Abstract
Artemisinin is a promising and potent antimalarial drug naturally produced by the plant Artemisia annua L. but in very low yield. Its artemisinin content is known to be greatly affected by both genotype and environmental factors. In this study, the production of artemisinin and leaf biomass in Artemisia annua L. was significantly increased by exogenous GA3 treatment. The effect of GA3 application on expression of proposed key enzymes involved in artemisinin yield was examined in both wild type (007) and FPS-overexpression (253-2) lines of A. annua. In the wild type (007) at 6 h post GA3 application there was an abrupt rise in FPS, ADS and CYP71AV1 expression and at 24 h a temporary and significant peak in artemisinin (1.45-fold higher than the control). After GA3 application in line 253-2, there was a dramatic rise in expression of FPS at 3 h, CYP71AV1 at 9 h and ADS at 72 h and accumulation of artemisinin after 7 days, which was a delay when compared with the wild type plant. Thus, increased artemisinin content from exogenous GA3 treatment was associated with increased expression of key enzymes in the artemisinin biosynthesis pathway. Interestingly, exogenous GA3 continuously enhanced artemisinin content from the vegetative stage to flower initiation in both plant lines and gave significantly higher leaf biomass than in control plants. Consequently, the artemisinin yield in GA3-treated plants was much higher than in control plants. Although the maximum artemisinin content was found at the full blooming stage [2.1% dry weight (DW) in 007 and 2.4% DW in 253-2], the highest artemisinin yield in GA3-treated plants was obtained during the flower initiation stage (2.4 mg/plant in 007 and 2.3 mg/plant in 235-2). This was 26.3 and 27.8% higher, respectively, than in non-treated plants 007 and 253-2. This study showed that exogenous GA3 treatment enhanced artemisinin production in pot experiments and should be suitable for field application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Artemisinin comprises a group of sesquiterpene lactones that have an endoperoxide group in their structures. Artemisinin and its derivatives are promising and potent antimalarial drugs that can meet the dual challenge posed by the appearance of drug-resistant parasites and the rapid progression of malaria. They are at the top of the list of new antimalarial drugs in terms of efficacy, low toxicity, resistance, cost, rapidity of action and first order pharmacokinetics (White 2008; Krishna et al. 2008). Artemisinin is commonly extracted from field-grown leaves of Artemisia annua L. which is native in some parts of China, Japan, Korea and Vietnam (Klayman 1985). However, the amount of artemisinin that exists in crude extracts of the natural plants is very low. Artemisinin content was previously reported to vary greatly from 0.003 to 0.21% in various plant preparations and from 0.01 to 0.8% of DW in individual plants, depending on clones and different cultivation conditions (Ferreira et al. 1995; Abdin et al. 2003). In addition, it was revealed that there is a dramatic increase of artemisinin content during the plant reproductive stage (Pras et al. 1991; Morales et al. 1993; Baraldi et al. 2008). Total organic synthesis is very complicated and gives low yields, making it economically unattractive (Avery et al. 1992). Consequently, the use of artemisinin-based combination therapies (ACTs) is more expensive by up to 20 times when compared to currently used monotherapies and alternative non-artemisinin-based combinations because of the imbalance between demand and supply (Mutabingwa 2005). In order to break through this obstacle, strategies have been developed to enhance artemisinin accumulation in plants and boost the yield per unit cultivation area. This would make plant production more efficient and sustainable than simply increasing the cultivation area. It has been demonstrated that plant growth behavior and production of artemisinin in plant parts of A. annua are affected not only by genotype but also by environmental factors (Ferreira et al. 1995). Irradiation (Wang et al. 2007), salinity stress (Qureshi et al. 2005; Qian et al. 2007), chilling stress (Feng et al. 2009) and DMSO elicitation (Mannan et al. 2010) have been reported to affect artemisinin production. In addition, phytohormones involved in the plant defense response [e.g., abscisic acid (Jing et al. 2009) and salicylic acid (Pu et al. 2009)] have also been shown to play important roles in artemisinin accumulation.
Gibberellic acid (GA3) is a plant hormone that regulates seed germination, stem elongation, leaf expansion, fruit development and plant flowering (Gomi and Matsuoka 2003). Application of exogenous GA3 can beneficially change the plant metabolic pathways. For example, high accumulation of antioxidant and ajmalicine occurred in Catharanthus roseus after GA3 treatment (Jaleel et al. 2007). There is also evidence that GA3 can significantly relieve NaCl-induced growth inhibition in rice (Wen et al. 2010). Interestingly, exogenous treatment with GA3 or a GA-like substance (triacontanol) was reported to induce significant increases in biomass and artemisinin content in hairy root cultures (Shukla et al. 1992; Smith et al. 1997). In 2005, Zhang et al. (2005) showed that the endogenous GA3 and artemisinin content both increased in leaves with the onset of flower bud development. They found that application of exogenous GA3 could increase artemisinin content to 0.64% DW which was threefold higher than in control plants. However, they did not study any associated changes in plant growth characteristics or in the expression of genes that might be associated with key enzymes in the artemisinin biosynthesis pathway. In the present study, the roles of GA3 on artemisinin production and biosynthesis were examined. The proposed key enzymes targeted for expression analysis were farnesyl pyrophosphate synthase (FPS), amorpha-4,11-diene synthase (ADS) and cytochrome P450 monooxygenase (CYP71AV1).
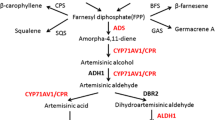
GA3 is diterpenoid compound synthesized from the terpenoid pathway, the same pathway used for artemisinin biosynthesis. Artemisinin is synthesized through the first intermediate FPP (C15) whereas GA3 is generated from GGPP (C20), which is the product of GPP and IPP condensation (Hedden and Phillips 2000; Liu et al. 2005; Bertea et al. 2005) as shown in Fig. 1. The overproduction of artemisinin and leaf biomass by the introduction of FPS gene was previously demonstrated in transgenic lines of A. annua that over-expressed FPS (Banyai et al. 2010). Thus, the effect of exogenous GA3 treatment was investigated in both wild type plants and plants that over-expressed FPS. The objective was to study the effect of GA3 on artemisinin production in both the low- and high-yield lines and in terms of gene expression patterns and plant growth behavior up to the reproductive stage.
Materials and methods
Plant cultivation and transplantation
Two lines of in vitro plants of Artemisia annua L., wild type code no. 007 and transgenic FPS-over-expressed line code no. 253-2 (Banyai et al. 2010), were maintained on 0.7% agar-solidified MS medium (Murashige and Skoog 1962) supplemented with 3% (w/v) sucrose and incubated under 25 ± 2°C, 60 ± 5% relative humidity and 16 h photoperiod with photosynthetic photon flux density of 60 ± 5 μmol m−2 s−1. Shoot segments were excised from 30 day-old plantlets and sub-cultured for multiplication by transferring regularly onto the same medium every 30 days. The in vitro plantlets (~5-cm height) were acclimated by transferring to photoautotrophic conditions. Under aseptic photoautotrophic culture, vermiculite filled with liquid MS medium (PGR-free and sugar free) was used as the supporting material. Air ventilation and CO2 exchange into cultured vessels were promoted through 45 μm-filter membranes (∅ = 1 cm) that were applied to each vessel cap. The cultures were acclimated for 2 weeks prior to transplantation to pots containing mixed soil (Klasman®, UK) and then incubated in plant growth chamber (model GS1700CH, Giant Star Ltd., Korea) under 25 ± 2°C, 60 ± 5% RH, 16 h photoperiod with 80 ± 5 μmol m−2 s−1 PPF, to observe growth and morphological characters.
GA3 treatment and sample collection
In the preliminary experiment, 10, 20, 40, 80 and 160 mg l−1 GA3 (GoldenGib®, S&P Formulator Co., TH) were applied to uniform 45 day-old wild type plants to determine the optimum concentration for artemisinin production. Since both 20 and 40 mg l−1 GA3 significantly increased artemisinin content at the same level, 20 mg l−1 concentration was used to investigate the effect of GA3 on plant growth, expression of proposed key enzyme genes and artemisinin production in A. annua. After transplantation, each pot containing a 45 day-old plant of either 007 or 253-2 was individually treated with 20 mg l−1 of GA3 by soil drenching. Water was applied in the same manner as a in the control experiment. The treated plants were incubated in a plant growth chamber (model GS1700CH, Giant Star Ltd., Korea) under 25 ± 2°C, 60 ± 5% RH, and 14 h photoperiod (6:00–20:00) with 80 ± 5 μmolm−2 s−1 PPF. They were fertilized with half-strength MS medium once a week and watered every other day. The expanded leaves at the positions of 5th to 15th from the shoot apex were collected at 1 (10:00), 3 (12:00), 6 (15:00), 9 (18:00), 12 (21:00), 24 (9:00), 48 (9:00), and 72 (9:00) h after treatment. Triplicate samples (0.1 g each) were kept in 1.5 ml microtubes prior to freezing in liquid nitrogen and storage at −80°C until used for gene expression analysis. For artemisinin evaluation, a similar sampling procedure was carried out at 12 (21:00), 24 (9:00), 48 (9:00), 72 (9:00) h and 7 (9:00), 14 (9:00), 28 (9:00) day after treatment. The harvested leaves (1.0 g each) were kept in aluminum foil, frozen in liquid nitrogen and stored at −80°C. Moreover, plant growth parameters in terms of shoot height, branching, no. of leaves, and leaf fresh weight were also recorded. A short photoperiod (8 h/days) was applied to plants in order to induce flowering. Leaves were collected for artemisinin analysis at the vegetative stage (28 days after treatment), the flower initiation stage (>50% of the plants showing signs of flower initiation) and the flower blooming stage (>70% of the flowers already open).
RNA extraction and gene expression analysis by real-time PCR
Total RNA was extracted using a modified guanidine thiocyanate method. DNase treatment (2 units, Promega) at 37°C for 30 min was applied to 2 μg of total RNA to eliminate any contaminating DNA in the samples. The reaction was stopped and then incubated at 65°C for 10 min. The treated RNA was then used as a template for cDNA synthesis. The cDNA was generated using an ImpromII kit (Promega) according to the manufacturer’s instructions. For qPCR amplification, the concentrated cDNA was diluted 1:5 and then 2 μl was used as a template. The internal reference gene in this experiment was Elongation factor 1-alpha (EF1-α), which was previously reported to be the stable housekeeping gene under biotic and abiotic stresses (Nicot et al. 2005). The specific primers for objective genes; farnesyl pyrophosphate synthase (FPS), amorpha-4, 11-diene synthase (ADS) and cytochrome P450 monooxygenase (CYP71AV1) are shown in Table 1. The 15 μl of qPCR amplification mix contained 7.5 μl of SYBR master mix (KAPA SYBR® FAST qPCR kit, USA), 10 μM of each primer and 2 μl of template. Sterile distilled water added to reach the 15 μl total volume. The quantitative assay was conducted by using an ABI7500 machine under the following conditions: 95°C for 60 s followed by 40 cycles of 95°C for 3 s and 56°C for 32 s. The standard curve of each gene was plotted between Ct and Log C0. Recombinant plasmids were constructed to contain the following individual genes of interest: pGEM-ADS, pGEM-CYP, pGEM-EF1 and pCAMBIAFPS. The plasmid DNA was extracted and digested with PstI prior to dilution at 10, 102, 103, 104, 105 and 106 copies/5 μl to plot standard curves. The copy number of interested gene was evaluated from individual standard curves. The relative expression level of each gene was normalized with the internal reference gene, EF1-α. The percentage of relative expression was calculated using the equation:
Artemisinin evaluation by HPLC
Detached leaves were immediately dipped into liquid nitrogen prior to storage at −80°C until used. Crude artemisinin was extracted according to the protocol modified from Van Nieuwerburgh et al. (2006). One gram of sample was dipped into 10 ml chloroform and immediately vortexed for 1 min. The solvent was evaporated in a fume hood at room temperature until absolutely dried prior to use as a crude extract. The artemisinin content was evaluated according to the procedure of Vandenberghe et al. (1995) with some modifications. Briefly, the crude extract was dissolved with 1 ml of methanol and then 200 μl of crude extract solution was separated into a new reaction tube. Exactly 800 μl of 0.2% (w/v) sodium hydroxide solution was added to the tube and mixed with a vortex mixer before incubation in a water bath at 50°C for 30 min. After cooling, 200 μl of methanol and 800 μl of 0.05 M acetic acid were added. The mixture was filtrated through a 0.45 μm Sartorious® membrane and then 10 μl of filtrate was subjected to HPLC (Water 717, USA) using a Luna 5 μm C18 100A column (150 mm × 4.6 mm2, Phenomenex, USA) with 1 ml min−1 flow rate. A mixture of 55% (v/v) acetonitrile in water containing 0.05% (v/v) formic acid was used as the mobile phase. The UV signal at 260 nm was monitored with a photodiode array detector. Authentic artemisinin (Khunming Pharmaceutical Corporation, China) was purified by re-crystallization in hexane and chloroform to be used as the standard in this experiment.
Results
Effect of GA3 on plant growth and flowering
After cultivation for 28 days in a growth chamber, a difference of plant growth was observed between 007 and 253-2 lines under both control and GA3 treatment (Table 2). In the control treatment, 007 showed a higher number of leaves than 253-2, but the fresh weight (FW) of upper part and leaves of 007 line was lower. GA3 marginally improved plant growth in both plant lines. Among five evaluated parameters, plant height and leaf FW were significantly increased in 007 after GA3 treatment whereas only the number of leaves was significantly enhanced in 253-2 (Table 2; Fig. 2a, b). All of the plants successfully produced flowers after exposure to a short photoperiod (8 h/days) (Fig. 2c, d). There was no significant difference in plant flowering period between control and GA3-treated plants in both lines. However, the transgenic line 253-2 was more sensitive to short day than the wild type 007, as shown in Table 3. Flower initiation occurred at 46 days (control) and 44 days (GA3) after treatment for 253-2 while it occurred at 72 days (control) and 73 days (GA3) for line 007. In all the plants, full blooming occurred 21-24 days later (Table 3). Leaf senescence was clearly observed when flower blooming began (Fig. 2e, f).
Morphology of A. annua plant code line 007 (wild type) compared with 253-2 (FPS-overexpressed line) at vegetative (a, b), flower initiation (c, d) and flower blooming (e, f) stages. The difference of plant height with or without GA3 treatment was clear after treatment of 007 for 28 days (a) but there was no significant difference with 253-2 (b). GA3-treated plants in both lines could produce flowers (c–f) after induction by short day period (8 h/days)
GA3 enhanced expression of proposed key enzyme genes
When mRNA expression of three proposed key enzyme genes, FPS, ADS and CYP71AV1 was determined by real-time PCR, it was higher in 253-2 than in 007 for all genes (Fig. 3). Among the three genes examined, the expression of ADS gene was lowest and FPS was highest in both plant lines. The changes in expression pattern after treatment differed between the two plant types. In 007, the increase in transcriptional levels occurred for all genes abruptly at 6 h after treatment (Fig. 3a–c). FPS and ADS declined thereafter but CYP71AV1 dropped sharply. At 6 h post GA3-treatment, the relative expression percentage for FPS, ADS and CYP71AV1 was 5-, 4- and 18-fold higher, respectively, than in the control plants. Although FPS and ADS expression decreased up to 48 h, it rose again at 72 h, but not to the previous high level. In 253-2, the expression of FPS increased significantly at 1 h post GA3-treatment and reached its highest level 3 h (twofold higher than the control). The level then decreased from 6 to 48 h (Fig. 3d). Without GA3 application, ADS expression was very low (less than 0.1%) while in GA3 treated plants it was 8-, 21-, 18.5-, 32- and 12-fold higher at 3, 6, 9, 12 and 24 h, respectively (Fig. 3e). The maximum ADS expression level with GA3 treatment was 247-fold higher than that of the control at 72 h post treatment. For CYP71AV1, a similar expression pattern was detected in both plant lines (Fig. 3c, f). The transcriptional levels post GA3 application suddenly increased and then suddenly dropped beginning at 6 h for 007 and 9 h for 253-2.
The percent relative expression of FPS (a, d), ADS (b, e) and CYP71AV1 (c, f) genes in the 007 and 253-2 lines of A. annua plants. The expression levels were compared between the control and 20 mg/l GA3 treatment at 0, 1, 3, 6, 9, 12, 24, 48 and 72 h after treatment. The data were averaged from 3 replicates containing three samples each and analyzed using a T-test for significant differences between GA3 treatments and the control. Error bar means ± SD. The single asterisk (*) represent a significant difference at P < 0.05 and a double asterisk (**) represents a highly significant difference at P < 0.01 between the control and GA3-treated group
Improvement of artemisinin production after GA3 treatment
GA3-treated plants showed higher artemisinin levels than did control plants in both plant lines (Figs. 4, 5), but the significant increases for the two types were observed at different times after treatment. In 007-treated plants, the increase in artemisinin level was biphasic. The first rise was observed from 12 to 24 h followed by a slow decrease up to 7 days followed by an additional increase up to 28 days. The highest artemisinin content (0.96% DW) during the first peak was obtained at 24 h after treatment. The second peak was detected from 14 to 28 days after treatment. The application of exogenous GA3 significantly increased the artemisinin production up to 0.99% DW 28 days after the treatment. It abruptly increased up to 1.8% in DW during the flower initiation period (Fig. 5a). Although the highest artemisinin levels (2.1% DW) were detected at the flower blooming stage, there was no significant difference of artemisinin content overall between the control and GA3-treated plants at that time (Fig. 5a). In 253-2, there was no significant difference of artemisinin content between GA3–treated and control plants up to 72 h after treatment (Fig. 4b). The boost in artemisinin production by GA3 first occurred at 7 days. Exogenous GA3 significantly improved artemisinin contents from 7 days after treatment until the flower initiation stage (Figs. 4b, 5b). High amounts of artemisinin were produced in GA3-treated plants 28 days after the treatment (1.8% in DW) and at the flowering initiation stage (2.0% in DW). However, the production of artemisinin in both control (2.2% in DW) and GA3 (2.4% in DW) treated plants was greatly enhanced at the flower blooming stage, at which time there was no significant difference in production between the treated and untreated plants. Although the maximum artemisinin content was obtained at full blooming stage in both lines, the biomass of leaves (gDW/plant) was drastically reduced after the flower blooming stage (Fig. 5a, b). Consequently, the artemisinin yield (mg/plant) was highest at the flower initiation stage (Fig. 5c, d). GA3 significantly increased both the artemisinin content and the leaf biomass. Therefore, the improvement of artemisinin yield was 2.38 mg/plant in 007 and 2.34 mg/plant in 253-2 and this was 1.2- and 1.3-fold higher, respectively, than in control plants.
The artemisinin content (%DW) affected by GA3 treatment in 007 (a) and 253-2 (b) lines. The data were averaged from 3 replicates containing three samples each and analyzed using a T-test for significant differences between GA3 treatments and the control treatment. Error bar means ± SD. The double asterisk (**) indicates a highly significant difference at P < 0.01 between the control and GA3-treated group
The effect of GA3 on artemisinin content, DW of leaves (a, b) and artemisinin yield (c, d) at different vegetative stages, at flower initiation and at full blooming in 007 and 253-2. The data were averaged from 3 replicates. Different letters within the same graph indicate significant differences in artemisinin yield analyzed by DMRT at P ≤ 0.01
Discussion
The transgenic FPS over-expressing line 253-2 was previously shown to give increased production of artemisinin (Banyai et al. 2010). In the present study, it was confirmed that expression of FPS in 253-2 was higher than that in the 007 control line. Moreover, the expression of ADS and CYP71AV1 in 253-2 line was also greater than that in the control with or without GA3 treatment (Fig. 3). This demonstrated that introduction of the FPS gene resulted in changes in the normal A. annua metabolic pathway leading to artemisinin. The abrupt increase in expression of FPS, ADS and CYP71AV1 after GA3 treatment also clearly demonstrated that GA3 up-regulated genes in the same biosynthesis pathway. It has been reported (Mansouri et al. 2009) that GA3 can increase the activity of HMG-CoA reductase (HMGR) that catalyzes an important control point in the mevalonate pathway (MVA). Although some sesquiterpenoids were built with C5 from the methyl-erythritol phosphate (MEP) pathway, most are synthesized via the MVA pathway (Enfissi et al. 2005; Liu et al. 2005). For artemisinin synthesis, it has been suggested that IPP from both MEP and MVA are used (Towler and Weathers 2007). Therefore, up-regulation of HMGR by exogenous GA3 might activate FPS, ADS and CYP71AV1 genes in the metabolic flow and consequently elevate the level of building blocks for artemisinin production. A role for exogenous GA3 in increasing salicylic acid (SA) biosynthesis has recently been reported in Arabidopsis (Alonso-Ramirez et al. 2009). In A. annua, SA might increase artemisinin content via a burst of reactive oxygen species (ROS) that could activate the expression of genes involved in artemisinin biosynthesis. Obvious enhancement was detected only for ADS transcription and there was little change seen for FPS and CYP71AV1 (Pu et al. 2009). Thus, the increase in artemisinin content after exogenous GA3 treatment may possibly be caused by up-regulation of gene expression in the biosynthesis pathway via multiple mechanisms and by a triggering of the ROS system through modulation of SA biosynthesis. In this study, dramatic changes in FPS, ADS and CYP71AV1 expression were observed 6 h after GA3 treatment in the 007 line but not until later in the 253-2 line (particularly for ADS) gene, which showed obvious increase 72 h after GA3 treatment (Fig. 3). This phenomenon could explain the late overproduction of artemisinin in 253-2 line that was detected 7 days after treatment when compared with 24 h in 007 line (Fig. 4). It also implies an important regulatory role of ADS gene in artemisinin biosynthesis.
Both GA3 and artemisinin are products of the isoprenoid biosynthesis pathway and they share the key intermediate FPP. Thus, the application of exogenous GA3 may divert carbon flow from GA3 to artemisinin. As a growth-promoting hormone, GA3 significantly enhanced not only artemisinin content but also total leaf biomass in A. annua (Fig. 5). Although artemisinin was previously reported to be overproduced also after treatment with ABA (Jing et al. 2009) and SA (Pu et al. 2009), the issue of any increase in plant biomass was not addressed. The highest artemisinin contents in treated plants with ABA and SA were 1.84% DW and 1.4% DW, which was 65 and 75.8% higher than the respective controls (Jing et al. 2009; Pu et al. 2009). The application of salinity stress was claimed to be a simple and efficient procedure for boosting artemisinin content (2–3% DW). However, a reduction of plant biomass caused by salt stress was also obtained (Qian et al. 2007).
After GA3 treatment in the present study, overproduction of artemisinin was obtained along with an increase in leaf biomass (Fig. 5a, b). The effect of GA3 on artemisinin production was investigated up to the reproductive stage to determine the optimum stage for plant harvesting since time of harvesting is related to artemisinin yield (Morales et al. 1993; Baraldi et al. 2008). Moreover, the phytotoxic activities of artemisinin on seed germination, root growth and chlorophyll content of A. annua and other monocotyledons and dicotyledons have been found (Bagchi et al. 1997; Dayan et al. 1999). Recently, it was confirmed that artemisinin is biosynthesized and accumulated in glandular trichomes of A. annua plants because of its high phytotoxicity to the plants themselves (Olsson et al. 2009). Accordingly, it is possibly that artemisinin is only produced and stored at limited levels in glandular trichomes that are mainly located in leaves. The highest artemisinin content was previously reported at the pre-flowering (Acton and Klayman 1985; Woerdenbag et al. 1993) or at full flowering stages (Pras et al. 1991; Morales et al. 1993). In our plant lines, the highest content was obtained at the full blooming stage (Fig. 5a, b), but this was accompanied by greatly decreased leaf biomass because of leaf senescence (Figs. 2, 5a–b). Although the artemisinin contents between the treatments (control or GA3) in both plant lines were different at the vegetative and flower initiation stages, there was no significant difference between them at the full blooming (Fig. 5a, b). The amount of artemisinin at the flower initiation stage in GA3-treated plant was quantified at up to 2.38 and 2.34 mg/plant in lines 007 and 253-2 and this was 26.3 and 27.8% higher, respectively, than in control plants (Fig. 5c, d). Therefore, the appropriate harvesting time for our plant lines was at the flower initiation stage, where the artemisinin yield was highest, especially when GA3 was applied.
The results above clearly revealed the potential of exogenous GA3 treatment for improvement of artemisinin production and leaf biomass, both of which can be further applied beneficially for field cultivation. Application of GA3 can boost the yield per unit cultivation area and further cause a potential reduction of ACTs price. In addition, more investigation on the relationship between GA3 and the artemisinin pathway would improve our knowledge of metabolic flow in the plant isoprenoid biosynthesis pathway and could possibly lead to further improvement in artemisinin production.
Abbreviations
- ADS:
-
Amorpha-4,11-diene synthase
- CYP71AV1:
-
Cytochrome P450 monooxygenase
- DW:
-
Dry weight
- EF1-α:
-
Elongation factor 1 alpha
- FPS:
-
Farnesyl pyrophosphate synthase
- FW:
-
Fresh weight
- GA:
-
Gibberellic acid
- qPCR:
-
Quantitative Real-time PCR
References
Abdin MZ, Israr M, Rehman RU, Jain SK (2003) Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med 69:289–299
Acton N, Klayman DL (1985) Artemisinin, a new sesquiterpene lactone endoperoxide from Artemisia annua. Planta Med 5:441–442
Alonso-Ramirez A, Rodriguez D, Reyes D, Jimenez JA, Nicolas G, Lopez-Climent M et al (2009) Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol 150:1335–1344
Avery MA, Chong WKM, Jennings-White C (1992) Stereoselective total synthesis of (+)-artemisinin, the antimalarial constituent of Artemisia annua L. J Am Chem Soc 114:974–979
Bagchi GD, Jain DC, Kumar S (1997) Arteether: a potent plant growth inhibitor from Artemisia annua. Phytochemistry 45:1131–1133
Banyai W, Kirdmanee C, Mii M, Supaibulwatana K (2010) Overexpression and sense suppression of farnesyl pyrophosphate synthase (FPS) gene affected artemisinin content and growth of Artemisia annua L. Plant Cell Tiss Organ Cult. doi: 10.1007/s11240-010-9775-8
Baraldi R, Isacchi B, Predieri S, Marconi G, Vincieri FF, Bilia AR (2008) Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem Syst Ecol 36:340–348
Bertea CM, Freije JR, van der Woude H, Verstappen FW, Perk L, Marquez V et al (2005) Identification of intermediates and enzymes involved in the early steps of artemisinin biosynthesis in Artemisia annua. Planta Med 71:0–7
Dayan FE, Hernandez A, Allen SN, Moraes RM, Vroman JA, Avery MA et al (1999) Comparative phytotoxicity of artemisinin and several sesquiterpene analogues. Phytochemistry 50:607–614
Enfissi EMA, Fraser PD, Lois LM, Boronat A, Schuch W, Bramley PM (2005) Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathway for the production of health-promoting isoprenoids in tomato. Plant Biotechnol J 3:17–27
Feng LL, Yang RY, Yang XQ, Zeng XM, Lu WJ, Zeng QP (2009) Synergistic re-channeling of mevalonate pathway for enhanced artemisinin production in transgenic Artemisia annua. Plant Sci 177:57–67
Ferreira JFS, Simon JE, Janick J (1995) Developmental studies of Artemisia annua: flowering and artemisinin production under greenhouse and field conditions. Planta Med 61:167–170
Gomi K, Matsuoka M (2003) Gibberellin signaling pathway. Curr Opin Plant Biol 6:489–493
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5:523–530
Jaleel CA, Gopi R, Manivannan P, Sankar B, Kishorekumar A, Panneerselvam R (2007) Antioxidant potentials and ajmalicine accumulation in Catharanthus roseus after treatment with gibberellic acid. Colloids Surf B Biointerf 60:195–200
Jing F, Zhang L, Li M, Tang Y, Wang Y, Wang Y et al (2009) Abscisic acid (ABA) treatment increases artemisinin content in Artemisia annua by enhancing the expression of genes in artemisinin biosynthetic pathway. Biologia 64:319–323
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from China. Science 228:1049–1055
Krishna S, Bustamante L, Haynes RK, Staines HM (2008) Artemisinins: their growing importance in medicine. Trends Pharmacol Sci 29:520–527
Liu Y, Wang H, Ye HC, Li GF (2005) Advances in the plant isoprenoid biosynthesis pathway and its metabolic engineering. J Interg Plant Biol 47:769–782
Mannan A, Liu C, Arsenault PR, Towler MJ, Vail DR, Lorence A et al (2010) DMSO triggers the generation of ROS leading to an increase in artemisinin and dihydroartemisinic acid in Artemisia annua shoot cultures. Plant Cell Rep 29:143–152
Mansouri H, Asrar Z, Mehrabani M (2009) Effects of gibberellic acid on primary terpenoids and ∆9-tetrahydrocannabinol in Cannabis sativa at flowering stage. J Integr Plant Biol 51:553–561
Morales MR, Charles DJ, Simon JE (1993) Seasonal accumulation of artemisinin in Artemisia annua. Acta Horticult 334:416–420
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Plant Physiol 15:473–497
Mutabingwa TK (2005) Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy. Acta Trop 95:305–315
Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56(421):2907–2914
Olsson ME, Olofsson LM, Lindahl AL, Lundgren A, Brodelius M, Brodelius PE (2009) Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annua L. Phytochemistry 70:1123–1128
Pras N, Visser JF, Batterman S, Woerdenbag HJ, Malingre TM, Lugt CB (1991) Laboratory selection of Artemisia annua for high artemisinin yielding types. Phytochem Anal 2:80–83
Pu GB, Ma DM, Chen JL, Ma LQ, Wang H, Li GF et al (2009) Salicylic acid activates artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep 28:1127–1135
Qian Z, Gong K, Zhang L, Lv J, Jing F, Wang Y et al (2007) A simple and efficient procedure to enhance artemisinin content in Artemisia annua L. by seeding to salinity stress. Afr J Biotechnol 6:1410–1413
Qureshi MI, Israr M, Abdin MZ, Iqbal M (2005) Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environ Exp Bot 53:185–193
Shukla A, Farooqi AHA, Shukla YN, Sharma S (1992) Effect of triacontanol and chlormequat on growth, plant hormones and artemisinin yield in Artemisia annua L. Plant Growth Regul 11:165–171
Smith TC, Weathers PJ, Cheetham RD (1997) Effect of gibberellic acid on hairy root cultures of Artemisia annua: growth and artemisinin production. In Vitro Cell Dev Biol-Plant 33:75–79
Towler MJ, Weathers PJ (2007) Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep 26:2129–2136
Van Nieuwerburgh FC, Vande Casteele SR, Maes L, Goossens A, Inze D, Van Bocxlaer J, Deforce DL (2006) Quantitation of artemisinin and its biosynthetic precursors in Artemisia annua L. by high performance liquid chromatography-electrospray quadrupole time-offlight tandem mass spectrometry. J Chromatogr A 1118:180–187
Vandenberghe DR, Vergauwe AN, Van Montagu M, Van den Eeckhout EG (1995) Simultaneous determination of artemisinin and its bioprecursors of Artemisia annua. J Nat Products 58:798–803
Wang ML, Jiang YS, Wei JQ, Wei X, Qi XX, Jiang SY et al (2007) Effects of irradiance on growth, photosynthetic characteristics, and artemisinin content of Artemisia annua L. Photosynthetica 46:17–20
Wen F, Zhang Z, Bai T, Xu Q, Pan Y (2010) Proteomics reveals the effects of gibberellic acid (GA3) on salt-stressed rice (Oryza sativa L.) shoots. Plant Sci 178:170–175
White NJ (2008) Qinghaosu (Artemisinin): the price of success. Science 320:330–334
Woerdenbag HJ, Luers JFJ, Van Uden W, Pras N, Malingre TM, Alfermann AW (1993) Production of the new antimalarial drug in shoot cultures of Artemisia annua L. Plant Cell Tissue Org Cult 32:247–257
Zhang YS, Ye HC, Liu BY, Wang H, Li GF (2005) Exogenous GA3 and flowering induce the conversion of artemisinic acid to artemisinin in Artemisia annua plants. Russ J Plant Physiol 52:58–62
Acknowledgments
This research was supported by Mahidol University grant, Thailand. The authors would like to thank the scholarship from The Royal Golden Jubilee PhD Program (RGJ) of the Thailand Research Fund. This work was conducted under the collaboration of Mahidol University and Chiba University for double degree program. Thanks to Prof. Timothy W. Flegel and FGS, MU for English language proof of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banyai, W., Mii, M. & Supaibulwatana, K. Enhancement of artemisinin content and biomass in Artemisia annua by exogenous GA3 treatment. Plant Growth Regul 63, 45–54 (2011). https://doi.org/10.1007/s10725-010-9510-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-010-9510-9