Abstract
Drought stress occurs frequently and severely as a result of global climate change, and it exerts serious effects on plants. 5-Aminolevulinic acid (5-ALA) plays a crucial role in conferring abiotic stress tolerance in plants. To enhance the drought tolerance of turfgrass and investigate the effects of 5-ALA on antioxidant metabolism and gene expression under drought stress conditions, exogenous 5-ALA was applied by foliar spraying before Kentucky bluegrass (Poa pratensis L.) seedlings were exposed to drought [induced by 10% polyethylene glycol (PEG)] stress for 20 days. 5-ALA pretreatment increased turf quality (TQ) and leaf relative water content (RWC) while reducing reactive oxygen species (ROS) production including H2O2 content and O2 •− generation rate, lipoxygenase (LOX) activity, and malondialdehyde (MDA) content under drought stress. 5-ALA pretreatment maintained ascorbate (AsA) and glutathione (GSH) contents and the ASA/DHA and GSH/GSSG ratios at high levels, and it enhanced the activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX), dehydroascorbate reductase (DHAR), and glutathione reductase (GR), which are crucial for scavenging drought-induced ROS. In addition, 5-ALA upregulated the relative expression levels of Cu/ZnSOD, APX, GPX, and DHAR but downregulated those of CAT and GR under drought stress. These results indicated that the application of 5-ALA might improve turfgrass quality and promote drought tolerance in Kentucky bluegrass through reducing oxidative damage and increasing non-enzyme antioxidant levels and antioxidant enzyme activity at transcriptional and posttranscriptional levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turfgrass growing under field conditions is often subjected to various environmental stresses. Drought is one of the most common stress factors, able to seriously influence the distribution, growth, and development of both cool-season and warm-season turfgrass species (Bian and Jiang 2009; Xu 2011). With increasing demands from agriculture, industry, and domestic use, as well as contamination of potable water, pressure to utilize more ecological and economical strategies in the management of turfgrass has gradually increased in recent years (Huang et al. 2014). Therefore, studies on how turfgrass responds to drought stress and methods for enhancing drought resistance will become increasingly important.
The production of reactive oxygen species (ROS) will increase when the plants are exposed to diversified stress conditions (Nahar et al. 2016). ROS, such as singlet oxygen (1O2), superoxide (O2 •−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•), can decrease cellular structural integrity and physiological and biochemical processes as a result of its effects on phosphatide peroxidation, protein degradation, and DNA fragmentation and thereby cause substantial cellular damage (Anjum et al. 2011; Mittler 2002).
To mitigate and cope with the deleterious effects of ROS, plants have developed a complex antioxidant defense system comprised of enzymatic and non-enzymatic antioxidants (Scandalios 1993). The enzymatic antioxidants include catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione S-transferase (GST), glutathione reductase (GR), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), and dehydroascorbate reductase (DHAR) (Xing et al. 2016). SOD is the first active enzyme in the ROS scavenger system for O2 •− disproportionating and results in H2O2 and O2 (Elstner 2003). H2O2 is then scavenged by CAT, APX, and GPX, but the functions of APX and GPX must rely on existing non-enzymatic antioxidants such as ascorbate (AsA) and glutathione (GSH) (Apel and Hirt 2004; Bowler et al. 1992). Along with the H2O2 scavenged, AsA and GSH will be oxidized to dehydroascorbate (DHA) and oxidized glutathione (GSSG), respectively (Apel and Hirt 2004). In the ascorbate-glutathione (AsA-GSH) cycle, maintaining the AsA/DHA and GSH/GSSG ratios at higher values is very important for scavenging ROS, while the intracellular AsA and GSH contents depend on the activities of MDHAR, DHAR, and GR (Loggini and Navari-Izzo 1999; Nishihara et al. 2003).
Kentucky bluegrass (Poa pratensis L.) belongs to the Gramineae family and is widely used in lawns, golf courses, landscapes, and sports fields as a prominent cool-season grass (Puyang et al. 2015a). Studies have indicated that Kentucky bluegrass is often subjected to drought stress, which results in limited plant growth, and thus reduces esthetic quality (Hu et al. 2012; Kackley et al. 1990). Therefore, it is crucially necessary to enhance the drought tolerance of this grass in arid and semi-arid areas.
5-Aminolevulinic acid (5-ALA) is an essential biosynthetic precursor of all organic heterocyclic tetrapyrrole molecules, such as chlorophyll, vitamin B12, billins, and heme, and it acts as a potential growth regulator in plants (Akram and Ashraf 2013; Naeem et al. 2011; Tanaka and Tanaka 2011). In addition, a number of reports showed that 5-ALA, in low concentrations, has great potential for mitigating abiotic stress-induced detrimental impacts on plant growth and development (Yang et al. 2014; Javed et al. 2011; Ali et al. 2013; Balestrasse et al. 2010). 5-ALA has been reported to improve salt tolerance in many crops and was found to be associated with maintenance of high K+/Na+ ratio, increase of antioxidant enzyme activity, activating AsA-GSH cycle, reduction of ROS, improvement in the content of chlorophyll, and the rate of photosynthesis (Naeem et al. 2011; Youssef and Awad 2008; Nishihara et al. 2003; Akram et al. 2012). The positive effects of 5-ALA for salt tolerance were also associated with the accumulation of organic acids, amino acids, and sugars (Yang et al. 2014).
However, the details of the physiological and metabolic mechanisms of ALA in drought stress tolerance still need to be elucidated (Akram and Ashraf 2013). Little information is available about the effects of exogenous 5-ALA application on perennial grass responses to drought stress. Such information is crucial for further understanding mechanisms regulating drought tolerance and for exploiting effective chemical products and management strategies to enhance turfgrass growth under drought stress. Therefore, the present study attempted to investigate the roles of exogenously applied 5-ALA in promoting Kentucky bluegrass tolerance to drought stress. To determine the overall tolerance response, we observed the growth, ROS formation, non-enzymatic antioxidant content, antioxidant enzyme activities, and gene expression levels of Kentucky bluegrass seedlings under drought stress.
Materials and methods
Plant culture and growth conditions
Seeds of Kentucky bluegrass (cv. MidnightII) were soaked overnight in tap water and then washed several times to rinse away the empty seeds floating on the water. The washed seeds were sown in plastic pots filled with sand and maintained in a growth chamber controlled at 25/20 °C (day/night) with a daily photoperiod cycle of 14 h (400 μmol m−2 s−1) and relative humidity of 65%. After 20 days of seeding, seedlings were transferred into half-strength Hoagland’s nutrient solution (Hoagland and Arnon 1950). The hydroponic culture period lasted for 5 weeks, and the nutrient solution was refreshed every 5 days during this period.
Treatments and sampling
Seedlings of uniform size were selected and divided into four groups to impose four treatment combinations: (1) control, only water; (2) 5-ALA, 10 mg L−1 5-ALA pretreatment; (3) drought, 10% polyethyleneglycol (PEG 6000); and (4) drought + 5-ALA, 10% PEG 6000 and 10 mg L−1 5-ALA. For the control and 5-ALA treatment, the seedlings were each foliar sprayed with water or 10 mg L−1 5-ALA (50 mL each pot) and then grown in half-strength Hoagland’s nutrient solution without stress. For the drought and drought + 5-ALA treatments, the seedlings were sprayed with water or 5-ALA and then grown in half-strength Hoagland’s nutrient solution with 10% PEG 6000. The foliar spraying started 3 days before the drought treatment. The application concentration of ALA (10 mg L−1) was the most effective based on turf quality (TQ) in preliminary tests using different concentrations (0.1, 1, 10, 25, 50, and 100 mg L−1) (data not shown). Leaf samples were collected at 5, 10, 15, and 20 days after drought treatment; frozen in liquid nitrogen; and stored at −80 °C until use.
A completely randomized block design with four treatments (control, 5-ALA, drought, and drought + 5-ALA) was used for this study. Each treatment had three replicates for three pots with multiple plants in each pot.
Turfgrass quality analysis
According to foliage color, uniformity, and density, turfgrass quality was rated on a scale of 1 to 9 visually, in which 1 is a completely desiccated and brown turf canopy; 6 is the minimal acceptable level; and 9 is a green, dense, and uniform turf canopy (Turgeon 1991).
Measurement of relative water content
About five leaves were collected early in the morning, and fresh weight (FW) was recorded immediately after the leaves were detached from the plant. These leaves were kept over deionized water for 24 h and allowed to attain turgidity. Turgid weight (TW) of the leaves was recorded, they were then dried for 72 h in an oven at 80 °C, and dry weight (DW) was noted. Relative water content (RWC) of the leaves was calculated using the following formula: RWC (%) = [(FW − DW) / (TW − DW)] × 100.
Detection of H2O2 and O2 •−
For H2O2 content estimation, 0.1 g was homogenized in 4 mL 0.1% (w/v) TCA in an ice bath and the extract was centrifuged at 12,000×g for 10 min at 4 °C. Then, 1 mL of supernatant was mixed with 0.2 mL ammonia and 0.1 mL 95% (v/v) hydrochloric acid containing 20% (v/v) TiCl4 and centrifuged at 10,000×g for 10 min at 4 °C after the sediment formation. The sediment was repeatedly washed with cold (−20 °C) acetone and centrifuged at 4 °C, following which the pellet was dissolved in 3 mL 1 M H2SO4. The resulting solution was determined spectrophotometrically at 410 nm (Liu et al. 2010).
The rate of O2 •− generation was measured following Schneider and Schlegel (1981). Leaves (0.1 g) were homogenized in 4 mL, 65 mM phosphate buffer (pH 7.8) and centrifuged at 5000×g for 15 min at 4 °C. One milliliter of 1 mM hydroxylamine chlorhydrate and 0.5 mL 65 mM phosphate buffer were added into 0.5 mL supernatant and incubated at 25 °C for 1 h. Then, 1 mL sulfanilamide (17 mM) and 1 mL a-naphthylamine (7 mM) were added to the mixture and incubated at 25 °C for 20 min. The absorbance of the supernatant was read at 530 nm after the addition of an equal volume of ether and centrifugation at 3000×g for 3 min.
Determination of lipid peroxidation
Membrane lipid peroxidation was determined by measuring malondialdehyde (MDA) content and lipoxygenase (LOX) activity. MDA content was determined using the thiobarbituric acid (TBA) method described by Heath and Packer (1968). LOX (EC 1.13.11.12) activity was measured according to the method of Doderer et al. (1992). Absorbance was recorded at 234 nm using linoleic acid as a substrate.
Estimation of AsA and GSH
Leaf tissue was extracted with 6% TCA and centrifuged at 15,000×g for 5 min (4 °C), and the supernatant was used for AsA and GSH assays (Kampfenkel et al. 1995).
AsA content was determined following a previously established method (Kampfenkel et al. 1995) with some modifications. Supernatant (0.2 mL) was neutralized with 0.5 mL of 0.2 M K-phosphate buffer (pH 7.4) and 0.1 mL dithiothreitol (DTT; reducing DHA to AsA). Based on the reduction of Fe3+ to Fe2+ in an acid solution, a red chelate can be formed between the Fe2+ and 2,2′-dipyridyl, and total AsA (AsA + DHA) content is evaluated by the absorbance at 525 nm. AsA was determined by replacing DTT with water. DHA was estimated from the difference between total AsA and AsA. Standard curves with known concentrations of AsA and DHA were used.
GSH content was determined following the method of Knörzer et al. (1996) with some modifications. Supernatant (0.2 mL) was neutralized with 1.4 mL of 50 mM K-phosphate buffer (pH 7.5). Based on enzymatic recycling, glutathione is oxidized by 2-nitrobenzoic acid (DTNB) and reduced by NADPH in the presence of GR, and total GSH content is evaluated by the rate of absorption changes at 412 nm. GSSG was determined after removal of GSH by 10% 2-vinylpyridine. GSH was estimated from the difference between total GSH and GSSG. Standard curves with known concentrations of GSH and GSSG were used.
Antioxidant enzyme extraction and activity assays
Leaf tissue (0.2 g) was homogenized with 4 mL of ice-cold extraction buffer, 100 mM potassium phosphate-buffered saline (PBS), pH 7.0, and 0.1 mM EDTA. The homogenate was filtered through muslin cloth and centrifuged at 16,000×g for 15 min at 4 °C. The supernatant was used as enzyme extract for enzyme activity assays (De Azevedo et al. 2005).
In the SOD (EC 1.15.1.1) activity (Jr and Fridovich 1987), the enzyme extract (30 μL) was added into 3 mL mixture (2.7 mL of 14.5 mM methionine, 90 μL of 50 mM PBS, 100 μL of 60 μM riboflavin, 100 μL of 2.25 mM nitro-blue tetrazolium, 10 μL of 30 μM EDTA), then reacted in an illumination incubator (4000 lx) for 20 min, and the absorbance was read at 560 nm.
In the CAT (EC 1.11.1.6) activity (Havir and Mchale 1987), the enzyme extract (100 μL) was mixed with 3 mL of 0.15 M PBS and 5 μL of 30% H2O2. Decrease in absorbance was recorded at 240 nm.
In the APX (EC 1.11.1.11) activity (Nakano and Asada 1981), the reaction mixture contained 50 mM PBS (pH 7.0), 0.5 mM AsA, 0.1 mM EDTA, 0.1 mM H2O2, and 30 μL of enzyme extract in a final volume of 2 mL. The reaction was started by adding H2O2. The activity was calculated from the recorded decrease in absorbance at 290 nm for 1 min.
In the DHAR (EC 1.8.5.1) activity (Nakano and Asada 1981), the reaction mixture consisted of 50 mM PBS (pH 7.0), 2.5 mM GSH, and 0.1 mM DHA, and the reaction was started by the addition of 50 μL of enzyme extract. Activity was measured by the decrease in absorbance at 265 nm in 1 min.
In the GPX (EC 1.11.1.9) activity (Elia et al. 2003), the reaction mixture contained 100 mM PBS (pH 7.0), 1 mM EDTA, 1 mM NaN3, 0.12 mM NADPH, 2 mM GSH, 1 μ GR, 0.6 mM H2O2 (as a substrate), and 30 μL of enzyme extract. The reaction was initiated with H2O2. Change in absorbance was recorded at 340 nm.
In the GR (EC 1.6.4.2) activity (Foyer and Halliwell 1976), the reaction mixture consisted of 0.1 M PBS (pH 7.0), 1 mM EDTA, 1 mM GSSG, 0.2 mM NADPH, and 50 μL of enzyme extract. The reaction was started by the addition of GSSG. Decrease in absorbance was recorded at 340 nm.
Gene expression analyses of antioxidant enzymes
The leaf tissues of Kentucky bluegrass with all treatments at 20 days were used for gene expression analysis of antioxidant enzyme-related genes. Total RNA was isolated from Kentucky bluegrass leaves and roots using the RNAsimple Total RNA Kit (TIANGEN) according to the kit instructions. The RNA quality and concentration were measured with a spectrophotometer (NanoVue™ plus, Wilmington, DE, USA), and samples with an A260/A280 ratio within 1.8–2.2 and an A260/A230 ratio of approximately 2.0 were retained. The removal of genomic DNA contamination and first-strand complementary DNA (cDNA) synthesis were performed using the PrimeScript™ RT Reagent Kit with gDNA Eraser (TaKaRa) following the manufacturer’s instructions. Gene-specific primers for Cu/ZnSOD, CAT, APX, DHAR, GPX, GR, and actin were designed and synthesized to detect gene expression in Kentucky bluegrass based on the sequences in NCBI, as shown in Table 1.
RT-qPCR was conducted with a LightCycle 96 (Roche) using SYBR® Premix DimerEraser™ (TaKaRa). Each 20 μL reaction mixture contained 2 μL cDNA, 10 μL ×2 SYBR® Premix DimerEraser, 0.6 μL each primer (10 μM), and 6.8 μL ddH2O. The amplification conditions were as follows: 95 °C for 300 s, followed by 40 cycles of 95 °C for 10 s, and 57 °C for 30 s. The melting curve was produced by varying the amplification temperature from 60 to 95 °C. No-template controls were included, and RT-qPCR analysis of each sample was performed in triplicate. The expression quantities of the Cu/ZnSOD, CAT, APX, DHAR, GPX, GR, and actin genes were calculated according to the relevant standard curve. The relative expression levels were standardized with actin and evaluated using the comparative ΔΔCT method.
Statistical analysis
All of the data from the four treatments were subjected to analysis of variance, and the mean separation was performed with the Fisher’s protected least significant difference (LSD) at a 0.05 significance level using SPSS version 19.0 for Windows (SPSS Inc., USA).
Results
Based on the preliminary experiment data, 5-ALA was screened at different concentrations and 10 mg L−1 was selected in this study, which positively alleviated drought-induced injury in Kentucky bluegrass (Fig. 1).
Turf quality and water status
The turf quality of Kentucky bluegrass was maintained at approximately 8.0 under non-drought treatment (control or 5-ALA treatment alone) from 0 to 28 days, while drought stress significantly reduced TQ after 5 days (Fig. 2a). The decline of TQ was less pronounced for drought + 5-ALA plants than drought alone treatment after 10 days. At 15 days after drought stress treatment, the TQ was not acceptable (<6.0) for plants with drought alone treatment, while drought + 5-ALA plants had an acceptable TQ of level 6.7. In addition, TQ was not significantly affected by 5-ALA treatment under non-drought treatment.
Effects of exogenous 5-aminolevulinic acid (5-ALA) on leaf relative water content (a) and turfgrass quality (b) of Kentucky bluegrass under drought stress conditions. The vertical bars at the top of the figure indicate the least significant difference (LSD) values for treatment comparison at a given day of treatment (P < 0.05)
Leaf RWC was maintained at approximately 95% in the control and 5-ALA treatments without drought stress during the experiment (Fig. 2b). A significant decrease in RWC was detected beginning at 5 days in the drought + 5-ALA and drought alone treatments. After 10 days of treatment, drought + 5-ALA plants maintained significantly higher RWC than drought alone treatment. At 20 days after treatment, 5-ALA pretreatment increased RWC by 19.7% when compared to 5-ALA non-treatment under drought stress conditions.
O2 •− generation rate and H2O2 content
The O2 •− generation rate increased in both the drought alone and drought + 5-ALA treatments beginning at 5 days (Fig. 3a). The application of 5-ALA (drought + 5-ALA) significantly reduced the O2 •− generation rate of plants, beginning at 5 days of drought stress, and the O2 •− generation rate remained lower in the drought + 5-ALA treatment than in drought alone during drought stress treatment. There were no significant differences in O2 •− generation rate between the control and 5-ALA-treated plants without drought conditions.
Effects of exogenous 5-aminolevulinic acid (5-ALA) on O2 •− generation rate (a), H2O2 content (b), MDA content (c), and LOX activity (d) in Kentucky bluegrass under drought stress conditions. The vertical bars at the top of the figure indicate the least significant difference (LSD) values for treatment comparison at a given day of treatment (P < 0.05)
From 5 to 20 days, a significant increase in H2O2 content was also observed in the drought treatment and drought + 5-ALA plants, showing a trend similar to the O2 •− generation rate (Fig. 3b). At 10, 15, and 20 days after drought stress treatment, the H2O2 content increased by 361.6, 362.9, and 439.1%, respectively, compared to the control. When exogenous 5-ALA was applied, the H2O2 content was reduced by 44.1, 39.8, and 41.5% at 10, 15, and 20 days, respectively, compared with 5-ALA non-treatment under drought stress conditions.
Lipid peroxidation
A significant increase in MDA content (Fig. 3c) and LOX activity (Fig. 3d) was detected, beginning at 10 days in the drought treatment and drought + 5-ALA plants compared to the control. 5-ALA pretreatment resulted in significantly lower MDA content and LOX activity during 20 days of drought stress, but it had no significant effects under non-drought conditions. At 20 days after drought treatment, MDA content and LOX activity decreased by 30.5 and 23.6%, respectively, after pretreatment with 5-ALA.
AsA and GSH homeostasis and their redox status
Compared with the control, a significant difference was observed in the effects of drought stress and 5-ALA on AsA and GSH contents of Kentucky bluegrass leaves (Fig. 4a, b) at 5 days after treatment; the AsA and GSH contents were markedly reduced in plants with drought alone treatment, while they showed a significant increase in plants with 5-ALA pretreatment. An immediately increase was induced by drought stress during the 10–20-day treatment period. The AsA content in plants with drought + 5-ALA treatment reached the highest level at day 10, which was 165.9 or 34.6% higher than the control or 5-ALA non-treatment under drought stress conditions, respectively. The GSH content of drought + 5-ALA treatment plants reached the highest level at 20 days after exposure (80.1% higher than drought-alone treatment).
Effects of exogenous 5-aminolevulinic acid (5-ALA) on non-enzymatic antioxidant content under drought stress conditions in Kentucky bluegrass, including AsA (a), GSH (b), DHA (c), GSSG (d), AsA/DHA ratio (e), and GSH/GSSG ratio (f). The bars with different letters are significantly different at P < 0.05. The error bars represent the SD values
As shown in Fig. 4c, d, drought stress induced a significant time response pattern for DHA and GSSG content when treated with drought and drought + 5-ALA. During the treatment period, drought stress caused an immediate increase in DHA and GSSG contents, which then decreased as the treatment time extended. 5-ALA pretreatment significantly alleviated the changes in DHA and GSSG contents when compared to 5-ALA non-treatment under drought stress conditions. The DHA content in plants with drought alone treatment was approximately five times more than that in drought + 5-ALA treatment plants at day 20.
A decrease in the AsA/DHA ratio was found in the whole treatment period under drought stress, but a significant increase in drought + 5-ALA treatment plants was only found at 15 days after treatment (Fig. 4e). In addition, little change was found from 5-ALA pretreatment at 5, 10, or 30 days after drought exposure. The ratio of GSH/GSSG decreased significantly after 5–10-day drought treatment but was induced by drought during the period from 15 to 20 days. When exogenous 5-ALA was applied, the GSH/GSSG ratio was significantly higher than in 5-ALA non-treatment under drought stress conditions from 5 to 20 days. The ratio of GSH/GSSG in plants under drought + 5-ALA treatment reached a higher level at day 20, which was 296.5 or 131.5% higher than the control or 5-ALA non-treatment under drought stress conditions, respectively.
Antioxidant enzyme activity
As shown in Fig. 5a, both drought treatment alone and drought + 5-ALA treatment caused a significant increase in leaf SOD activity of Kentucky bluegrass, beginning at 10 days. Although the decline in SOD activity was detected in drought + 5-ALA treatment plants at 20 days after treatment, SOD activity in drought + 5-ALA treatment plants remained significantly higher than in other treatments from 10 to 20 days. At 15 days after treatment, SOD activity in drought + 5-ALA treatment plants reached the highest value, showing markedly higher activity than the control drought treatment alone.
Effects of exogenous 5-aminolevulinic acid (5-ALA) on antioxidant enzyme activity under drought stress conditions in Kentucky bluegrass, including SOD (a), CAT (b), APX (c), GPX (d), DHAR (e), and GR (f). The vertical bars at the top of the figure indicate the least significant difference (LSD) values for treatment comparison at a given day of treatment (P < 0.05)
During the period of drought stress treatment, CAT activity of Kentucky bluegrass increased from 0 to 15 days and then decreased (Fig. 5b). Drought stress raised CAT activity by 80.8% relative to the control at day 15. Exogenous 5-ALA pretreatment increased CAT activity by 29.6% when compared to 5-ALA non-treatment plants under drought stress conditions at 15 days after treatment.
There was a significant difference in drought-mediated effect on APX activity in leaves of Kentucky bluegrass (Fig. 5c). APX activity increased significantly at 10 days after drought stress but was inhibited by drought during the period from 0 to 5 days, while the increase of APX activity in 5-ALA non-treatment plants under drought stress was much less than that in 5-ALA pretreatment. The major difference between 5-ALA pretreatment and non-treatment was found at 10 days after treatment with drought causing a significant increase of 64.1% in 5-ALA pretreatment plants, while the increase in 5-ALA non-treatment plants was almost negligible when compared with the control.
Similar responses to drought stress or 5-ALA addition were found in the activity of GPX (Fig. 5d), DHAR (Fig. 5e), and GR (Fig. 5f) in the leaves of Kentucky bluegrass. Drought stress induced a consistent increase in GPX, DHAR, and GR activities from 0 to 10 days, and the addition of 5-ALA sustained higher activity than non-addition. A slight decrease in the activities was detected at day 15, and then, the activities showed a significant increase at day 20. At 20 days after treatment, the effects of drought stress and 5-ALA addition on GPX, DHAR, and GR activities were the most significant, and GPX, DHAR, and GR activities in drought + 5-ALA treatment plants were 197.0, 210.9, and 276.6% higher than that in the control and 69.4, 119.1, and 87.9% higher than that in drought treatment alone, respectively. In general, the SOD, CAT, APX, GPX, DHAR, and GR activities were not significantly affected by 5-ALA pretreatment under non-drought treatment.
Gene expression level of antioxidant enzymes
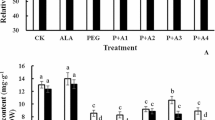
As shown in Fig. 5, drought stress induced an increase in the relative expression level of antioxidant enzyme genes compared to the control at day 20. Under drought stress, exogenous 5-ALA pretreatment caused a significant increase in Cu/ZnSOD (Fig. 6a), APX (Fig. 6c), DHAR (Fig. 6d), and GPX (Fig. 6e), while it inhibited the expression levels of GR (Fig. 6f) and CAT (Fig. 6b) when compared to drought stress treatment alone. The changes of the relative gene expression levels in Cu/ZnSOD, CAT, and GR responding to drought stress were much less than those in APX, DHAR, and GPX. The relative expression levels of APX, DHAR, and GPX in drought + 5-ALA pretreatment plants increased by 219.9, 647.9, and 270.9%, respectively, compared with the control, while it induced an increase of 96.3, 70.9, and 61.2%, respectively, in Cu/ZnSOD, CAT, and GR.
Effects of exogenous 5-aminolevulinic acid (5-ALA) on gene expression level of antioxidant enzymes under drought stress conditions in Kentucky bluegrass at 20 days after treatment, including SOD (a), CAT (b), APX (c), DHAR (d), GPX (e), and GR (f). CK control, ALA 5-ALA pretreatment, D drought treatment alone, D + ALA drought stress + 5-ALA pretreatment. The vertical bars at the top of the figure indicate the least significant difference (LSD) values for treatment comparison at a given day of treatment (P < 0.05)
Discussion
Drought is a common environmental factor that greatly inhibits turfgrass growth and development (Huang et al. 2014). It has been widely reported that foliar 5-ALA mitigates the adverse impacts due to drought stress in a number of plants (Al-Khateeb 2006; He et al. 2013; Liu et al. 2016). In the present study, the growth parameters (TQ) and water status (RWC) of Kentucky bluegrass indicated that pretreatment with exogenous 5-ALA at 10 mg L−1 effectively mitigated growth damage induced by drought stress. This is in agreement with previous studies showing that foliar application of 5-ALA reduces the decline of TQ and RWC under salinity stress conditions (Yang et al. 2014). To our knowledge, this is the first report of positive effects of 5-ALA on turfgrass responses to drought stress.
Drought stress induces a loss of balance between the light reactions and the Calvin–Benson cycle, which makes deplete electron carriers from chloroplasts and mitochondria, resulting in the production of ROS by the transfer of electrons to molecular oxygen (Mittler et al. 2004; Nahar et al. 2016). Kentucky bluegrass plants subjected to drought stress enhanced ROS production; free radicals such as superoxide radical (expressed as O2 •− generation rate) and non-radical compounds (H2O2) significantly increased under drought stress (Fig. 3a, b). LOX, an oxidative and lipid-degrading enzyme, is associated with lipid peroxidation (Doderer et al. 1992). MDA, which is a product of lipid peroxidation, is associated with the accumulation of ROS, resulting in the damage of cell membrane integrity in plants (Puyang et al. 2015b). Our results indicated that LOX activity and MDA content increased dramatically at 10 days after drought treatment (Fig. 3c, d). However, plants pretreated with 5-ALA showed reduced O2 •− generation rate, contents of H2O2 and MDA, and LOX activity, which reduced subsequent oxidative damage (Figs. 2 and 3) responses to drought stress. Similar results on mitigating drought-induced oxidative damage by 5-ALA pretreatment were obtained in a previous study (He et al. 2013; Liu et al. 2016).
Antioxidant enzymes are the most direct and effective method to scavenge ROS in plants (Apel and Hirt 2004). Being the first line of the enzymatic defense system, SOD converts toxic O2•− to the more stable H2O2 (Elstner 2003). In the present study, Kentucky bluegrass seedlings subjected to drought stress showed higher SOD activity and higher O2•− generation rate (Figs. 4a and 3a), consistent with other reports (Puyang et al. 2015a). Furthermore, 5-ALA pretreatment enhanced SOD activity compared to the drought treatment alone. It has been reported that the application of 5-ALA enhances SOD activity (Akram et al. 2012). In addition, similar increases of CAT, APX, DHAR, GPX, and GR activities were detected under drought stress (Fig. 4). Some reports indicated that 5-ALA could improve CAT and APX activities as a result of 5-ALA enhancing the synthesis of heme, and CAT and APX all contain the heme structure (Ot and Jones 1975; Yu and Weinstein 1997). The results of this study also demonstrated that 5-ALA pretreatment increased the activities of CAT and APX.
APX, GPX, DHAR, and GR are potential enzymes that participate in the AsA-GSH cycle playing indispensable roles in scavenging ROS and maintaining the AsA and GSH levels (Noctor and Foyer 1998). APX is engaged in the ROS-scavenging process by converting AsA to DHA (Gill and Tuteja 2010), which caused the decline of APX activity and AsA content under drought stress at day 5 (Figs. 4a and 5c), but the AsA content increased (Fig. 4c), following the decrease of the AsA/DHA ratio (Fig. 3c). The activity of DHAR was able to regenerate AsA (Asada 1992). With the DHAR activity increasing, the AsA content of 5-ALA + drought treatment plants increased significantly from 10 to 20 days compared to the other treatments (Figs. 4a and 5e). GPX and GR also participated in the ROS-scavenging process by the conversion between GSH and GSSG (Apel and Hirt 2004). In contrast to the control, the contents of GSH and GSSG and the ratio of GSH/GSSG showed results similar to those of AsA and DHA under drought stress and 5-ALA pretreatment (Fig. 4b, d, f). Loggini and Navari-Izzo (1999) reported that higher ratios of ASA/DHA and GSH/GSSG could more effectively scavenge drought-induced ROS. In our study, exogenous 5-ALA pretreatment enhanced the ratios of ASA/DHA and GSH/GSSG (Fig. 4e, f) under drought stress when compared to the control or drought treatment alone. Some reports also demonstrated that the application of 5-ALA was capable of increasing the ratios of ASA/DHA and GSH/GSSG, which is one of the reasons 5-ALA efficiently scavenges ROS under abiotic stress (Nishihara et al. 2003; Liu et al. 2011). Higher ratios of ASA/DHA and GSH/GSSG were obtained by 5-ALA pretreatment, which could be the result of 5-ALA improving the antioxidant enzyme activity of the AsA-GSH cycle and enhancing the cycle’s efficiency.
In our study, the expression levels of Cu/ZnSOD, CAT, APX, DHAR, GPX, and GR were upregulated under drought stress (Fig. 6). This is in agreement with the results wherein drought stress caused an increase in SOD, CAT, APX, DHAR, GPX, and GR activities (Fig. 5). In addition, 5-ALA pretreatment significantly upregulated the expression levels of Cu/ZnSOD, APX, DHAR, and GPX, which were consistent with the higher activity of these enzymes. Similar results were found in the study of Liu et al. (2011), which reported that the expression levels of some antioxidant enzymes were upregulated in response to PEG-mediated stress, and 5-ALA pretreatment was able to significantly improve upregulation. However, the changes in the expression levels of antioxidant enzymes were relatively complex as a result of impacts from cultivars, tissues, stress intensity, and stress duration (Zhou and Shimizu 2010). Our study revealed that the activities of CAT and GR in drought + 5-ALA treatment plants were significantly higher than in drought treatment alone at 20 days after treatment, while the relative expression levels of CAT and GR were markedly lower than in drought treatment alone (Figs. 5b, f and 6b, f). Inconsistent results between the relative expression level of antioxidant enzyme genes and enzyme activity were also observed in Kentucky bluegrass in response to drought or salt stress (Bian and Jiang 2009; Puyang et al. 2015b; Xu 2011). These results indicated that the activities of several antioxidant enzymes may be regulated at the posttranscriptional level but not at the transcriptional level, resulting in different regulation modes among different genes in Kentucky bluegrass (Puyang et al. 2015b).
Conclusion
The present study revealed that the exogenous application of 5-ALA enhanced the tolerance of Kentucky bluegrass plants in response to drought stress. 5-ALA pretreatment had vital roles in alleviating oxidative damage induced by drought stress, which significantly reduced the generation of ROS, LOX activity, and MDA content. 5-ALA was able to mitigate oxidative damage because it sustained non-enzyme antioxidants (AsA and GSH levels), enhanced the activity of antioxidant enzymes, and improved the expression level of antioxidant enzyme genes at the transcriptional or posttranscriptional level. Further transcriptome, proteome, and metabolome research may be necessary to identify the molecular and metabolic pathways by which 5-ALA alleviates drought stress in turfgrass and other plant species. In addition, field tests should be conducted to further examine the effectiveness of 5-ALA at promoting drought tolerance of plants under natural environmental conditions.
Abbreviations
- 5-ALA:
-
5-Aminolevulinic acid
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid (ascorbate)
- BSA:
-
Bovine serum albumin
- CAT:
-
Catalase
- CDNB:
-
1-Chloro-2,4-dinitrobenzene
- DHA:
-
Dehydroascorbate
- DHAR:
-
Dehydroascorbate reductase
- DTNB:
-
5,5′-Dithio-bis (2-nitrobenzoic acid)
- EDTA:
-
Ethylenediaminetetraacetic acid
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- GPX:
-
Glutathione peroxidase
- LOX:
-
Lipoxygenase
- MDHAR:
-
Monodehydroascorbate reductase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NTB:
-
2-nitro-5-thiobenzoic acid
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
References
Akram NA, Ashraf M (2013) Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J Plant Growth Regul 32:663–679
Akram NA, Ashraf M, Al-Qurainy F (2012) Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci Hortic-Amsterdam 142:143–148
Ali B, Tao Q, Zhou Y, Gill RA, Ali S, Rafiq MT, Xu L, Zhou W (2013) 5-Aminolevolinic acid mitigates the cadmium-induced changes in Brassica napus as revealed by the biochemical and ultra-structural evaluation of roots. Ecotoxicol Environ Saf 92:271
Al-Khateeb SA (2006) Promotive effect of 5-aminolevulinic acid on growth, yield and gas exchange capacity of barley (Hordeum vulgare L.) grown under different irrigation regimes. J King Saud Unive Agric Sci 18(18):103–111
Anjum SA, Xie XY, Wang LC, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6:2026–2032
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K (1992) Ascorbate peroxidase - a hydrogen peroxide scavenging enzyme in plants. Physiol Plant 85:235--241
Balestrasse KB, Tomaro ML, Batlle A, Noriega GO (2010) The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 71:2038–2045
Bian S, Jiang Y (2009) Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci Hortic-Amsterdam 120:264–270
Bowler C, Montagu MVA, Inze D (1992) Superoxide-dismutase and stress tolerance. Annu Rev Plant Biol 43:83–116
De Azevedo NA, Prisco JT, Eneas-Filho J, Medeiros JV, Gomes-Filho E (2005) Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J Plant Physiol 162:1114–1122
Doderer A, Kokkelink I, Van DVS, Valk BE, Schram AW, Douma AC (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta 1120:97–104
Elia AC, Galarini R, Taticchi MI, Dörr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
Elstner EF (2003) Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol 33:73–96
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Havir EA, Mchale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455
He J, Li MF, Wu F, Li SP, Zhou SY (2013) Physiological response of banana seedling to exogenous ALA under drought stress. J South Agric:745–750
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:357–359
Hu L, Wang Z, Huang B (2012) Growth and physiological recovery of Kentucky bluegrass from drought stress as affected by a synthetic cytokinin 6-benzylaminopurine. Crop Sci 52:2332–2340
Huang B, DaCosta M, Jiang Y (2014) Research advances in mechanisms of turfgrass tolerance to abiotic stresses: from physiology to molecular biology. Crit Rev Plant Sci 33:141–189
Javed N, Ashraf M, Akram NA, Al-Qurainy F (2011) Alleviation of adverse effects of drought stress on growth and some potential physiological attributes in maize. Photochem Photobiol 87:1354–1362
Jr BW, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Kackley KE, Grybauskas AP, Dernoeden PH, Hill RL (1990) Role of drought stress in the development of summer patch in field-inoculated Kentucky bluegrass. Phytopathology 80:655–658
Kampfenkel K, Van Montagu M, Inze D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Knörzer OC, Burner J, Boger P (1996) Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol Plantarum 97:388–396
Liu ZJ, Guo YK, Bai JG (2010) Exogenous hydrogen peroxide changes antioxidant enzyme activity and protects ultrastructure in leaves of two cucumber ecotypes under osmotic stress. J Plant Growth Regul 29:171–183
Liu D, Pei ZF, Naeem MS, Ming DF, Liu HB, Khan F, Zhou WJ (2011) 5-Aminolevulinic acid activates antioxidative defence system and seedling growth in Brassica napus L. under water-deficit stress. J Agron Crop Sci 197:284–295
Liu M, Li J, Niu J, Wang R, Song J, Lv J, Zong X, Wang S (2016) Interaction of drought and 5-aminolevulinic acid on growth and drought resistance of Leymus chinensis seedlings. Acta Ecol Sin 36:180–188
Loggini B, Navari-Izzo F (1999) Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol 119:1091–1099
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Gollery M, Van BF (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Naeem MS, Rasheed M, Liu D, Jin ZL, Ming DF, Yoneyama K, Takeuchi Y, Zhou WJ (2011) 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta Physiol Plant 33:517–528
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Mahmud JA, Suzuki T, Fujita M (2016) Insights into spermine-induced combined high temperature and drought tolerance in mung bean: osmoregulation and roles of antioxidant and glyoxalase system Protoplasma 1–16
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nishihara E, Kondo KParvez MM, Takahashi K, Watanabe K, Tanaka K (2003) Role of 5-aminolevulinic acid (ALA) on active oxygen-scavenging system in NaCl-treated spinach (Spinacia oleracea). J Plant Physiol 160:1085–1091
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Plant Biol 49:249–279
Ot CP, Jones (1975) Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol 55:485–490
Puyang X, An M, Xu L, Han L, Zhang X (2015a) Antioxidant responses to waterlogging stress and subsequent recovery in two Kentucky bluegrass (Poa pratensis L.) cultivars. Acta Physiol Plant 37:1–12
Puyang X, An M, Han L, Zhang X (2015b) Protective effect of spermidine on salt stress induced oxidative damage in two Kentucky bluegrass (Poa pratensis L.) cultivars. Ecotoxicol Environ Saf 117:96–106
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Schneider K, Schlegel HG (1981) Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochem J 193:99–107
Tanaka R, Tanaka A (2011) Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim Biophys Acta 1807:968–976
Turgeon AJ (1991) Turfgrass management. 3rd ed
Xing X, Zhou Q, Xing H, Jiang H, Wang S (2016) Early abscisic acid accumulation regulates ascorbate and glutathione metabolism in soybean leaves under progressive water stress. J Plant Growth Regul:1–12
Xu L (2011) Antioxidant enzyme activities and gene expression patterns in leaves of Kentucky bluegrass in response to drought and post-drought recovery. J Am Soc Hortic 136:247–255
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649–654
Yang Z, Chang Z, Sun L, Yu J, Huang B (2014) Physiological and metabolic effects of 5-aminolevulinic acid for mitigating salinity stress in creeping bentgrass. PLoS One 9:e116283–e116283
Youssef T, Awad MA (2008) Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid-based fertilizer. J Plant Growth Regul 27:1–9
Yu GH, Weinstein JD (1997) Heme synthesis and breakdown in isolated developing pea chloroplasts. Plant Physiol Biochem 35:223–234
Acknowledgements
This work was funded by National Natural Science Foundation of China (NSFC) (project no. 3131160482).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Niu, K., Ma, X., Liang, G. et al. 5-Aminolevulinic acid modulates antioxidant defense systems and mitigates drought-induced damage in Kentucky bluegrass seedlings. Protoplasma 254, 2083–2094 (2017). https://doi.org/10.1007/s00709-017-1101-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1101-4