Abstract
Ruthenia Medic is tolerant to drought, cold, high salinity, resistance to trampling and high quality features. Inter-simple sequence repeat (ISSR) and simple sequence repeat (SSR) molecular markers were employed for the first time to access the genetic diversity and relationships of 30 wild Ruthenia Medic accessions obtained from Inner Mongolia in the present study. A total of 94 bands were amplified by ten ISSR primers, of which 83 (88.5 %) were polymorphic, and 57 polymorphic bands (80.4 %) were observed in 69 bands amplified by ten SSR primers. Shannon’s information index (I = 0.487), and average expected heterozygosis (He = 0.329) generated by ISSR primer were higher than that of SSR analysis (I = 0.372, He = 0.231). The study indicated that ISSR were more effective than SSR markers for assessing the degree of genetic variation of Ruthenia Medic. UPGMA cluster analysis revealed inconsistencies in the clustering patterns, as the Mantel’s test between the dendrograms for ISSR and SSR data indicated a poor fit for the ISSR and SSR data types (r = 0.0970). Whereas the pattern of clustering of the genotypes remained relatively the same in ISSR and combined data of ISSR and SSR. The results of principal components analysis also supports their UPGMA clustering. These results have an important implication for Ruthenia Medic germplasm characterization, improvement, and conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicago ruthenica (L.) Trautv. is an diploid (2n = 2x = 16) perennial legume species widely distributed in China as including 10 provinces of northern China, especially in Helongjiang grasslands, Inner Mongolia dry grasslands, Xinjiang hill grasslands, and the Qinghai-Tibet plateau grasslands, which commonly grows on sunny hillsides, dry stony slopes, embankments, and in mixed grass steppes and moist meadows, at times in large numbers. (Small and Jomphe 1989; Gen et al. 1995; Hao and Shi 2006). This species has been collected mostly from the Transbaikal region to the Pacific Ocean, and south to about 34°N latitude (Small 2011). It is tolerant to drought, high salinity, and alkaline environments. Therefore, it is regarded as a promising legume for forage in arid and semi-arid areas. It held that Ruthenia Medic seen far more capable of existing on poor stony soils under conditions of the continental North than Medicago sativa L., and so has potential application in low-input agricultural systems (Small 2011).
Ruthenia Medic is expected to become a high quality raw materials resources for improved alfalfa resistance genes (Campbell et al. 1997, 1999; Li et al. 2012). Evaluation of genetic diversity and relationships among various accessions is fundamental importance for plant breeding programs. This information can provide predictive estimates of genetic variation within a species, thus facilitating breeding material selection (Qi et al. 2008). Molecular markers have been shown to be a very powerful tool for genotype characterization and estimation of genetic diversity. In recent years, various molecular markers, including random amplified polymorphic (RAPD) (Deshwall et al. 2005; Teklewold and Becker 2006), amplified fragment length polymorphisms (AFLP) (Laurentin and Karlovsky 2006; Wang et al. 2010a), and simple sequence repeats (SSRs or microsatellites) (Beyene et al. 2006; Wang et al. 2010b) have been used to detect genetic diversity in different plant. The ISSR and SSR marker systems are widely used to evaluate genetic diversity (Ariss and Vandemark 2007; Uysal et al. 2010; Yang et al. 2010a; Iwata et al. 2005; Ofori et al. 2008; Panguluri 2007). These studies have given important clues in understanding species relationship, which may further assist in developing and planning breeding strategies. However, reports on genetic diversity using molecular markers are rarely available in the Ruthenia Medic germplasm (Li et al. 2008a, b; Li et al. 2009, 2012). The objectives of this study were to assess genetic diversity of 30 Ruthenia Medic accessions collected from Inner Mongolia using ISSR and SSR molecular markers.
Materials and methods
Plant materials and DNA extraction
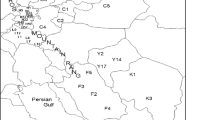
A total of 30 accessions Ruthenia Medic germplasm were analyzed in this study (Table 1). All accessions were obtained from National Medium-term Gene Bank of Forage Germplasm in The Institute of Grassland Research of Chinese Academy of Agricultural Science (CAAS), Inner Mongolia, Hohhot, China.
Total genomic DNA was extracted from 10 young leaves of each accession following the CTAB method described by Doyle and Doyle (1987). DNA quality and quantity were checked in 1 % agarose gels and UN4802 Spectrophotometer (Unico, USA), respectively. Genomic DNA was stored at −20 °C for further analysis.
ISSR-PCR
ISSR primers used in this study were synthesized by Shanghai Sangon Biological Engineering Technology & Service Co., Ltd. (China), according to the primer set published by the University of British Columbia (UBC). Eighty ISSR primers were initially screened, and ten of them, which yielded bright and discernible bands, were used for the analysis (Table 2). ISSR amplification reactions were carried out in a 25 μL reaction volume containing 10× reaction buffer, 2.0 mM Mg2+, 0.6 mM of each dNTP, 0.9 μM for primer, 1.5 U of Taq DNA polymerase and 0.5 ng of genomic DNA template. Amplification was performed in an Eppendorf Master cycler Gradient PCR (Eppendorf, Germany) under the following conditions: 3 min at 94 °C, followed by 30 s at 94 °C, 45 s at 52 °C, and 1.5 min at 72 °C for 35 cycles, and 10 min at 72 °C. PCR products were separated by electrophoresis using 1.5 % agarose gel and stained using EB and photographed by Nikon D200 (Japan).
SSR-PCR
The SSR primer pairs mainly originated from 89 Truncatula (Medicago truncatula Gaertn.) (Bernadette et al. 2003) and alfalfa (M. sativa L.) (Diwan et al. 1997). Ten of them, which yielded bright and discernible bands, were used for the analysis (Table 2). SSR amplification reactions were carried out in 25 μL reaction volume containing 10× reaction buffer, 5.5 μL Mg2+, 0.75 μL of each dNTP, 0.75 μL for forward and reverse primer, respectively, 5.0 U of Taq DNA polymerase and 408 ng of genomic DNA template. Amplification was performed in an Eppendorf Master cycler Gradient PCR under the following conditions: 3 min at 94 °C, followed by 25 s at 94 °C, 30 s at 55–66 °C, and 30 s at 72 °C, and 35 cycles, and a final extension of 10 min at 72 °C, the stored at 4 °C. The amplification products by 6 % denaturing polyacrylamide gel electrophoresis, constant power 80 W, 30 min. And then silver staining, decolorization and fixed, washed, and finally stained and developing.
Data scoring and statistical analysis
The ISSR and SSR bands were scored as present (1) or absent (0) across 30 accessions for each primer or primer combination. POPGENE v1.32 (Yeh et al. 1997) was used to calculate various genetic diversity parameters, including the percentage of polymorphic bands (PPB), Shannon’s information index (I) and Nei’s gene diversity (He). A dendrogram was constructed based on Jaccard’s similarity coefficients (GS) using the un-weighted pair group method with arithmetic average (UPGMA) with the SAHN module of NTSYS-pc 2.20 (Rohlf 2000). The COPH and MXCOMP programs were used to calculate good-ness-of-fit between the cluster analysis and original matrix. A principal coordinate analysis to construct a two-dimensional array of eigenvectors was performed using the DCENTER module of the NTSYS-pc2.20 program. Finally, a Mantel test was made with 1,000 permutations to determine the significance level between ISSR and SSR, ISSR and ISSR + SSR, and SSR and ISSR + SSR based on the Jaccard’s coefficient using NTSYS-pc 2.20 software.
Results and analysis
ISSR analysis
A total of 94 scored bands were generated using the 10 primer previously screened across 30 Ruthenia Medic accessions (Table 2). The number of amplified bands per primer ranged from 8 (UBC809, UBC834, UBC836) to 13 (UBC822) with an average of ten bands per primer (Table 3). Of the 94 amplified bands, 83 were polymorphic (88.5 %), ranging from 6 (UBC809, UBC836) to 11(UBC822), with a mean of 8.3 polymorphic bands per primer. The percentage of polymorphic bands across the primers ranged from 75 % (UBC809, UBC836) to 100 % (UBC813, UBC845), with an average of 88.0 % polymorphism. The Shannon’s information index (I) and gene diversity (He) were conducted to further understand the genetic diversity of Ruthenia Medic from different origins and from the complete set of accessions. The Shannon’s information index (I) and gene diversity (He) of Ruthenia Medic were 0.487 and 0.329, respectively. Genetic similarity coefficients were obtained with UPGMA algorithm using Jaccard’s coefficient. The GS ranged from 0.514 (MR-8 and MR-24) to 0.885 (MR-2 and MR-5).
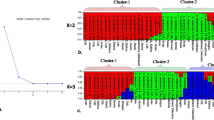
The dendrogram grouped the 30 accessions into four main clusters (with a GS of 0.64) (Fig. 1a). Accessions within Cluster I were further divided into two subcluster. The subcluster I-1 comprised 12 accessions including eight accessions from Xilingol League, MR-15, MR-16 and MR-17 from Xing an League, MR-18 from Hulunbeier City. Subcluster I-2 contained seven accessions, MR-21, MR-22 and MR-23 from Wulanchabu City, MR-25 and MR-26 from Baotou City, MR-27 and MR-28 from Hohhot City. Cluster II comprised four accessions including MR-13 and MR-14 from Tongliao City, MR-19 and MR-20 from Hulunbeier City. Cluster III contained six accessions including MR-9, MR-10 and MR-11 from Chifeng City, MR-12 from Tongliao City, MR-29 and MR-30 from Hohhot City. Accession MR-24 from Wulanchabu City formed cluster IV. The results of the principal components analysis (PCA) were comparable to the cluster analysis (Fig. 2a). The first three components explain 35.24 % of the total variation.
Two-dimensional plot (with vectors) of principal coordinate analysis of 30 Ruthenia Medic accessions using ISSR (a), SSR (b) and combined ISSR + SSR (c). The numbers correspond to those listed on Table 1
SSR analysis
A total of 89 Truncatula and alfalfa different primer combinations were employed. Only ten pairs of them could produce clear and reproducible bands and were chosen in the following study (Table 2). A total of 69 bands were observed,of which 57 were polymorphic (80.4 %), ranging from 3 (MTIC233, MTIC347, MTIC258) to 10 (MTIC432) (Table 3). Average number of bands and polymorphic bands per primer combinations were 6.9 and 5.7, respectively. Percentage of polymorphic bands (PPB) ranged from 60 % (MTIC258) to 100 % (MTIC432) with an average polymorphism of 80.4 % across all the accessions.
The Shannon’s information index (I) and gene diversity (He) of Ruthenia Medic were 0.372 and 0.231, respectively. The dendrogram using UPGMA analysis based on Jaccard’s GS of the SSR markers of 30 accessions is showed in Fig. 1b. The GS varied from 0.7257 (MR-8 and MR-14) to 0.9571 (MR-11 and MR-12). The 30 Ruthenia Medic accessions were grouped into four clusters (with a GS of 0.705). Cluster I comprised five accessions, three accessions from Xilingol League, MR-12 from Tongliao City, MR-30 from Hohhot City. Cluster II comprised 22 accessions and was further divided into two subcluster. The subcluster II-1 contained seven accessions, MR-3 and MR-4 accessions from Xilingol League, MR-16 and MR-17 accessions from Xing an League, MR-18 and MR-19 accessions from Hulunbeier City, MR-22 accession from Wulanchabu City. Subcluster II-2 contained MR-7 from Xilingol League, three accessions from Chifeng City, two accessions from Tongliao City, one from Xing an League, one from Hulunbeier City, three accessions from Wulanchabu City, two from Baotou City, two from Hohhot City. Cluster III comprised MR-5 from Xilingol League, MR-27 from Hohhot City. Cluster IV comprised MR-8 from Xilingol League. The results of the principal components analysis (PCA) were basically consisting with the cluster analysis (Fig. 2b). The first three components explain 30.53 % of the total variation.
Combined (ISSR and SSR) data
In order to obtain more accurate genetic estimates, combined analysis was carried out using all the ISSR and SSR data together. The Jaccard’s GS ranged from 0.5952 (MR-8 and MR-24) to 0.9030 (MR-11 and MR-12). The Jaccard’s GS matrix based on combined data was used to generated a dendrogram showing genetic relationships among the accessions. The dendrogram grouped the 30 accessions into four main clusters (Fig. 1c). Eight accessions from Xilingol League formed cluster I. Cluster II is a biggest cluster consisting of 15 accessions and was further divided into two subcluster. Cluster II-1 included two from Tongliao City, three from Xing an League, MR-18 from Hulunbeier City, three accessions from Wulanchabu City, two accessions from Baotou City, two accessions from Hohhot City. Cluster II-2 included MR-19 and MR-20 which were from Hulunbeier City, respectively. The cluster III comprised three accessions from Chifeng City, MR-12 from Tongliao City, MR-29 and MR-30 from Hohhot City. Cluster IV comprised one accession from Wulanchabu City. The results of the principal components analysis (PCA) were comparable to the cluster analysis (Fig. 2c). The first three components explain 28.19 % of the total variation.
Discussion
Assessment of the genetic variability within a wild forage has important consequences in plant breeding and the conservation of genetic resources, and molecular markers offer an approach to unveil the genetic diversity among accessions based on nucleic acid polymorphisms. In the present study, two marker systems, ISSR and SSR, were applied for the first time to assess the level and pattern of genetic diversity of 30 Ruthenia Medic accessions from Inner Mongolia. Wide genetic variability among different accessions of Ruthenia Medic was observed in the study (Table 3). This high level of polymorphism in Ruthenia Medic has been reported before for its morphology level in previous studies (Wang et al. 2008; Li et al.2011). In comparison to other legume forage species, the genetic diversity of Ruthenia Medic was higher than that of alfalfa (M. sativa) (ISSR,PPB = 60 %) (Touil et al. 2008) and red clover (Trifolium pratense) (RAPD, PPB = 74.2 %) (Ulloa et al. 2003). It was also lower than that found for other legume forage species such as Astragals rhizanthus (ISSR, PPB = 92.3 %) (Anand et al. 2010), and goat’s rue (Caragana korshinskii) (AFLP, PPB = 93.9 %) (Wang et al. 2007). The applicability of ISSR and SSR as genetic markers to characterize the genetic diversity of Ruthenia Medic was compared. ISSR markers showed higher PPB (88.5 %) than the SSR markers (80.4 %). The average Nei gene diversity is also more higher for ISSR than that for SSR markers (Table 3) based on The Shannon’s information index, Similar results were also observed in Elymus species (Li et al. 2005), Tobacco (Nie and Liu 2011). The present study showed clustering of accessions based on ISSR and SSR markers was not similar. The correlation between two Jaccard’s similarity coefficient value resulted in low correlation between SSR and ISSR + SSR (r = 0.0191) and high for ISSR and ISSR + SSR (r = 0.956) and ISSR and SSR data (r = 0.0970). This shows that ISSR data is slightly more close to ISSR + SSR combined data. A possible explanation for the difference in resolution of two marker systems is that the two marker techniques targeted different portions of the genome. The ISSR markers scattered throughout the genome which revealed the diversity of the entire genome, while SSR markers only amplified target region of open reading frame (ORF), the functional regions (Yang et al. 2010b; Gimenes et al. 2007). These differences may also be attributed to marker sampling errors and/or the level of polymorphism detected, reinforcing the importance of the number of loci and their coverage of the overall genome in obtaining reliable estimates of genetic relationships among cultivars (Loarce et al. 1996). Although a part of accessions (e.g. from Xilingol League) are consistently grouped together in all four cluster analysis, the dendrograms did not show clear clustering pattern of geographically closer accessions, which indicated that the association between genetic similarity and geographical distance was less significant. Similar results were reported in Azuki bean (Yee et al. 1999). Our results indicate the presence of great genetic variability among Ruthenia Medic accessions. Genetic variation among Ruthenia Medic accessions based on ISSR and SSR analysis could be useful to select parents to be crossed for generating appropriate populations intended for both genome mapping and breeding purposes. The variation of Ruthenia Medic is poorly understood and basic systematic analyses of the different variants that have been recognized need to be carried out. Although there has been recent acquisition of germplasm,there is still a need for additional germplasm collection of the apparently wide range of ecotypes of this species and evaluation of their agronomic potential. There also needs to be an evaluation of the possibility of gene transfer to alfalfa, particularly those characteristics favoring drought and cold resistance (Small 2011). Ruthenia Medic occurs in remote areas of Asia that are subject to genetic erosion, and so conservation evaluation is desirable. The very recent collections acquired by the National Medium-term Gene Bank of Forage Germplasm will ensure that much of the genetic variation will be available in the future (Small 2011).
References
Anand KK, Srivastava RK, Chaudhary LB (2010) Analysis of genetic diversity in Astragalus rhizanthus Benth. ssp. rhizanthus var. rhizanthus (Fabaceae) using molecular markers from India. J Bot 2010:1–9
Ariss JJ, Vandemark GJ (2007) Assessment of genetic diversity among nondormant and semidormant alfalfa populations using sequence related amplified polymorphisms. Crop Sci 47:2274–2284
Bernadette J, Sandrine F, Philippe B, Gaelle C, Sylvain S, Thierry H, Christian H (2003) Construction of two genetic linkage maps in cultivated tetraploid alfalfa (Medicago sativa) using microsatellite and AFLP markers. BMC Plant Biol 3:9
Beyene Y, Botha AM, Myburg AA (2006) Genetic diversity among traditional Ethiopian highland maize accessions assessed by simple sequence repeat (SSR) markers. Genet Resour Crop Evol 53(8):1579–1588
Campbell TA, Bao G, Xia ZL (1997) Agronomic evaluation of Medicago ruthenica collected in Inner Mongolia. Crop Sci 37:599–604
Campbell TA, Bao G, Xia ZL (1999) Completion of the agronomic evaluations of Medicago ruthenica [(L.) Ledebour] germplasm collected in Inner Mongolia. Genet Resour Crop Evol 46:477–484
Deshwall RPS, Singh R, Malik K, Randhawa GJ (2005) Assessment of genetic diversity and genetic relationships among 29 populations of Azadirachta indica A. Juss. using RAPD markers. Genet Resour Crop Evol 52:285–292
Diwan N, Bhagwat AA, Bauchan GR, Cregan PB (1997) Simple sequence repeat (SSR) DNA markers in alfalfa and perennial and annual Medicago species. Genome 40:887–895
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Gen HZ, Wu YF, Cao ZZ (1995) Chinese alfalfa. China Agriculture Press, Beijing 14
Gimenes MA, Hosino AA, Barbosa AVG, Palmieri DA, Lopes CR (2007) Characterization and transferability of micro-satellite markers of cultivated peanut (Arachis hypogaea). BMC Plant Biol 7:9
Hao JH, Shi FL (2006) Study on drought resistance of Medicago ruthenica accessions. Chine J Grassland 28:39–42
Iwata H, Imon K, Tsumura Y, Ohsawa R (2005) Genetic diversity among Japanese indigenous common buckwheat (Fagopyrum esculentum) cultivars as determined from amplified fragment length polymorphism and simple sequence repeat markers and quantitative agronomic traits. Genome 48:367–377
Laurentin HE, Karlovsky P (2006) Genetic relationship and diversity in sesame (Sesamum indicum L.) germplasm collection using amplified fragment length polymorphism (AFLP). BMC Genet 7:1–10
Li YX, Li SS, Li LH (2005) Comparison of genetic diversity of twelve Elymus species using ISSR and SSR markers. Sci Agric Sin 38(8):1522–1527
Li HY, Li ZY, Mi FG, Lu XX, Shi WG (2008a) Appraisal of Medicago ruthenica Germplasms by SSR markers. Acta Agriculturae Boreali-sinica 23(3):67–71
Li HY, Mi FG, Ning HM, Shi WG, Zhao CG (2008b) Genetic diversity of Medicago ruthenica based on SSR markers. Chin J Grassland 30(2):34–38
Li ZY, Shi WG, Li HY, Li XY, Liu L (2009) Genetic diversity and relationship of three Medicago ruthenica ecotypes from Inner Mongolia. Acta Bot Boreal Occident Sin 29(12):2421–2428
Li HY, Li ZY, Mi FG, Shi WG, Cai LY, Wang CH, Xing JJ (2011) The genetic diversity of Medicago ruthenica were analyzed by phenotype and SSR marker in China. J Northwest A&F Univ 39(8):1–9
Li ZY, Li HY, Shi FL, Wang XL, Zhao CG, Shi WG, Cai LY (2012) Genetic Diversity Analysis of Medicago ruthenica by ISSR in China. J Plant Genet Resour 13(1):48–51, 56
Loarce Y, Gallego R, Ferrer E (1996) A comparative analysis of genetic relationships between rye cultivars using RFLP and RAPD markers. Euphytica 88:107–115
Nie Q, Liu R (2011) Genetic diversity of 23 tobacco germplasm resources by SSR and ISSR analysis. Southwest China J Agric Sci 24(1):15–19
Ofori A, Becker HC, Kopisch-ObuchF J (2008) Effect of crop improvement on genetic diversity in oilseed Brassica rapa (turnip–rape) cultivars, detected by SSR markers. J Appl Genet 49:207–212
Panguluri SK (2007) RAPD and ISSR fingerprinting in cultivated chickpea (Cicer arietinum L.) and its wild progenitor Cicer reticulatum Ladizinsky. Genet Resour Crop Evol 54:1235–1244
Qi XH, Yang JH, Zhang MF (2008) AFLP-based genetic diversity assessment among Chinese vegetable mustards (Brassica juncea (L.) Czern.). Genet Resour Crop Evol 55:705–711
Rohlf FJ (2000) NTSYS-PC version 2.10 s. Numerical taxonomy and multivariate analysis system. Exeter Publications, Setauket
Small E (2011) Alfalfa and relatives. Evolution and classification of Medicago. NRC Research Press, Ottawa, pp 567–570
Small E, Jomphe M (1989) A synopsis of the genus Medicago (Leguminosae). Can J Bot 67:3260–3294
Teklewold A, Becker HC (2006) Geographic pattern of genetic diversity among 43 Ethiopian mustard (Brassica carinata A. Braun) accessions as revealed by RAPD analysis. Genet Resour Crop Evol 53:1173–1185
Touil L, Guesmi F, Fares K, Ferchichi A (2008) Genetic diversity of some mediterranean populations of the cultivated alfalfa (Medicago sativa L.) using ISSR markers. Biotechnology 7(4):808–812
Ulloa O, Ortega F, Campos H (2003) Analysis of genetic diversity in red clover (Trifolium pratense L.) breeding populations as revealed by RAPD genetic markers. Genome 46(4):529–535
Uysal H, Fu YB, Kurt O, Peterson GW, Diederichsen A, Kusters P (2010) Genetic diversity of cultivated flax (Linum usitatissimum L.) and its wild progenitor pale flax (Linum bienne Mill.) as revealed by ISSR markers. Genet Resour Crop Evol 57(7):1109–1119
Wang Z, Gao HW, Wu YQ, Han JG (2007) Genetic diversity and population structure of Caragana korshinskii revealed by AFLP. Crop Sci 47:1737–1743
Wang JE, Wang Z, Wang YQ, Gao HW, Chapurin VF (2008) Morphological diversity of Galega officinalis L. germplasm resources. J Plant Genet Resour 9(2):201–205
Wang Z, Kenworthy KE, Wu YQ (2010a) Genetic diversity of common carpetgrass revealed by amplified fragment length polymorphism markers. Crop Sci 50:1366–1374
Wang Z, Wu YQ, Martin DL, Gao HW, Samuels T, Tan CC (2010b) Identification of vegetatively propagated turf bermudagrass cultivars using simple sequence repeat markers. Crop Sci 50:2103–2111
Yang GS, Wang Y, Sun ChY, Jlang SC, Zhang MP (2010a) SSR analysis of genetic diversity of Panax ginseng. Med Plant 1(7):50–53
Yang Y, Pan YZ, Gong X, Fan M (2010b) Genetic variation in the endangered Rutaceae species Citrus hongheensis based on ISSR fingerprinting. Genet Resour Crop Evol 57(8):1239–1248
Yee E, Kidwell KK, Sills GR, Lumpkin TA (1999) Diversity among selected Vigna angularis (Azuki) accessions on the basis of RAPD and AFLP markers. Crop Sci 39:268–275
Yeh FC, Yang R-C, Boyle TBJ, Ye Z-H, Mao JX (1997) POPGENE, the user-friendly shareware for population genetic analysis. Molecular Biology and Biotechnology Centre. University of Alberta, Canada
Acknowledgments
This study was supported by Ministry of Agriculture, Crop Germplasm Resources Protection and Utilization Project (NB2012-2130135-33), Ministry of Agriculture Forage Germplasm Resources Protection Program (2012-43). The authors wish to thanks an anonymous reviewer for his/her valuable suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Hy., Li, Zy., Cai, Ly. et al. Analysis of genetic diversity of Ruthenia Medic (Medicago ruthenica (L.) Trautv.) in Inner Mongolia using ISSR and SSR markers. Genet Resour Crop Evol 60, 1687–1694 (2013). https://doi.org/10.1007/s10722-012-9950-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-012-9950-3