Abstract
As unique secondary metabolites of Ginkgo biloba L. and relics of Ginkgoaceae, terpene trilactones (TTLs) possess great medicinal value. In this study, we investigated the effects of ultraviolet (UV) irradiation, cold stress (4 °C), and exogenous hormone treatment on TTLs and key genes expression in ginkgo leaves in annual potted ginkgo seedlings subjected to different stress treatments. The processing conditions were as follows: UV treatment (1500 μJ/m2) for 0, 8, 16, 24, and 48 h; cold stress at 4 °C, 400 µmol photons m−2 s−1; 100 μM abscisic acid (ABA); 100 μM methyl jasmonate (MeJA); 10 mM salicylic acid (SA); and 10 mM ethephon (ETH) treatments for 0, 2, 4, 6, and 8 days. Results of evaporative light-scattering detector–high-performance liquid chromatography showed that UV, cold stress, ABA, SA, MeJA, and ETH treatments increased the TTL content at various levels and up to 21.9%. The relationships among the expression levels of the key enzyme genes GbDXS, GbGGPPS, GbLPS, and GbMVD in the TTL biosynthesis pathway were also analyzed. Changes in the TTL content and expression levels of the key genes were significantly and positively correlated with TTL content. UV, cold stress, ABA, SA, MeJA, and ETH were found to increase the TTL content by upregulating the expression of the key genes. This study aimed to reveal the molecular mechanism through which TTLs are synthesized. The results can also provide theoretical guidance for the application of treatment methods to increase TTL content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gingko biloba, also known as a “living fossil,” is one of the existing ancient plants and a special species in China. G. biloba contains several medicinal ingredients, including terpene trilactones (TTLs) and flavonoids. TTLs are components unique to this species and primarily include ginkgolides and bilobalide. Ginkgolides are potent antagonists of platelet-activating factor and can be used to treat cardiovascular and cerebrovascular diseases. Bilobalide has a protective function against neuronal damages and can be used to treat demyelinating brain, spinal cord, and neurological diseases (Diamond and Bailey 2013; Serrano-García et al. 2013). However, the TTL content of G. biloba is considerably low and cannot meet the requirements for industrial production (Ye et al. 2020a). Therefore, exploring a suitable method to increase TTL content could benefit the industry of G. biloba.
Plant terpenoids, including TTLs, are synthesized primarily through two pathways. The classical mevalonate (MVA) pathway in the cytoplasm is responsible for the biosynthesis of sesquiterpenoids, triterpenoids, and steroids; the other pathway is the 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway in the chloroplasts and is responsible for the formation of monoterpenoids, diterpenoids, and tetraterpenoids (Ye et al. 2020b). The terpenoid content in plants can be increased by upregulating the expression of the key enzyme genes in the terpenoid biosynthesis pathway. Methyl jasmonate (MeJA) induces artemisinin biosynthesis by upregulating the expression of the genes involved and the transcription factor (Xiang et al. 2014). Similar studies in G. biloba have been reported (Xu et al. 2011; Kim et al. 2012; Liao et al. 2016). The key enzyme genes of the MEP pathway (GbDXS, GbGGPPS, and GbLPS) and the MVA pathway (GbHMGR and GbMVD) have been isolated and identified from G. biloba (Liao et al. 2004; Gong et al. 2006; Kim et al. 2012; Liao et al. 2015, 2016).
Exogenous stress can regulate the metabolism and accumulation of several secondary metabolites by regulating the expression levels of key genes in the biosynthesis pathways in plants. Spraying chlorocholine chloride on the ginkgo leaves significantly upregulates the expression of four key enzyme genes (GbDXS, GbDXR, GbGGPPS, and GbLPS) in the TTL biosynthesis pathway, thereby increasing TTL content (Xu et al. 2011). MeJA can elevate the transcription levels of GbCMK2 and GbIDS2 in G. biloba and transfer the primary metabolites to the secondary metabolites and upregulate the GUS expression in transgenic plants; this feature shows that MeJA treatment can enhance TTL biosynthesis (Kim et al. 2012). The PkHMGR and PkDXS genes have cold stress and light responses in the biosynthesis of picroside; cold stress and light induce the expression levels of PkHMGR and PkDXS, thereby elevating the picroside content in Picrorhiza scrophulariiflora (Kawoosa et al. 2010). The transcript levels of GbAACT and GbMVK significantly increased in response to MeJA and salicylic acid (SA) treatments, and the increase corresponded to the elevation in the TTL content (Chen et al. 2017). The biosynthesis of TTLs can be altered through the responses of GbHMGR2 and GbHMGR3 to cold, MeJA, SA, ethephon (ETH), and abscisic acid (ABA) treatments (Rao et al. 2019). In our previous studies, we showed that GbMVD responds positively to MeJA and SA treatments (Liao et al. 2016).
We previously showed that the GbMVD promoter has a MeJA-responsive cis-acting regulatory, ethylene-responsive, ABA-responsive elements, and light-responsive transcription factor binding sites (Liao et al. 2016). The GbLPS promoter comprises ABA-responsive and MeJA-responsive cis-acting regulatory elements (Kim et al. 2012). In this study, the effects of UV, cold, ABA, SA, MeJA, and ETH treatments on TTL concentration, the roles of these treatments in TTL biosynthesis, and the corresponding molecular mechanisms were investigated.
Materials and Methods
Plant Materials and Growth Conditions
The samples were annual seedlings of G. biloba in pots filled with culture soil (humus soil:perlite:peat 1:1:1). All independent seedlings of the same genotype “Jiafoshou” were collected from G. biloba trees with uniform growth conditions and the same age. All seedlings were placed in a greenhouse with 16 h of day (25 °C, 400 μmol photons m−2 s−1) and 8 h of night (18 °C) photoperiod at relative humidity of 70%.
Treatment Method
UV irradiation was performed as follows. A UV light box at 1500 μJ/m2 was used, and all leaves were collected at 0, 8, 16, 24, and 48 h after UV treatment. Cold treatment was performed under the following conditions: 4 °C light incubator (400 μmol photons m−2 s−1) and leaf collection at 0, 2, 4, 6, and 8 days after cold treatment. The conditions for exogenous hormone treatment were as follows. ABA (100 μM), MeJA (100 μM), SA (10 mM), and ETH (10 mM) were dissolved in their corresponding solvents and sprayed on the surfaces of the G. biloba seedlings. In the control, the solvent was sprayed on the plants. Each treatment had three biological replicates with ten strains per replicate. All samples were collected, immediately frozen in liquid nitrogen, and prepared for further use.
RNA Extraction and cDNA Preparation
The total RNAs were extracted with the TaKaRa MiniBEST Plant RNA Extraction kit (TaKaRa, China), and potential RNAs were removed using the RNase-free Recombinant DNase I. The RNA concentrations were measured spectrophotometrically with Nano Drop One (Thermo Fisher Scientific, USA). The RNA from each sample was reversely transcribed into the cDNA by using a PrimeScript™ II 1st Strand cDNA biosynthesis kit (TaKaRa, China).
Total TTLs Detected Using an Evaporative Light-Scattering Detector–High-Performance Liquid Chromatograph
The total TTL content was detected through the evaporative light-scattering detector–high-performance liquid chromatography (HPLC-ELSD) method established in our laboratory. The specific operations were as follows. G. biloba leaves were collected and dried at 50 °C, and 2 g of the dry powder was extracted with 30 mL of ethyl acetate in 600 W ultrasonic bath for 15 min. The sample was transferred to a filter paper bag and placed in a Soxhlet extractor. Then, 20 mL of ethyl acetate and two drops of 2% HCl were added. The samples were condensed and refluxed at 90 °C for 1.5 h, filtered, and evaporated to dryness in vacuum at 90 °C. Finally, the residue was redissolved in 5 mL of methanol. The solution was filtered through a 0.45 μm organic membrane filter and divided into three parts. Then, the filtered solutions were analyzed through Thermo UltiMate 3000 HPLC system and Thermo ELSD 6000 detector. High-performance liquid chromatography was performed using a Thermo C-18 column (5 μm, 4.6 mm × 250 mm) with methanol. Tetrahydrofuran was prepared using water (20:8:72) mixture as mobile phase at a flow rate of 0.8 mL min−1 and column temperature of 40 °C. ELSD conditions comprised a drift tube temperature at 108 °C. The carrier gas was air, and the gas flow rate was 3.1 L min−1. The authentic control samples were purchased from Yuanye Bio-Technology Co. (China). The injection volume was 2 μL, and three replications (n ≥ 3) were performed. The statistical significance of TTL content was analyzed with SPSS 22.0. All tests were performed in triplicate, and data are represented as means ± SD (n = 3).
Quantitative Real-Time PCR Detected
The qRT-PCR volumes were 20 μL, containing 10 μL of 2 × ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China), forward and reverse primers with a final concentration of 0.2 μM, 2 μL of cDNA, and ddH2O added in the PCR solution to reach 20 μL. The qRT-PCR was conducted using a BIOER FQD-96A cycler (BIOER, Hangzhou, China) under the following conditions: 95 °C for 30 s; followed by 40 cycles of 95 °C for 10 s, 60 °C for 10 s; 1 cycle of 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. The relative expression level of each gene was normalized with the internal reference gene for 18 s. The relative expression was calculated using the 2-△△Ct, which was performed to calculate the relative changes in gene expression determined by the qRT-PCR experiments (Livak and Schmittgen 2001). The primers used for the qRT-PCR and gene accession number are listed in Table 1. The qRT-PCR data were technically replicated with error bars, representing mean ± SD (n = 3).
Statistical Analysis
Data were analyzed with one-way ANOVA by using SPSS 22.0 for Windows (SPSS Inc., USA). The means were compared with Duncan’s multiple range tests. P value less than 0.05 was considered to be statistically significant.
Results
Effects of UV Treatment on TTL Content and Gene Relative Expression Levels
The differences in TTL content were nonsignificant when G. biloba plants were treated with UV for 0 h (217.29 μg/g DW), 8 h (212.92 μg/g DW), and 16 h (221.52 μg/g DW) but showed significant improvement after 24 h (P < 0.05) and 48 h (P < 0.01) of UV irradiation (Fig. 1). The TTL content was 239.17 μg/g DW in plants treated with UV for 24 h and 264.85 μg/g DW in plants treated with UV for 48 h, with increases of 10.0% and 21.9%, respectively, relative to the TTL content in the control (217.29 μg/g DW). The results indicated that UV irradiation for 24 h could significantly increase TTL content.
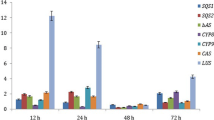
The qRT-PCR revealed the response levels of key genes involved in the biosynthesis of TTLs under UV irradiation. The expression levels of GbDXS, GbGGPPS, GbLPS, and GbMVD were significantly increased in different periods after UV irradiation for 0–48 h. The expression level of GbDXS was minimally changed in response to UV irradiation at 16 h after treatment, increased thereafter, and reached the highest level at 48 h. The expression level of GbGGPPS also significantly increased from 16 to 48 h and reached the highest level at 16 h. The expression levels of GbLPS and GbMVD significantly increased from 16 to 48 h, and the highest values were obtained at 24 h (Fig. 2).
The TTL content of the G. biloba leaves increased with prolonged UV irradiation, and the highest production was obtained at 48 h of UV irradiation. The highest expression levels of GbDXS, GbGGPPS, GbLPS, and GbMVD were recorded at 48, 16, 24, and 24 h of treatment, respectively. The trend of GbDXS expression level was consistent with that of TTL accumulation. That is, UV treatment time was proportional to the TTLs and GbDXS expression level. The peaks expression of GbGGPPS, GbLPS, and GbMVD appeared before the peak of the TTL content (Fig. 3). These results demonstrated that GbDXS positively responded to UV at the final stage of treatment, and GbGGPPS, GbLPS, and GbMVD positively responded to UV at the middle stage of treatment.
Effects of Cold Treatment on TTL Content and Expression of Key Genes
The differences among the TTL contents of G. biloba under cold treatment on 0 day (250.96 μg/g DW), 2 days (250.12 μg/g DW), and 4 days (260.35 μg/g DW) were nonsignificant. By contrast, TTL production showed significant improvement (P < 0.05) after cold treatment for 6 and 8 days (Fig. 4). The TTL contents were 281.86 μg/g DW in the plants after cold treatment for 6 days and 287.23 μg/g DW in those treated with cold temperature for 8 days, with increases of 12.3% and 14.5%, respectively, relative to the TTL content of the control (250.96 μg/g DW) (Fig. 4). The results suggested that cold treatment for 6 days could enhance TTL biosynthesis.
The qRT-PCR results revealed the response levels of the key genes involved in the biosynthesis of TTLs under cold treatment. The expression level of GbDXS remained at the control level between 0 and 4 days, after which this expression was significantly induced between 6 and 8 days and reached the highest level at 6 days. Changes in the expression level of GbGGPPS in response to cold temperature at 2 days after treatment was minimal. The gene was significantly induced between 4 and 8 days, reaching the highest level at 4 days. The expression level of GbLPS remained at the control level between 0 and 4 days. This gene was significantly induced between 6 and 8 days, reaching the highest level at 8 days. The expression level of GbMVD was significantly induced between 2 and 8 days and reached the highest level at 8 days (Fig. 5). Thus, cold treatment could increase the expression levels of GbDXS, GbLPS, GbGGPPS, and GbMVD.
The highest TTL content was determined at 8 days after cold treatment, and the highest expression levels of GbDXS, GbGGPPS, GbLPS, and GbMVD were recorded at 6, 4, 8, and 8 days of treatment. The peaks of GbLPS and GbMVD expression levels were consistent with the peak of TTL content. The peaks of GbGGPPS and GbDXS expression levels were obtained before the peak of the TTL content was reached (Fig. 6). GbDXS and GbGGPPS positively responded to UV at the beginning of treatment, whereas GbLPS and GbMVD positively responded to UV at the end stage of treatment. Thus, cold treatment could increase the expression of key genes in the biosynthesis of TTLs and thereby elevate the TTL content in G. biloba.
Effects of Hormone Treatment on the TTL Content and Expression of Key Genes
No significant differences in TTL contents were observed when the plants were treated with ABA for 0 day (258.15 μg/g DW), 2 days (262.72 μg/g DW), 4 days (244.31 μg/g DW), 6 days (259.17 μg/g DW), and 8 days (251.03 μg/g DW). Differences in the TTL contents were nonsignificant when the plants were treated with SA for 0 day (259.42 μg/g DW), 2 days (259.96 μg/g DW), 4 days (261.25 μg/g DW), and 6 days (270.25 μg/g DW). However, the TTL contents significantly improved (P < 0.05) after SA treatment for 8 days (283.41 μg/g DW), with 9.2% increase relative to that of the control (259.42 μg/g DW). Differences in the TTL contents were nonsignificant when the plants were treated with MeJA for 0 day (260.16 μg/g DW), 2 days (267.65 μg/g DW), and 4 days (269.42 μg/g DW), whereas TTL contents significantly improved (P < 0.05) after MeJA treatment for 6 days (286.07 μg/g DW) and 8 days (297.85 μg/g DW), with increases of 10.0% and 14.5%, respectively, relative to the TTL content of the control (260.16 μg/g DW). Differences in the TTL contents were nonsignificant when the plants were treated with ETH for 0 day (259.28 μg/g DW), 2 days (261.04 μg/g DW), 4 days (274.29 μg/g DW), and 6 days (278.32 μg/g DW). By contrast, TTL content significantly improved (P < 0.05) after ETH treatment for 8 days (285.79 μg/g DW), with 10.2% increase relative to that of the control (259.28 μg/g DW) (Fig. 7). The results suggested that ABA, SA, MeJA, and ETH treatments enhanced the biosynthesis of TTLs.
The qRT-PCR revealed the response levels of the key genes involved in the biosynthesis of TTLs under exogenous hormone treatments. In the ABA treatment, the expression level of GbDXS minimally changed at 2 days after treatment and had the highest expression level on 4 days. From 6 to 8 days, the expression level of GbDXS returned to the same level with that of the control. The expression level of GbGGPPS was significantly induced between 2 and 8 days and reached the highest level on 6 days. The expression level of GbLPS had no significant difference when the plants were treated with ABA from 0 to 8 days. The expression level of GbMVD significantly increased from 4 to 8 days and reached the highest level on 8 days(Fig. 8). These results indicated that ABA can significantly increase the expression levels of key genes in the TTL biosynthesis but cannot significantly increase the TTL content. Thus, TTL biosynthesis is a complex process. In addition to the regulation of some key genes, this biosynthesis might contain other substances, such as transcription factors.
The relative expression levels of DXS, GGPPS, LPS, and MVD in G. biloba L. treated by ABA, SA, MeJA, and ETH in 8 days. Notes: * means that the level of gene expression is significantly higher than the control (P < 0.05); ** means that the level of gene expression is significantly higher than the control (P < 0.01)
In the SA treatment, the expression level of GbDXS increased as the processing time was extended from 2 to 8 days and reached the highest level on 8 days. The expression level of GbGGPPS was significantly induced between 2 and 8 days and reached the highest level on 4 days. The expression level of GbLPS had no significant changes from 2 to 6 days but showed significant improvement on 8 days, reaching the highest level on 8 days. The expression level of GbMVD increased as the processing time was prolonged from 2 to 8 days and reached the highest level on 8 days (Fig. 8). The peak of GbGGPPS expression level appeared before the TTL content reached its peak level. The peaks of GbDXS, GbLPS, and GbMVD expression levels were consistent with the peak of TTL content (Fig. 9).
The expression level of GbDXS under MeJA treatment had no significant difference after 2 days but showed highly significant changes from 4 to 8 days, reaching the highest level on 6 days. The expression level of GbGGPPS exhibited a highly significant improvement between 2 and 8 days and reached the highest level on 6 days. The expression level of GbLPS significantly improved between 2 and 8 days and reached the highest level on 6 days. However, the expression level of GbMVD had no significant change after 2 days of MeJA treatment but showed highly significant improvement between 4 and 8 days, reaching the highest level on 6 days (Fig. 8). The peaks of the expression levels of the four key genes in the TTL biosynthesis were consistent on 6 days (Fig. 9). Thus, the genes in the TTL biosynthetic pathway coordinated with each other and may be regulated by transcription factors.
The expression level of GbDXS under ETH treatment showed significant improvement between 2 and 8 days and reached the highest level on 4 days. The expression level GbGGPPS had no significant changes from 0 to 4 days but improved significantly between 6 and 8 days, reaching the highest level on 6 days. The expression level of GbLPS significantly improved between 2 and 8 days and reached the highest level on 2 days. The expression level of GbMVD showed highly significant improvement between 2 and 8 days and reached the highest level on 8 days (Fig. 8). The peak of the GbMVD expression level was consistent with the TTL content. The peaks of GbGGPPS, GbDXS, and GbLPS expression levels were recorded before TTL content reached its peak level (Fig. 9). These results indicated that GbDXS, GbLPS, and GbGGPPS positively responded to ETH at the beginning of treatment, whereas GbMVD positively responded to ETH at the end of treatment.
Discussion
TTL content increased with prolonged UV irradiation, reaching the highest level at 48 h and increased by 21.9% compared with the control. Light quality affected the biosynthesis and accumulation of TTLs, changes in light intensity and quality during TTL biosynthesis considerably increased TTL content, and the highest increase was observed in plants treated with purple film (Leng et al. 2002). The GbHMGS1 expression was significantly induced by UV-B, and the TTL content was continuously induced and increased in G. biloba (Meng et al. 2017). However, only changes in TTL content after treatment were previously studied. According to the qRT-PCR data in this study, the physiological changes and intrinsic molecular mechanism of UV irradiation increased the TTL content. UV-B irradiation can significantly increase the amount of suspended cells and secondary metabolites in a suspension culture system of Tripterygium wilfordii (Zhu et al. 2013). This finding is consistent with the results in the present study. Thus, the secondary metabolite content of plants could be increased by UV treatment. UV irradiation could significantly induce the expression of the four key genes, and the expression level of GbDXS was elevated with prolonged UV irradiation. The expression levels of the three other key genes peaked within 0 h and 48 h. In other words, UV irradiation improved TTL content, which was most probably achieved by increasing the expression levels of the key enzyme genes.
Cold stress is one of the main abiotic stresses in plant growth, and plant growth and physiological metabolism are directly affected by this stress. Plants respond to cold stress in several ways, particularly through metabolic regulation. The response of G. biloba to cold treatment continuously increased within 8 days, reached its maximum level at 8 days, and increased by 14.5% compared with the control. The qRT-PCR results also showed that cold treatment significantly increased the expression levels of GbDXS, GbGGPPS, GbLPS, and GbMVD. The GbLPS and GbMVD enzyme genes were at the end of the TTL biosynthesis pathway (Kim et al. 2012; Buhaescu and Izzedine 2007). Thus, the peaks were consistent with the peak of TTL content. GbDXS and GbGGPPS were close to the front in the TTL biosynthesis pathway (Gong et al. 2006; Liao et al. 2004). Thus, the peaks of these genes appeared earlier than that of the TTL content. A previous study has shown that the contents of some terpenoid compounds in basil can be partially enhanced by increasing the expression levels of genes involved in their biosynthesis under cold treatment (Senji and Mandoulakani 2018).
ABA is an important plant hormone and plays a key role in the regulation of plant response to stress environment, fruit ripening, and plant secondary metabolism. This molecule also plays an important role in the induction of bisbibenzyl biosynthesis in Marchantia polymorpha (Kageyama et al. 2015). ABA concentration was increased from 0 to 6 μM, and the production of total phenolics, flavonoids, and sucrose was enhanced (Ibrahim and Jaafar 2013). In this study, ABA treatment did not significantly increase the TTL content. However, the qRT-PCR results showed that ABA treatment significantly increased the expression levels of GbDXS, GbGGPPS, GbLPS, and GbMVD. This phenomenon was due to multiple factors. The effects of ABA treatment may vary on the different plants. These findings indicated that TTL biosynthesis is a complex process, and the secondary metabolism of plants should be explored and verified from several perspectives.
In plants, SA is an elicitor that triggers the secondary metabolism pathway (Dong et al. 2010). The results of this study showed that the response to SA increased continuously within 8 days in G. biloba, reaching its maximum level on 8 days and increased by 9.2% compared with that of the control. The qRT-PCR results showed that GbDXS, GbGGPPS, and GbMVD expression levels reached a significant level after SA treatment for more than 2 days. GbLPS expression level reached a significant level on 8 days. Adding exogenous SA during the suspension culture of G. biloba cells induced the cells to produce TTLs and released them into the medium (Kang et al. 2006). SA could induce the expression of the transcription factor WRKY, thereby regulating the expression of key enzyme genes directly involved in artemisinin biosynthesis (Kumari and Pandey-Rai 2018). GbHMGS2 transcription in the MVA pathway was also efficiently induced by SA (Meng et al. 2018). Plants respond to hormones in various ways. Some plants can directly regulate the transcriptional expression of related genes, whereas others regulate the expression of key genes in secondary metabolite biosynthesis by regulating transcription factors (Kawoosa et al. 2010; Zhang et al. 2014).
MeJA triggers the pathway of secondary metabolism in plants (Pauwels et al. 2008). As a signaling substance, jasmonates are natural compounds produced in several plants, and more than 30 kinds of these compounds have been discovered. MeJA is extensively used as an exogenous phytohormone, because it has good volatility, is not ionized, and easily penetrates the cell membrane. The results showed that MeJA treatment increased the expression levels of GbDXS, GbGGPPS, GbLPS, and GbMVD, and all genes reached their maximum levels on 6 days. TTL content continuously increased with prolonged treatment, reaching the highest value on 8 days. Adding exogenous MeJA to the cell culture of G. biloba resulted in a significant increase in the ginkgolide content and GbDXS expression (Gong et al. 2006). In our previous study, MeJA treatment was found to increase the transcription level of GbMVD and TTL content in G. biloba (Liao et al. 2016). MeJA increased the TTL content in G. biloba and showed similar effects on TTL accumulation in the G. biloba suspension cell culture (Kang et al. 2006). MeJA treatment could increase the abundance of GbLPS transcript in the G. biloba root (Kim et al. 2012). Similar data in other plants have been reported. Artemisinin accumulation was significantly induced by MeJA, and the artemisinin content reached its maximum level with an increase of 49% after 8 days of treatment (Wang et al. 2009). Treating Pinus massoniana with 0.2 mmol L−1 MeJA can increase the relative content of terpenoids, especially monoterpenes and diterpenes, in pine needles (Yao et al. 2018).
Ethylene is a gas hormone that plays an important role in plant growth, development, maturity, and senescence. In this study, the plants were treated with ETH instead of ethylene. In our previous study, the results showed that ETH could upregulate the expression of GbMVD and increase TTL content in G. biloba (Liao et al. 2016). In the current study, ETH treatment induced the expression of GbDXS, GbGGPPS, GbLPS, and GbMVD and increased TTL content. This result is consistent with those of previous studies. In summary, exogenous stress treatment could increase TTL content in G. biloba and may be achieved by increasing the expression level of the key enzyme genes in the TTL biosynthesis pathway. This phenomenon may be related to the cis-acting elements and motifs in the GbDXS, GbGGPPS, GbLPS, and GbMVD promoters responding to UV light, cold stress, and hormones (Kim et al. 2012; Liao et al. 2016).
Conclusion
The effects of UV irradiation, cold stress, and exogenous hormone treatments on the TTL content in G. biloba were investigated. Under the designed treatment conditions, UV light treatment for 24 h significantly increased the TTL content, which reached its maximum level at 48 h and was 21.9% higher than that of the control. After 8 days of cold treatment (4 °C), the highest TTL content was obtained, which was 14.5% higher than that of the control. The difference in TTL content under ABA treatment (100 μM) was nonsignificant. MeJA treatment (100 μM) for 6 days significantly increased TTL content, which reached its maximum level after 8 days and was 14.5% higher than that of the control. The TTL content under SA treatment (10 mM) reached a significant level on 8 days and was 9.2% higher than that of the control. The TTL content under ETH treatment (10 mM) reached a significant level on 8 days and increased by 10.2% compared with that of the control. The efficacy of treatments in decreasing order was MeJA, SA, ETH, and ABA. The results of this study may serve as a theoretical basis and technical guidance to improve the TTL production.
References
Buhaescu I, Izzedine H (2007) Mevalonate pathway: a review of clinical and therapeutical implications. ClinBiochem 40:575–584. https://doi.org/10.1016/j.clinbiochem.2007.03.016
Chen QW, Yan JP, Meng XX, Xu F, Zhang WW, Liao YL, Qu JW (2017) Molecular cloning, characterization, and functional analysis of acetyl-CoA C-acetyltransferase and mevalonate kinase genes involved in terpene trilactone biosynthesis from Ginkgo biloba. Molecules 22:74. https://doi.org/10.3390/molecules22010074
Diamond BJ, Bailey MR (2013) Ginkgo biloba: indications, mechanisms, and safety. Psychiatr Clin North Am 36:73–83. https://doi.org/10.1016/j.psc.2012.12.006
Dong J, Wan G, Liang Z (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148(2–3):99–104. https://doi.org/10.1016/j.jbiotec.2010.05.009
Gong YF, Liao ZH, Guo BH, Sun XF, Tang KX (2006) Molecular cloning and expression profile analysis of Ginkgo biloba DXS gene encoding 1-deoxy-D-xylulose 5-phosphate synthase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Planta Med 72(04):329–335. https://doi.org/10.1055/s-2005-916234
Ibrahim M, Jaafar H (2013) Abscisic acid induced changes in production of primary and secondary metabolites, photosynthetic capacity, antioxidant capability, antioxidant enzymes and lipoxygenase inhibitory activity of Orthosiphon stamineus Benth. Molecules 18(7):7957–7976. https://doi.org/10.3390/molecules18077957
Kageyama A, Ishizaki K, Kohchi T, Matsuura H, Takahashi K (2015) Abscisic acid induces biosynthesis of bisbibenzyls and tolerance to UV-C in the liverwort Marchantia polymorpha. Phytochemistry 117:547–553. https://doi.org/10.1016/j.phytochem.2015.05.009
Kang SM, Min JY, Kim YD, Kang YM, Park DJ, Jung HN, Kim SW, Choi MS (2006) Effects of methyl jasmonate and salicylic acid on the production of bilobalide and ginkgolides in cell cultures of Ginkgo biloba. Vitro Cell Dev Plant 42(1):44–49. https://doi.org/10.1079/IVP2005719
Kawoosa T, Singh H, Kumar A, Sharma SK, Devi K, Dutt S, Vats SK, Sharma M, Ahuja PS, Kumar S (2010) Light and temperature regulated terpene biosynthesis: hepatoprotective monoterpene picroside accumulation in Picrorhiza kurrooa. Funct Integr Genomic 10(3):393–404. https://doi.org/10.1007/s10142-009-0152-9
Kim JH, Lee KI, Chang YJ, Kim SU (2012) Developmental pattern of Ginkgo biloba levopimaradiene synthase (GbLPS) as probed by promoter analysis in Arabidopsis thaliana. Plant Cell Rep 31(6):1119–1127. https://doi.org/10.1007/s00299-012-1232-1
Kumari A, Pandey-Rai S (2018) Enhanced arsenic tolerance and secondary metabolism by modulation of gene expression and proteome profile in Artemisia annua L. after application of exogenous salicylic acid. Plant Physiol Biochem 132:590–602. https://doi.org/10.1016/j.plaphy.2018.10.010
Leng PS, Su SC, Wang TH, Jiang XN, Wang SS (2002) Effects of light intensity and light quality on photosynthesis, flavonoid glycosides and terpene lactones in Ginkgo biloba L. J Plant Resour Environment 1:1–4. https://doi.org/10.3724/SP.J.1145.2013.00280
Liao ZH, Chen M, Gong YF, Liang G, Tan QM, Feng XQ, Sun XF, Tan F, Tang KX (2004) A new geranylgeranyl diphosphate synthase gene from Ginkgo biloba, which intermediates the biosynthesis of the key precursor for ginkgolides. DNA Seq 15:153–158. https://doi.org/10.1080/10425170410001667348
Liao YL, Xu F, Huang XH, Zhang WW, Cheng H, Li LL, Cheng SY, Shen YB (2015) Promoter analysis and transcriptional profiling of Ginkgo biloba 3-hydroxy-3-methylglutaryl coenzyme a reductase (GbHMGR) gene in abiotic stress responses. Not Bot Horti Agrobot 43(1):25–34. https://doi.org/10.15835/nbha4319416
Liao YL, Xu F, Huang XH, Zhang WW, Cheng H, Wang XH, Cheng SY, Shen YB (2016) Characterization and transcriptional profiling of Ginkgo biloba mevalonate diphosphate decarboxylase gene (GbMVD) promoter towards light and exogenous hormone treatments. Plant Mol Biol Rep 34(3):566–581. https://doi.org/10.1007/s11105-015-0947-x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Meng XX, Song QL, Ye JB, Wang LL, Xu F (2017) Characterization, function, and transcriptional profiling analysis of 3-hydroxy-3-methylglutaryl-CoA synthase gene (GbHMGS1) towards stresses and exogenous hormone treatments in Ginkgo biloba. Molecules 22(10):1706. https://doi.org/10.3390/molecules22101706
Meng XX, Xu F, Song QL, Ye JB, Liao YL, Zhang WW (2018) Isolation, characterization and functional analysis of a novel 3-hydroxy-3-methylglutaryl-coenzyme A synthase gene (GbHMGS2) from Ginkgo biloba. Acta Physiol Plant 40(4):72. https://doi.org/10.1007/s11738-018-2650-7
Pauwels L, Morreel K, Witte ED, Lammertyn F, Montagu MV, Boerjan W, Inzée D, Goossens A (2008) Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc Natl Acad Sci 105(4):1380–1385. https://doi.org/10.1073/pnas.0711203105
Rao S, Meng XX, Liao YL, Yu T, Cao J, Tan JP, Xu F, Cheng SY (2019) Characterization and functional analysis of two novel 3-hydroxy3-methylglutaryl-coenzyme A reductase genes (GbHMGR2 and GbHMGR3) from Ginkgo biloba. Sci Rep 9:14109. https://doi.org/10.1038/s41598-019-50629-8
Senji BM, Mandoulakani BA (2018) The impact of cold stress on genes expression pattern of mono-and sesquiterpene biosynthesis and their contents in Ocimum basilicum L. Phytochemistry 156:250–256. https://doi.org/10.1016/j.phytochem.2018.09.006
Serrano-García N, Pedraza-Chaverri J, Mares-Sámano JJ, Orozco-Ibarra M, Cruz-Salgado A, Jiménez-Anguiano A, Sotelo J, Trejo-Solís C (2013) Antiapoptotic effects of EGb761. Evid-Based Compl Alt 2013:1–18. https://doi.org/10.1155/2013/495703
Wang H, Ma C, Li Z, Ma L, Wang H, Ye H, Xu G, Liu B (2009) Effects of exogenous methyl jasmonate on artemisinin biosynthesis and secondary metabolites in Artemisia annua L. Ind Crop Prod 31(2):214–218. https://doi.org/10.1016/j.indcrop.2009.10.008
Xiang L, Zhu S, Zhao T, Zhang M, Liu W, Chen M, Lan X, Liao Z (2014) Enhancement of artemisinin content and relative expression of genes of artemisinin biosynthesis in Artemisia annua by exogenous MeJA treatment. Plant Growth Regul 75(2):435–441. https://doi.org/10.1007/s10725-014-0004-z
Xu F, Zhang WW, Sun NN, Li LL, Chen SY, Wang Y (2011) Effect of chlorocholine chloride on photosynthesis, soluble sugar and terpene trilactones of Ginkgo biloba. Acta Hort Sin 38(12):2253–2260. https://doi.org/10.16420/j.issn.0513-353x.2011.12.033
Yao RL, Li HJ, Zhang XN, Wang Y (2018) Effects of MeJA on terpenoid synthetase activities and their cytochemical localizations in Pinus massonina. Guihaia 38(7):876–885. https://doi.org/10.11931/guihaia.gxzw201707015
Ye JB, Zhang X, Tan JP, Xu F, Cheng SY, Chen ZX, Zhang WW, Liao YL (2020a) Global identification of Ginkgo biloba microRNAs and insight into their role in metabolism regulatory network of terpene trilactones by high-throughput sequencing and degradome analysis. Ind Crop Prod 148:112289. https://doi.org/10.1016/j.indcrop.2020.112289
Ye JB, Mao D, Cheng SY, Zhang X, Tan JP, Zheng JR, Xu F (2020b) Comparative transcriptome analysis reveals the potential stimulatory mechanism of terpene trilactone biosynthesis by exogenous salicylic acid in Ginkgo biloba. Ind Crop Prod 145:112104. https://doi.org/10.1016/j.indcrop.2020.112104
Zhang JH, Wang YZ, Sun HY, Yang L, Jiang FC (2014) Effect of exogenous salicylic acid on the expression of antioxidant enzymes and CBF transcription factors in apricot flower at low temperature. Plant Physiol J 50(2):171–177. https://doi.org/10.13592/j.cnki.ppj.2014.02.014
Zhu CS, Feng MX, Miao GP, Feng JT, Zhang X (2013) Effects of ultraviolet-b(UV-B) radiation and ultrasonic treatment on the content of three secondary metabolites in Tripterygium wilfordii Hook.F. cell suspension cultures. J Agr Biotechnol 21(9):1052–1059. https://doi.org/10.3969/j.issn.1674-7968.2013.09.006
Funding
This study was supported by the National Natural Science Foundation of China (No. 31901344 and 31370680).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
Cold stress, ultraviolet irradiation, abscisic acid, salicylic acid, methyl jasmonate, and ethephon can upregulate the expression of the key genes GbDXS, GbGGPPS, GbLPS, and GbMVD in the terpene trilactone (TTL) biosynthesis pathway and increase the TTL content. These phenomena revealed the intrinsic mechanism of the TTL biosynthesis. This study can serve as a theoretical basis and technical guidance to improve TTL production.
Rights and permissions
About this article
Cite this article
Zheng, J., Zhang, X., Fu, M. et al. Effects of Different Stress Treatments on the Total Terpene Trilactone Content and Expression Levels of Key Genes in Ginkgo biloba Leaves. Plant Mol Biol Rep 38, 521–530 (2020). https://doi.org/10.1007/s11105-020-01220-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-020-01220-3