Abstract
We have investigated the response of two peanut cultivars (TEGUA and UTRE) with different growth habits and branching pattern structures to different nitrogen (N) sources, namely, N-fertilizer or N2 made available by symbiotic fixation, and analysed the pattern of nitrate reductase (NR) activity in these cultivars. Nitrate and amino acid contents were also examined under these growth conditions. In terms of nitrogen source, cv. TEGUA showed a better response to inoculation with Bradyrhizobium sp. SEMIA 6144 at 40 days after planting, while cv. UTRE responded better to N-fertilizer (5 mM KNO3). Both cultivars showed different patterns of NR activity in the analyzed plant organs (leaves, roots, and nodules), which were dependent on the N source. When nitrogen became available to the plant through symbiotic N2 fixation, the patterns of NR activity distribution were different in the two cultivars, with cv. TEGUA showing a higher NR activity in the nodules than in the leaves and roots, and cv. UTRE showing no difference in terms of NR activity among organs. The nitrate and amino acid contents showed a similar trend between the two cultivars, with the highest nitrate content in the leaves of fertilized plants and the highest amino acid content in the nodules. The high nitrate content of the leaves of cv. UTRE indicated the better response of this cultivar to N-fertilizer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nitrogen (N) requirement of legumes can be met by both inorganic N assimilation and symbiotic N2 fixation, and in practice, legumes obtain their N through both processes. Nitrate (NO3 −) is often the major source of inorganic nitrogen available from soil or fertilizer, and it is reduced to ammonia in a two-step process, of which the first step is the reduction of nitrate to nitrite (NO2 −). This reaction is catalysed by nitrate reductase (NR; EC 1.6.6.4), an inducible enzyme whose activity depends on the availability of nitrate and light (Campbell 1999).
The distribution of NR activity in various parts of the plant varies according to plant genotype, age and nitrate supply (Caba et al. 1995). In the Phaseoleae tribe, nitrate is reduced mainly in the leaves, with low NR activity in the root and nodules (Andrews et al. 1990). Nitrate reduction in the nodules is only a small fraction of the total NR activity in the plant. The use of NO3 − from soil or fertilizer and atmospheric nitrogen (N2) by symbiotic association with rhizobia, either simultaneously or in a complementary way by nodulated legumes, is a unique characteristic of higher plants. At high concentrations, nitrate inhibits both nodulation and N2 fixation in almost all legume species. However, a low concentration of nitrate favours the initial establishment of the nodules, perhaps as an additional source of nitrogen (Becana and Sprent 1987).
Peanut (Arachis hypogaea L.) is an important crop worldwide and is a direct source of subsistence food and several other food products. Argentina, one of the major producers of peanut, concentrates about 92% of its production in the central and southern region of the province of Córdoba. Like all legumes, peanut can meet its nitrogen demand from different sources, such as NO3 − from the soil or fertilizer and N2 by symbiotic association with rhizobia. Peanut is usually nodulated with rhizobia belonging to the Bradyrhizobium genus (Boogerd and van Rossum 1997; Taurian et al. 2002). Peanut rhizobia initiate infection at the bases of the root hairs in the axils of emerging lateral roots through spaces between epidermal cells and subsequently proliferate in intercellular spaces before invading the cortical cells—a process termed “crack entry”. After achieving entry, rhizobial cells occupy the space between the epidermal and cortical cells, spreading further through the root cortex via an intercellular matrix, eventually entering the plant cells.
The peanut plant has a distinct main stem and a variable number of lateral branches. The carriage of the laterals determines the growth habit of the plant. Two distinct forms of growth habit, postrate and erect, have long been recognized and have provided the basis for both agronomic and taxonomic classifications. In earlier classifications, the postrate bunch form was associated with the alternate branching pattern (subspecies hypogaea) and the erect habit with sequential branching pattern (subspecies fastigiata) (Krapovickas 1973).
Peanut cultivars differ botanically (Virginia, Spanish and Valencia types), with each type characterized by a different growth habit and branching pattern structures. The new cultivars that have been obtained from crossing these botanical types have no specific distribution and have been developed to meet environmental demands, such as a short cycle, resistance to drought, high oleic acid content, among others. Differences among cultivars in terms of nodulation (nodule weight and numbers) and the ability of symbiotic N2 fixation have been detected (Wynne et al. 1983).
The objectives of the study reported here were to investigate the response of two peanut cultivars (TEGUA and UTRE) with different growth habits and branching pattern structures to different nitrogen sources, namely, N-fertilizer (5 mM KNO3) or N2 made available by inoculation with Bradyrhizobium sp. SEMIA 6144, and to analyse the pattern of NR activity distribution as well as the nitrate and amino acid contents in these plants under specific growth conditions.
Materials and methods
Bacterial strain and growth conditions
Bradyrhizobium sp. SEMIA 6144, which is able to infect peanut (Arachis hypogaea L.), was obtained from the Microbial Resource Centres (MIRCEN), Brazil. Stock cultures were maintained on YEMA (yeast extract mannitol–agar) supplemented with Congo red (Vincent 1970). Cultures were grown in YEM (yeast extract mannitol) medium at 28°C on a gyratory shaker at 150 rpm. The number of viable cells was determined as colony-forming units (cfu) by the drop-plate method on YEMA plates using bacterial cultures that had been incubated for 48 h (Somasegaran and Hoben 1994).
Peanut cultivars
The peanut cultivars used in the experiments were (1) TEGUA (Virgina botanical type, postrate growth habit and alternate branching pattern, cycle 150 days), kindly supplied by the nursery El Carmen S.A, General Cabrera-Córdoba and (2) UTRE (cross between Spanish and Virgina botanical types, erect growth habit and sequential branching pattern, emergency at low temperature, cycle 130 days), obtained from the peanut improvement programme of Universidad Nacional de Río Cuarto.
Peanut seeds were surface-sterilized following the method described by Vincent (1970). Sterilized seeds were germinated at 28°C in petri dishes (on one layer of Whatman No. 1 filter paper and moist cotton) until the radicle reached a length of 2–3 cm. Individual seedlings growing from sterile seeds were then aseptically transferred to pots containing sterile volcanic sand. The plants were divided into three experimental groups, consisting of (1) control plants; (2) plants fertilized with 5 mM KNO3; (3) plants inoculated with 4 ml of Bradyrhizobium sp. SEMIA 6144 (1 × 108 cfu ml−1). The pots were placed in a greenhouse under controlled environmental conditions (light intensity 200 μmol m−2 s−1, photoperiod 16/8-h light/dark, constant temperature 28°C, relative humidity 50%), harvested at growth stage R1 (flowering; approximately 40 days after planting) and used for the determinations of shoot dry weight, shoot-N content, dry weight of nodules and number of nodules. The shoots and nodules were dried at 70°C for 72 h to a constant weight. The nitrogen content in the shoots was determined according to the procedure proposed by Nelson and Sommers (1973). The amount of nitrogen derived from symbiotically fixed nitrogen was estimated according to International Centre of Agricultural Research in the Dry Area (ICARDA 1990) by applying the following calculation:
Amount of fixed N = (N content of inoculated plant) − (N content of control plant).
Extraction of NR and activity measurement
Leaves, roots and nodules of peanut plants harvested at growth stage R1 were ground in liquid nitrogen and extracted in 4 ml g−1 (fresh weight) buffer A (50 mM Hepes-KOH, pH 7.6, 10 mM MgCl2, 1 mM DTT, 5 μM FAD, 1 μM leupeptin) at 4°C. The mixture was incubated for 15 min on ice, and the homogenate was centrifuged for 10 min at 12,000 g at 4°C. The supernatant was used immediately for NR activity measurements according to the method described by Pigaglio et al. (1999) with some modifications. The NR activity measurements were carried out in buffer B (50 mM Hepes-KOH, pH 7.6, 10 mM MgCl2, 140 μM NADH, 5 mM KNO3). In some experiments, EDTA (15 mM) was added to the reaction buffer. The reaction was initiated by adding 300 μl of extract and was carried out for 10 min at 27°C in a total volume of 1 ml. To estimate the amount of nitrite formed, 1 ml each of 1% sulfanilamide in 3 N HCl and 0.02% N-(1-naphthyl)ethylenediamine (NNEDA) were added to the reaction mixture and the absorbance read at 540 nm on a spectrophotometer (GENESYS 2; Thermo Scientific, Waltham, MA). The specific activity of NR was expressed as nanomoles NO2 − per minute per milligram protein The activation state of NR corresponds to the ratio of NR specific activity measured in the presence of free magnesium ions to that measured in the presence of EDTA. This activation state was expressed as a percentage and reflects the amount of active NR in an extract.
Nitrate, amino acid and protein determinations
Leaves, roots and nodules of peanut plants were dried at 70°C for 72 h to a constant weight, and the nitrate content was determined using the method of Cataldo et al. (1975). Amino acids were quantitatively extracted and estimated according to the ninhydrin method using the amino acid l-leucine as the standard (Rosen 1957). Protein was estimated by the Coomassie blue staining method with bovine serum albumin as the standard (Bradford 1976).
Statistical analysis
Data were analysed statistically by one-way analysis of variance, and means were compared with Duncan’s multiple mean comparison test. A level of P < 0.05 was accepted as significant. The correlation analysis was determined using Pearson’s coefficient.
Results
Estimation of growth and nitrogen fixation in peanut cultivars
Growth and nitrogen fixation in the two peanut cultivars varied among the vegetative and reproductive structures. Cultivar TEGUA, which has a postrate growth habit and alternate branching pattern, showed a significant increase in shoot dry weight and nitrogen content in the inoculated treatment as compared to the other two treatments (control and fertilized) (Table 1). Pearson’s coefficient revealed that shoot dry weight was correlated with nitrogen content (r = 0.91), nodule number (r = 1) and nodule dry weight (r = 0.90). Nodule dry weight was correlated with nodule number (r = 0.93). Cultivar UTRE, which has an erect growth habit and sequential branching pattern, showed a significant increase in shoot dry weight and nitrogen content in the fertilized treatment relative to the other two treatments. A comparison of shoot dry weight with nitrogen content revealed a high correlation (r = 0.98). The amount of nitrogen derived from symbiotically fixed nitrogen in cvs. TEGUA and UTRE was 44.64 ± 2.92 and 5.62 ± 0.48 mg plant−1, respectively.
NR activity in peanut cultivars
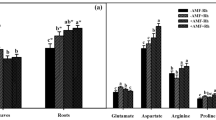
The application of N-fertilizer increased NR activity in the leaves of the two peanut cultivars compared to those of the control plants. There were no differences between the inoculated and fertilized plants of cv. UTRE in terms of NR activity in the leaves and roots. Plants of the two cultivars had different patterns of NR activity distribution when inoculated with Bradyrhizobium sp. SEMIA 6144, with those of cv. TEGUA having a higher NR activity in the nodules than in the leaves and roots, while there was no difference in NR activity in the organs of cv. UTRE (Fig. 1a, b).
Nitrate reductase (NR) activity in control, fertilized and inoculated peanut cultivars. a TEGUA, b UTRE. Data are given as the mean ± standard error (SE) (n = 5). Data for leaves or roots, respectively, with the same letter above each column do not differ significantly at P < 0.05 according to Duncan’s multiple mean comparison
Nitrate and amino acids contents in peanut cultivars
In both cultivars, the nitrate content was higher in the leaves of fertilized plants than in those of inoculated plants (Fig. 2a, b), and the total amino acid content was higher in nodules than in the leaves and roots (Fig. 3a, b).
Amino acid content in control, fertilized and inoculated peanut cultivars. a TEGUA and b UTRE. Data are given as the mean ± SE (n = 5). Data for leaves or roots, respectively, with the same letter above each column do not differ significantly at P < 0.05 according to Duncan’s multiple mean comparison
Discussion
Peanut has a high symbiotic nitrogen-fixing capacity and, relative to other tropical legumes, it accumulates a larger amount of nitrogen. A high degree of nodulation has been correlated with symbiotic effectiveness of the inoculated strain (Sprent 1994). Our findings on the estimation of nitrogen content and nodulation (nodule weight and numbers) revealed differences that correlated with the growth habits and branching pattern of the peanut cultivars. Thus, cv. TEGUA, with a postrate growth habit and alternate branching pattern, showed a better response to inoculation, while cv. UTRE cultivar, with an erect growth habit and sequential branching pattern, presented a better response to N-fertilizer. Cox et al. (1982) also found that peanut cultivars with a sequential branching pattern had a high growth in response to N-fertilizer although these researchers did not evaluate the response of these cultivars to symbiotically fixed nitrogen.
It is well documented that net nitrogen fixation is the product of rhizobial and plant genotypes as well as of environmental conditions (Sessitsch et al. 2002). As such, the efficiency of a rhizobial strain could change when it interacts with different plant genotypes. However, little is known about the genetic compatibility between rhizobia and their host plant in terms of nitrogen fixation, which is the agronomically and ecologically important result of such an interaction. Chen et al. (2003) found that different peanut cultivars varied in their ability to nodulate when inoculated with a given Bradyrhizobium strain. In our study, Bradyrhizobium sp. SEMIA6144 was able to nodulate both cultivars (TEGUA and UTRE), although the nodulation efficiency was higher in cv. TEGUA, possibly due to differences between cultivars in terms of their ability to interact symbiotically with this rhizobial strain.
The peanut cultivars studied here differed in terms of the pattern of NR activity distribution throughout the plants. Both fertilized and inoculated plants of cv. UTRE plants showed a high NR activity in the leaves and roots. In contrast, only fertilized plants of cv. TEGUA showed a high NR activity in the leaves and roots despite the fact that the nitrogen content of the shoots was similar to that found in control plants.
Studies carried out on the pattern of NR activity distribution in species of bean (Phaseolus lunatus and Phaseolus vulgaris) inoculated with rhizobia have revealed differences in the NR activity of leaves, roots and nodules (Silveria et al. 2001). Our results also revealed differences in the NR activity in these organs of cv. TEGUA plants inoculated with Bradyrhizobium sp. SEMIA 6144, with the highest NR activity found in the nodules. These results agree with those reported by Polcyn and Lucinski (2001) who found that NR activity was higher in the nodules of yellow lupine than in other parts of the plant. On the other hand, Ayala (1997) suggested that NR activity in the nodules of peanut plants could be used as a measure of nitrogen fixation and the effectiveness of rhizobial strains. Our results on NR activity in nodules indicate that Bradyrhizobium sp. SEMIA 6144 is an effective strain for use as an inoculant of peanut.
Several published studies have demonstrated that enhancement of nitrogen fixation is due to the presence of active NR in bacteroids. Symbiotic associations in which NR function is complementary to that nitrogenase are possible since bacteroids of many rhizobial strains are capable of performing dissimilatory nitrate reduction (Chamber-Pérez et al. 1997). Symbiotic associations of Rhizobium strains characterized by a high NR activity have been found to be less susceptible to inhibition by nitrate (Lucinski et al. 2002). Studies aimed at elucidating how the addition of nitrate affects the process by which Bradyrhizobium sp. SEMIA 6144 infects peanut plants and bacteroidal NR activity are ongoing.
Nitrate assimilation in the leaves or roots is dependent on the concentration of nitrate in the soil and the nature of the plant. In peanut plants, the nitrate concentration in different organs showed a similar trend between the two cultivars, with the highest nitrate content observed in the leaves of fertilized plants. In plants of cv. UTRE, the high nitrate content in the leaves and roots indicated a better response of this cultivar to N-fertilizer. A positive correlation between NR activity and nitrate content in these organs was also found.
An earlier study on ammonium assimilation found that the enzyme activities of GS and GOGAT (glutamate synthase) clearly increased in cv. TEGUA plants inoculated with Bradyrhizobium sp. SEMIA 6144 (Terzo et al. 2005). The increase in amino acid contents found in our study may be related to a more effective ammonium assimilation since this process is involved in the synthesis of amino acids. Considering that nodule GS and GOGAT are plant gene products whose expression can be influenced by the nodule stage development and effectiveness (Vance et al. 1988; Suganuma et al. 1999), it is possible to suggest that a higher availability of fixed N may modulate the gene expression of the enzyme in the nodule.
This is the first study on the response of two peanut cultivars with different growth habits and branching patterns to different nitrogen sources, namely N-fertilizer or symbiotically fixed N. We found that cv. TEGUA plants responded strongly to biological nitrogen fixation, and those of cv. UTRE responded strongly to N-fertilizer. Both cultivars showed different patterns of NR activity distribution in the analysed organs (leaves, roots and nodules) that was dependent on the nitrogen source supplied. Cultivar TEGUA plants provided symbiotic N2 fixation showed a higher NR activity in the nodules than in the leaves and roots, while no difference in NR activity was found in these organs cv. UTRE plants.
Knowing that there are differences among cultivars in terms of response to different nitrogen sources is important in terms of agronomical practices; for example, when to apply a N-fertilizer or whether to inoculate with Bradydrhizobium sp. in order to obtain a higher crop yield. This knowledge is useful both for the production system and for the environment. For the production system, this knowledge may lead to lower costs and fewer activities during planting. For the environment, the use of inoculants as the nitrogen source to the plant will facilitate soil conservation measures and diminish the use of environmentally harmful N-fertilizers.
Abbreviations
- CFU:
-
Colony-forming units
- ICARDA:
-
International Centre of Agricultural Research in the Dry Area
- MIRCEN:
-
Microbial Resource Centres
- NR:
-
Nitrate reductase
- YEM:
-
Yeast extract mannitol
- YEMA:
-
Yeast extract mannitol agar
References
Andrews M, Faria SM, McInroy SG, Sprent J (1990) Constitutive nitrate reductase activity in the leguminosae. Phytochem 29:49–54
Ayala L (1997) Estudio de algunos aspectos de la fijación simbiótica de nitrógeno por el maní (Arachis hypogaea). Agron Trop 27:427–449
Becana M, Sprent JI (1987) Nitrogen fixation and nitrite reduction in the root nodules of legumes. Physiol Plant 70:757–765
Boogerd F, Van Rossum D (1997) Nodulation of groundnut by Bradyrhizobium: a simple infection process by crack entry. FEMS Microbiol Rev 21:5–27
Bradford M (1976) A rapid sensitive method for the quantification the microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–276
Caba JM, Lluch C, Ligero F (1995) Distribution of nitrate reductase activity in Vicia faba: effect of nitrate and plant genotype. Physiol Plant 93:667–672
Campbell WH (1999) Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Annu Rev Plant Physiol Mol Biol 50:277–303
Cataldo D, Haroon N, Schrader L, Young V (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80
Chamber-Pérez MA, Camacho-Martínez M, Soriano-Niebla JJ (1997) Nitrate reductase activities of Bradyrhizobium sp. in tropical legumes: effects of nitrate on O2 diffusion in nodules and carbon costs of N2 fixation. J Plant Physiol 150:92–96
Chen Q, Zhang X, Terfework Z, Kaijalainen S, Li D, Lindström K (2003) Diversity and compatibility of peanut (Arachis hypogaea L.) bradyrhizobia and their host plants. Plant Soil 255:605–617
Cox F, Adams F, Tucker B (1982) Liming, fertilization and mineral nutrition. In: Pattee HE, Young CT (eds) Peanut science and technology. American Peanut Research and Education Society, Yoakum
ICARDA (1990) Chickpea biological nitrogen fixation. In: ICARDA (ed) Food legume improvement program. International Center of Agricultural Research in the Dry Area, Annual Reporter, Aleppo, pp 96–107
Krapovickas A (1973) Evolution of the genus Arachis. In: Moav R (ed) Agricultural genetics-selected topics. National Council of Research and Development, Jerusalem, p 131–151
Lucinski R, Polcyn W, Ratajczak L (2002) Nitrate reduction and nitrogen fixation in symbiotic association Rhizobium-legumes. Acta Biochim Polonica 49:537–546
Nelson D, Sommers L (1973) Determination of total nitrogen in plant material. Agron J 65:109–112
Pigaglio E, Durand N, Meyer C (1999) Conserved acidic motif in the N-terminal domain of nitrate reductase is necessary for the inactivaction of the enzyme in the dark by phosphorylation and 14–3–3 binding. Plant Physiol 119:219–229
Polcyn W, Lucinski R (2001) Functional similarities of nitrate reductase as a source of nitrogen in soybean, Glicyne max (L). Physiol Plant 158:829–834
Rosen H (1957) A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys 67:10–15
Sessitsch A, Howieson JG, Perret X, Antoun H, Martnez-Romero E (2002) Advances in Rhizobium research. Crit Rev Plant Sci 21:323–378
Silveria JA, Matos JC, Cecatto VM, Viegas RA, Oliveira JT (2001) Nitrate reductase activity, distribution, and response to nitrate in two contrasting Phaseolus species inoculated with Rhizobium spp. Environ Exp Bot 46:37–46
Somasegaran P, Hoben H (1994) Quantifying the growth of Rhizobia. In: Somasegaran P, Hoben H (eds) Handbook for Rhizobia. Springer, New York, pp 47–57
Sprent J (1994) Nitrogen fixation. In: Smartt J (ed) The groundnut crop: a scientific basis for improvement. Chapman and Hall, London, pp 255–280
Suganuma N, Watanabe M, Yamada T, Isaura T, Yanamoto K, Nishimura M, Toniyama K (1999) Involvement of ammonia in maintenance of cytosolic glutamine synthetase activity in Pisum sativum nodules. Plant Cell Physiol 40:1053–1060
Taurian T, Aguilar OM, Fabra A (2002) Characterization of nodulating peanut rhizobia isolated from a native soil population in Córdoba, Argentina. Symbiosis 33:59–72
Terzo E, Natera V, Isola MC, Fabra A, Franzoni L, Castro S (2005) Effect of low pH on the enzyme activities of the ammonium assimilation pathways in the symbiotic association Bradyrhizobium sp.-peanut (Arachis hypogaea L.). Symbiosis 40:1–6
Vance CP, Egli MA Griffith SM (1988) Plant regulated aspects of nodulation and N2 fixation. Plant Cell Environ 11:413–427
Vincent J (1970) A manual for the practical study of root nodule bacteria. International biological programme. Handbook No. 15. Blackwell Scientific, Oxford
Wynne JC, Elkan GH, Isleib TG, Schneeweis TJ (1983) Effect of host plant, Rhizobium strain and host x strain interaction on symbiotic variability in peanut. Peanut Sci 10:110–114
Acknowledgments
We thank the Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto for providing financial assistance for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delfini, R., Belgoff, C., Fernández, E. et al. Symbiotic nitrogen fixation and nitrate reduction in two peanut cultivars with different growth habit and branching pattern structures. Plant Growth Regul 61, 153–159 (2010). https://doi.org/10.1007/s10725-010-9461-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-010-9461-1