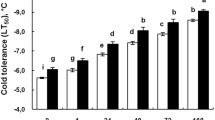
The leaf relative electric conductivity increased significantly with time under cold stress (Table 1). This increasing rate was higher in YL 6 than in GT 28. After exogenous ABA application, the relative permeability of cell membranes in the two sugarcane varieties declined significantly in the both varieties.
As a result of low temperature stress, the MDA content in both varieties increased first then decreased. During the low temperature treatment period (3–14 DAT), the MDA content was significantly higher in YL 6 than in GT 28. After exogenous ABA application, MDA content in both varieties decreased significantly, and it was decreased from 10.4 to 27.9 % treatment compared to the control from 1 to 14 DAT. Similarly, MDA content in GT 28 in the ABA treatment decreased from 8.3 to 20.3 % during the same period of time compared to the control. Significant decreases were observed at 7, 10, and 14 DAT.
Plants protect their tissues from low-temperature damage by accumulating proline in the cells leading to a better osmotic adjustment. As cold stress increased over the treatment period, the proline content increased significantly in the both varieties. The proline content was significantly higher in GT 28 than in YL 6 during the low temperature treatment period (3–14 DAT). After exogenous ABA application, the proline content significantly increased by 13.0 and 24.2 % at 7 and 10 DAT in GT 28, respectively, and by 24.26, 22.08, and 16.07 % at 7, 10, and 14 DAT in YL 6, respectively.
Endogenous Hormones
During low temperature stress, the ABA content in the leaves of the both varieties significantly increased (Table 2). Notably, the ABA content increases were higher in GT 28 than in YL 6. At 10 and 14 DAT, the ABA content in GT 28 with ABA application increased by 26.0 and 24.7 %, respectively. In YL 6 ABA-treated plants, the most significant increase in ABA content were at 3, 7, 10, and 14 DAT, corresponding to 26.6, 45.8, 23.9, and 32.3 %, respectively.
As the cold treatment duration increased, the trends of GA3 content in the both varieties varied. In GT 28, the GA3 content first decreased and then increased slightly, but in YL 6, the GA3 content decrease was continuous. Interestingly, the GA3 content was still higher in YL 6 than in GT 28. After exogenous ABA application, the GA3 content of the treatment groups decreased overall compared with the control groups, GT 28 treatment group exhibiting the greatest rate of decline.
Under cold stress, the IAA content in leaves of the two varieties also changed. The trend of the IAA contents over time fluctuated; GT 28 reached the highest IAA content at 7 DAT, while YL 6 reached the highest IAA content at 10 DAT. After exogenous ABA application under cold treatment for GT 28, from 1 to 7 DAT, the IAA content was higher in the treatment group than in the control group, but from 10 to 14 DAT, the IAA content was lower in the treatment group than in the control group. The above described phenomenon was reversed for YL6.
Similar to the trend of IAA contents, the trend in ZR content in leaves of the both varieties also fluctuated (Table 2). After exogenous ABA application, the ZR content decreased in the both varieties. The ZR content decrease in GT 28 ranged from 5.9 to 48.1 % from 1 to 14 DAT under cold stress, while the ZR content decrease in YL 6 was from 5.2 to 33.9 % from 1 to 14 DAT under cold stress. The most significant decrease was observed at 3, 10, and 14 DAT for GT 28 and at 1, 7, and 14 DAT for YL 6.
Hormone Ratio
The ratio of ABA/GA3, ABA/IAA, and ABA/ZR for each treatment and variety are summarised in Table 3. Under prolonged cold stress, the changes in ABA/GA3, ABA/IAA, and ABA/ZR ratio in leaves of the two sugarcane varieties showed an increasing trend. The ABA/GA3, ABA/IAA, and ABA/ZR ratio in leaves were higher in the treatment group than those in the control group. Also the ABA/GA3 ratio in GT 28 was higher than that of YL 6.
Discussion
Free radicals induced by cold stress damage cell membranes (Fridovich 1978). Under cold stress, OH− and O2 − free radicals increase in cells, which increase membrane lipid peroxidation, leading to membrane damage and destruction (Wang et al. 1988). This experiment showed that, under cold stress, lipid peroxidation was enhanced and triggered cell-produced MDA accumulation. Cold stress also led to increased membrane permeability, which then caused electrolyte leakage outside the membrane; therefore the relative electric conductivity increased. This result is consistent with the results of Sun et al. (2012) and Chen (1992). Cell water-holding capacity and biological macromolecule stability are enhanced by proline. After cold stress treatment, the proline content in sugarcane significantly increased; increase in cultivar GT 28 was considerably higher than those of cultivar YL 6. Thus, the accumulation of proline is associated with improving cold tolerance in sugarcane, similar to results reported by Zhang et al. (2011). Under cold stress, the control groups of the two cultivars of sugarcane leaves exhibit differences in cell membrane permeability, MDA content, and proline content. Cell membrane permeability and MDA content were lower while proline content was higher in GT 28 compared to those in YL 6. In the treatment groups, the osmotic adjusting proline content increased under cold stress, which reduced the MDA content and improved the cell membrane. Previous research indicated that spraying ABA improved cold resistance (Zhu 1995; Huang et al. 2002). In previous research, it was reported that because exogenous ABA promoted the transportation of water from roots to leaves, it improved cell membrane permeability and enhanced the stability of the membrane to reduce electrolyte leakage (Shi et al. 2006; Xin and Li 1992). In the present study, the ABA content increased continuously over time as the plants innately regulated the exogenous ABA effects. According to Lang et al. (1994) and Wang et al. (2009), during cold treatment cold resistance was stronger when the ABA content was higher.
GA3 was the first hormone suspected to be related to cold resistance (Donald and Kathryn 2001). GA3 content decreased in the both varieties, and the cold resistant variety GT 28 exhibited a more rapid decrease than YL 6. In regulating plant stress, the endogenous hormones are likely stress signal factors, therefore playing the role of defense mechanism signals in the plant system, with ABA acting as the positive signal and GA3, ZR, and IAA acting as the negative signals (Zhang et al. 2002). After ABA application, the GA3 content decreased in the treatment groups greater than the control groups, but the content of IAA and ZR did not exhibit any consistent trends. In the present study, the ABA/GA3, ABA/IAA, and ABA/ZR ratio in the treatment groups were higher than the ratio in the control groups, especially under cold stress, where all the ratios in the treatment groups increased significantly. This indicates that various hormones mutually promote each other under normal circumstances but restrict this mutual promotion when the sugarcane experiences cold stress. Exogenous ABA application reduced GA3 and ZR content but increased ABA content, ABA/GA3 ratio, ABA/IAA ratio, and ABA/ZR ratio. Our data strongly suggests that ABA applications improve sugarcane cold resistance.
References
Bates, L.S., R.P. Waldren, and I.D. Teare. 1973. Rapid determination of free proline for water stress studies. Plant and Soil 39(1): 205–207.
Chen, S.Y. 1992. Low temperature stress and peroxidation of membrane lipid in Sugarcane. Journal of Fujian Agriculture and Forestry University (Natural Science Edition) 21(2): 22–26.
Deng, X.K., D.R. Qiao, L. Li, X. Yu, N.S. Zhang, G.P. Lei, and Y. Cao. 2005. The effect of chilling stress on physiological characters of Medicago sativa. Journal of Sichuan University (Natural Science Edition) 42(1): 190–194.
Donald, E.R., and E.K. Kathryn. 2001. How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annual Review of Plant Physiology and Plant Molecular Biology 52: 67–88.
Fridovich, L. 1978. The biology of oxygen radicals. Science 201: 875–880.
Guy, C.L. 1990. Cold acclimation and freezing stress tolerance: role of protein metabolism. Annual Review of Plant Physiology and Plant Molecular Biology 41: 187–223.
He, H.Y., L. Xue, L.P. Tian, and Y.L. Chen. 2008. Effect of low temperature stress on the chlorophyll contents and chlorophyll fluorescence parameters in muskmelon seedling leaves. Northern Horticulture 4: 121–127.
Huang, Y.Z., J.Y. Xu, C.J. Chen, and X.Y. He. 2002. Comparative tests on drought resistance and frost resistance of several new sugarcane varieties. Journal of Guangxi Agriculture and Biological Science 21(2): 101–104.
Lang, V., E. Mantyla, B. Welin, B. Sundberg, and E.T. Palva. 1994. Alterations in water status, endogenous abscisic acid content, and expression of rabl8 gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiology 104: 1341–1349.
Li, Y.R., and L.T. Yang. 2009. New developments in sugarcane industry and technologies in China since 1990s. Southwest China Journal of Agricultural Sciences 22(5): 1469–1476.
Ohkawa, H., N. Ohishi, and Y. Yagi. 1979. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Analytical Biochemistry 95(2): 351–358.
Perras, M., and F. Sarhan. 1989. Synthesis of freezing tolerance proteins in leaves, crown and roots during cold acclimation of wheat. Plant Physiology 89: 577–585.
Qiu, Y.P., and D.Q. Yu. 2009. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environmental and Experimental Botany 65(1): 35–47.
Shi, C.J., Y. Liu, and T. Jing. 2006. Review on Stress-resistance of phytohormone. World Forestry Research 5(19): 21–26.
Sun, F., L.T. Yang, X.N. Xie, G.L. Liu, and Y.R. Li. 2012. Effect of chilling stress on physiological metabolism in chloroplasts of seedlings of sugarcane varieties with different chilling resistance. Acta Agronomica Sinica 38(4): 1–8.
Wang, A.G., C.B. Shao, G.H. Luo, J.Y. Guo, and H.G. Liang. 1988. Senescence and peroxidation of membrane lipid in mitochondria of soybean hypocotyl. Acta Phytophysiologica Sinica 14(3): 269–273.
Wang, X., J. Yu, Y. Yang, J. Chang, and Z.F. Li. 2009. Changes of endogenous hormones of winter wheat varieties with different cold-resistances under low temperature. Journal of Triticeae Crops 29(5): 827–831.
Xin, Z., and P.H. Li. 1992. Abscisic acid-induced chilling tolerance in maize suspension-cultured cells. Plant Physiology 99: 707–711.
Zhang, B.Q., L.T. Yang, and Y.R. Li. 2011. Comparison of physiological and biochemical characteristics related to cold resistance in sugarcane under field conditions. Acta Agronomica Sinica 37(3): 496–505.
Zhang, M.S., B. Xie, and F. Tan. 2002. Relationship between changes on endogenous hormone of sweetpotato under water stress and drought resistance. Scientia Agricultura Sinica 35(5): 498–501.
Zhang, Y.S., X. Huang, and Y.F. Chen. 2009. Plant physiology experiment, 128–129. Beijing: High Education Press.
Zhou, B.Y., and Z.F. Guo. 2005. Effect of ABA and its biosynthesis inhibitor on chilling resistance and anti-oxidant enzymes activity. Acta Pratacultural Science 14(6): 94–96.
Zhu, Q.Z. 1995. Study on the identification of cold resistance in sugarcane. Guangxi Agricultural Sciences 6: 264–265.
Acknowledgments
The present study was supported by the Grants from the National High Technology Research and Development Program (“863” Program) of China (2013AA102604), International Scientific Cooperation Program of China (2013DFA31600), the National Key Technology R&D Program (2008BADB8B00), Guangxi Special Funds for Bagui Scholars and Distinguished Experts (2013), Guangxi Natural Science Foundation (2011GXNSFF018002, 2012GXNSFDA053011, 2013NXNSFAA019073, 2014GXNSFBA118087), and Guangxi R&D Program (Gui Ke Chan 1123008-1, Gui Ke Gong 1222009-1B, Gui Ke He 1347004-2).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, X., Chen, MH., Yang, LT. et al. Effects of Exogenous Abscisic Acid on Cell Membrane and Endogenous Hormone Contents in Leaves of Sugarcane Seedlings under Cold Stress. Sugar Tech 17, 59–64 (2015). https://doi.org/10.1007/s12355-014-0343-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-014-0343-0