Abstract
Eighty-seven Phaseolus vulgaris landraces, still cultivated in Calabria (Italy), were investigated in order to study the patterns of common bean genetic diversity in this region, to better understand the evolutionary development of beans in Europe and to properly manage these genetic resources. Four American accessions and five Italian varieties were also included. Different markers, such as 12 microsatellites, seed traits, phaseolins and 100-seed weight were combined with different statistical approaches. For each microsatellite, expected (H e ) and observed (H o ) heterozygosities, polymorphism information content (PIC), probability of identity (PI) and homozygosity were calculated. Furthermore, in Calabrian group of bean landraces, total (N a ) and private (N pa ) number of alleles, observed (H o ), expected heterozygosities (H e ) and allelic richness (AR) were calculated. Genetic distances among landraces were estimated using Nei’s coefficient and a cluster analysis using the UPGMA algorithm was performed. The results clearly indicated that: (1) Calabrian germplasm showed a high level of diversity (H e = 0.595); (2) Mesoamerican and Andean gene pools were clearly distinguished in Calabrian germplasm, with the Andean gene pool predominating (83 %); (3) Calabrian landraces were largely hybridized within and between the gene pools. A model-based approach, using the STRUCTURE software, was adopted. Six groups, including 4 of Andean origin and one of Mesoamerican origin were identified. Even more interesting, a small group (8 %) showed a distinct genetic structure, in which interspecific hybridizations with runner bean (Phaseolus coccineus L.) could have occurred. Nevertheless, a relatively high proportion of Calabrian bean landraces (12.6 %) was derived from intra and interspecific hybridizations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The common bean (Phaseolus vulgaris L.), originally from Latin America, is an important food legume that provides a source of protein in the diet of many people around the world (Broughton et al. 2003) and is mainly cultivated in eastern and southern Africa and Latin America. Europe has only 1 % of the worldwide land that is committed to bean production (FAOSTAT 2009). Common bean was introduced into Europe from two centers of domestication, Central and South America, where the Mesoamerican and the Andean cultivated gene pools were originated. Gene pool diversity is based on seed size, phaseolin seed protein patterns, morphological traits, allozymes and DNA markers (Gepts et al. 1986; Koenig and Gepts 1989; Singh et al. 1991a, b; Singh et al. 1991c; Tohme et al. 1996; Beebe et al. 2000; Beebe et al. 2001). Within each gene pool, different groups of landraces or ‘races’ have been identified according to their morphological traits and agro-ecological adaptations (Singh et al. 1991a, b; Gepts 1988). The dissemination and evolution of the common bean in Europe from both centers of domestication have not been completely clarified yet due to a wide geographic diffusion among different climatic environments and the divergent selection criteria for agronomic traits chosen by farmers (Piergiovanni et al. 2006). In addition, the spread of the crop from South America into Europe has caused a genetic erosion of European common bean germplasm, whose incidence has to be further verified as reported by Angioi et al. (2010). Recently, using phaseolin and molecular markers, the highest incidence ever of the Andean gene pool (around 70 %) in common bean has been found across all European countries (Logozzo et al. 2007; Angioi et al. 2010) and at the regional level (Limongelli et al. 1996; Escribano et al. 1998; Sicard et al. 2005; Angioi et al. 2009).
In addition, there has been a high percentage (44 %) of hybridization between the Andean and Mesoamerican gene pools reported, with different frequencies being found in Central and Southern Europe (Angioi et al. 2010). In Spain and Italy, for example, this hybridization seems to be rare, although recently, some evidence for hybridization has been shown in Sardinia, Italy (Angioi et al. 2009). Furthermore, interspecific cross hybridizations between common and runner beans (Phaseolus coccineus L.) could have taken place (Sicard et al. 2005; Papa et al. 2006; Spataro et al. 2011). The runner bean, introduced into Europe along with the common bean (Gepts and Debouck 1991; Santalla et al. 2004), is a perennial outcrossing crop often cultivated in small gardens in Western and Southern Europe (Acampora et al. 2007; Spataro et al. 2011).
In Italy, many farmers maintained their old local landraces, populations or varieties which were well adapted to the pedoclimatic conditions of their limited geographical areas, and exchanged their seeds with surrounding areas, mainly in local markets (Piergiovanni et al. 2000b). Protecting this germplasm, from regions where agriculture still maintains a traditional structure and where the fixation of local ecotypes has taken place, should be considered a high priority and needs to be collected, characterized and evaluated for potential utilization.
In recent years, molecular markers have developed into an important tool in the analysis of genetic diversity (Pallottini et al. 2004; Kwak and Gepts 2009; Burle et al. 2010). Furthermore, the knowledge of genetic distances among the landraces has become determinant in improving the productivity of bean varieties by molecular assisted breeding programs.
With the aim of reducing genetic erosion, the focus of the present study was to collect and characterize a large collection of common bean landraces that are still cultivated in Calabria (Italy) in order to clarify the origin and the genetic diversity of this germplasm. These results have allowed to: (1) clarify the bean dissemination process in Europe; (2) identify the original gene pool (Andean and Mesoamerican) of the cultivated Calabrian common bean; (3) evaluate the cross hybridization frequency between the two gene pools of origin in the Calabrian common bean and (4) identify rare events of natural interspecific hybridization among Phaseolus species in Europe.
Materials and methods
Plant material
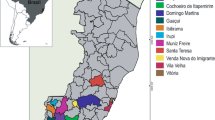
Eighty-seven bean landraces (each seed lot obtained from a single farmer) from different areas of Calabria (Fig. 1) along with five Italian commercial varieties and four American accessions were analyzed. The American genotypes and the Calabrian landraces were kindly provided by Centro Internacional de Agricoltura Tropical (CIAT, Colombia) and by Agenzia Regionale Sperimentazione e Servizi in Agricoltura (ARSSA—Calabria, Italy), respectively. The collection was grouped according to the type and the site of origin (Table 1).
Morpho-phenotypic seed analysis
The following seed traits on twenty seeds for each landrace were measured with a millimeter (mm) adjustment caliper: width, end to end length and side to side height at the hilum. Seed color, coat pattern and shape were scored for all landraces according to the IBPGR/IPGRI (1982) descriptors list. According to Angioi et al. (2009), a trait called ‘prevalent’ was adopted for seed classification, for which 3 states are possible: (1) darker color as background and lighter color as stripes; (2) darker color and lighter color equally distributed and (3) lighter color as background and darker color as stripes. In addition, the 100-seed weight (g) was also measured. The American genotypes and the Italian varieties were not analyzed for morpho-phenotypic seed traits due to the limited number of seeds available.
Phaseolin analysis
Five seeds of each landrace were analyzed. The seed coats were manually removed before grinding and phaseolin was extracted according to Limongelli et al. (1996). One-dimensional SDS-PAGE of the extracts was performed, as reported by Ma and Bliss (1978), using 17 % (w/v) polyacrylamide slab gels.
DNA extraction and microsatellite analysis
Total genomic DNA was isolated from young fresh leaves of each bean landrace (one plant/landrace) and, after lyophilization, stored at −80 °C until required. The CTAB (cetyl-trimethyl-ammonium bromide) extraction method was used (Lodhi et al. 1994) with some modifications. Briefly, the extract was treated with DNase-free RNase (Roche Diagnostics, Germany) and quantified in agarose gels (1 %) using standard lambda DNA as a comparison.
Twelve microsatellite (SSR) loci from several linkage groups, chosen from among the most utilized bean, were selected based on their dispersal map location (Yu et al. 2000; Blair et al. 2003; Guerra-Sanz 2004) and on the evaluation of 150 microsatellites previously tested for polymorphism and PCR conditions (Blair et al. 2006). The genetic linkage map location can be found in the original publications (Blair et al. 2003; Yu et al. 2000; Gaitàn-Solìs et al. 2002), while repeat motif, primer sequences and annealing temperatures are reported in Table 2.
The PCR amplifications were carried out with a GeneAmp 2700 Thermal Cycler (Applied Biosystems) under varying annealing temperatures (Ta), depending on the primer pairs. One of each pair was fluorescently labelled with FAM, JOE or TAMRA. PCR reactions were carried out in reaction volumes of 15 μl containing 20 ng of genomic DNA and 0.2 μM each of forward and reverse primers, in a 2X Qiagen multiplex Master Mix (Qiagen GmbH, Hilden, Germany). Reactions were performed under the following conditions: 15 min at 95 °C; 35 cycles of 30 s at 94 °C, 1 min 30 s at specific Ta, 1 min at 72 °C, followed by 10 min at 72 °C. Amplification products were checked and quantified by electrophoresis on 3 % agarose using Low DNA mass Ladder (Invitrogen). The fragments were separated by capillary electrophoresis and genotyped with an ABI PRISM 3500 Genetic Analyzer.
Data analysis
Alleles in base pairs were estimated by comparing the fragment peaks with the internal size standard using the default method for band calling with SSR, and the expected repeat size. Electropherograms were verified visually using Gene Mapper version 4.1 software to ensure that the proper selection of multiplex markers had been made. The pairwise genetic distances for phylogenetic relationships among landraces were estimated using Nei’s (1973) coefficient, cluster analysis was performed according to the UPGMA (Unweighted Pair-Group Method with Arithmetical Averages) algorithm and a dendrogram was generated (PowerMarker version 3.25; Liu and Muse 2005). A consensus tree was created in nexus format for viewing in Tree-View (Page 1996), the nodes being supported by bootstrap analysis (1,000 replicates).
Genetic diversity was estimated by comparing the number of alleles per locus (N a ), number of effective alleles (N e ), allele size range, number of private alleles (N pa ), allelic richness (AR), expected (H e) and observed heterozygosities (H o) using GeneALex 6 (Peakall and Smouse 2006) and FSTAT version 2.9.3.2 (Goudet 2002) software.
Microsatellite screening ability (MSA) was calculated using the probability of identity (PI) (Paetkau et al. 1995) and the polymorphic information content (PIC) (Weber 1990) as follows:
where pi and pj are the frequencies of the ith and jth allele and n is the number of alleles.
These indices, ranging from 0.0 to 1.0, were calculated using the IDENTITY software (Wagner and Sefc 1999 version 1.0; Centre for Applied Genetics, University of Agricultural Sciences, Vienna, Austria).
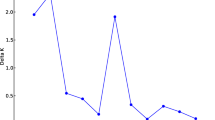
To identify the genetic groups within the Calabrian bean collection and the relationships with American and Italian genotypes, STRUCTURE version 2.2 software (Pritchard and Wen 2003) was utilized. This Bayesian approach to analysis uses no a priori classification and assigns samples to K populations based on the allele frequencies at each locus. The estimate of the most likely number of genetic groups (K, ranged from 1 to 10) was performed following the procedure of Evanno et al. (2005), which proposed an ad hoc statistic, ΔK. Program settings used the admixture ancestry and correlated marker frequency models. Alpha was inferred from the data and lambda was set to 1 (Pritchard and Wen 2003; Evanno et al. 2005). For each K, 20 independent runs (500,000 burn-in, 1,000,000 Marchov Chain Monte Carlo) were carried out.
Results
Seed traits and phaseolin
According to the IBPGR/IPGRI (1982) descriptor list, 34 diverse seed morphotypes were described (Table S1). The frequency of morphotypes ranged from 1.3 to 13.8 % (Fig. 2). Eight out of 34 morphotypes (23.5 %) showed a higher that 5.0 % frequency, 55 % of analyzed seeds were grouped as 00712 ‘white-oval’ (13.8 %), 00714 ‘white reniform’ (7.5 %), 00313 ‘maroon cuboid’ (7.5 %), 2(12)613 ‘pale-cream, red striped, cuboid’ (6.3 %), 00113 ‘black cuboid’ (5.1 %), 00713 ‘white cuboid’ (5.1 %), 21314 ‘maroon, black striped, reniform’ (5.1 %) and 72313 ‘maroon, broad striped, cuboid’ (5.1 %) (Fig. 3). Twenty-four landraces were included in the morphotypes 00711 (3), 00712 (11), 00713 (4) and 00714 (6), which were all characterized by a white coat color. Moreover, according to the seed trait analysis, 77 landraces out of 87 (88.5 %) were homogeneous while only ten (11.5 %) were heterogeneous, generally showing 2 morphotypes, one of which was considered predominant and selected as representative for frequency data counting.
The landraces showing the greatest spread of seed-morphotype (00712 ‘white oval’), such as “Posa di montagna”, “Sbraca pasta” and “Bianco”, were frequently indistinguishable from Cannellino nano, an Italian commercial variety. According to the seed trait analysis, differentiation among Calabrian landraces was high (FST = 0.557), especially considering that some morphotypes (19) consisted of a single landrace.
The phaseolin pattern was analyzed for all bean germplasm (Table S1, Fig. S1). Phaseolin C (56.3 %) and T (18.4 %) types, typical of the Andean gene pool, were found in most landraces, while the S type (11.5 %), from the Mesoamerican gene pool, was present in a limited number of landraces. Unexpectedly, in a few landraces (6.9 %), an unknown phaseolin pattern similar to that observed in wild runner bean by Gepts et al. (1986), never reported in the common bean, was observed. On the basis of these results, since P. coccineus and P. vulgaris differ in germination method, being hypogeal and epigeal, respectively (CIAT 1986), germination type for all landraces was analyzed. Only five (“Ciota serpiata”, “A fava”, “Favarula nera”, “Favu” and “Quarantino”) out of 87 landraces showed hypogeal germination (data not shown), the first evidence that these landraces, cultivated as common bean in Calabria, might be instead assigned to P. coccineus or to interspecific hybridization between the 2 Phaseolus species. At the flowering stage, red flowers were observed in “Ciota serpiata” and “Favarula nera” landraces, flower color reported as typical from P. coccineus (CIAT 1986). The 100-seed weight was also analyzed to further distinguish among landraces and their provenances. A significant difference (t test: P = 0.001) between 100-seed weight means (31 vs. 48 g) was observed in landraces from Mesoamerican and Andean origin, respectively (Table S1).
Taking into account seed traits and biochemical results, seven landraces showed an average 100-seed weight of 85 g, further, 6 out of the same 7 landraces harbored the not classified phaseolin type, while only one (“Ciota Serpiata”) clearly showed a T type pattern.
SSRs based genetic diversity
A genetic diversity analysis on all germplasm using 12 widespread SSR loci was carried out. Four SSRs (PV-AG004, BMd-1, BMd-18 and BMd-51) were monomorphic, thus the statistical analyses were performed on the eight polymorphic SSRs of which the main genetic parameters are reported in Table 3. A total of 57 alleles were detected with an average of 7.1 alleles per locus, ranging from 73 to 260 bp. The expected heterozygosity (H e ) ranged from 0.181 to 0.827 (mean = 0.625) while H o ranged from 0.162 to 0.483 (mean = 0.326), which was expected for an autogamous species. According to previous studies (Kwak and Gepts 2009), the PIC, which defines the ability of each SSR locus to distinguish among landraces, ranged from 0.167 to 0.793. The PI, which indicates the probability of the identity between 2 landraces harbouring different alleles at an analyzed locus, ranged from 0.103 to 0.710, while the PI value calculated for overall loci was 9.14 × 10−6 (Table 3). Since the high level of PIC, along with lower PI values, indicated a greater SSR ability to discriminate among landraces, the results indicated that the BM160 locus was the best SSR for discriminating among bean landraces (PIC = 0.793 and PI = 0.103). The percentage of homozygosity (Hom) in each locus, as expected for an autogamous species, ranged from 53.1 to 85.4 % (Table 3). The data, including the number of alleles, shows that the heterozygotic profiles for each locus ranged from 2 to 15, with an average of 8 (Table 3).
The genetic parameters already analyzed in Table 3 were recalculated for overall polymorphic SSRs and across each group of “genotypes”. The H e of the Calabrian common bean group was 0.595, the total number of alleles (N a ) ranged from 11 to 49 (landraces), and the number of private alleles ranged from 0 to 19 (landraces). Allelic richness (AR) showed values ranging from 1.5 to 2.5 (landraces), confirming the high rate of Calabrian germplasm diversity.
A dendrogram that included all bean landraces by cluster analysis (Nei 1973) and UPGMA algorithm was generated (Fig. 4a). Considering the large number of landraces investigated, based on the SSR analyzed only one putative synonym (“Quartu e luna” – “Menza luna”) was found. On the other hand, several couple or groups of landraces (“Cocò Bianca” and “Cocò gialla”; “Borlotto paesano”, “Borlotto locale”, “Borlotto spaddrera” and “Borlotto spineto”; “Cannellino”, “Cannellino bianco” and “Cannellina”), with similar names but different genetic profiles were also found.
a Dendrogram of genetic relationships among the 96 genotypes generated with Nei’s coefficient (Nei 1973) and UPGMA cluster analysis. Origin of Calabrian common bean landraces: (CS) Cosenza, (CZ) Catanzaro, (VV) Vibo Valentia, (RC) Reggio Calabria, (unk) unknown. In bold italics landraces of putative hybrid origin. Underlined Italian and American tester; (c) climbing, (d) dwarf. b Hierarchical organization of genetic relatedness of 96 bean genotypes analyzed by the STRUCTURE program. Each color represents one population (Group 1 white dashed; Group 2 dark gray; Group 3 light gray; Group 4 white; Group 5 black; Group 6 white pointed) and the length of the colored segment shows the estimated membership proportion of each sample to designed group. c Phaseolin types for each landrace are reported (C and T phaseolin types are from the Andes, S type is from Mesoamerica, nc-not classified)
According to genetic distances, 3 discrete clusters, (A), (B) and (C), were obtained (Fig. 4a). Cluster (A) included the Mesoamerican genotypes (BAT93 and G12783) together with 10 landraces from Calabria, most of which (50 %) came from Cosenza province. Cluster B included most (4) of the seven landraces that were taken from Cosenza which, in terms of morphological traits (seed and plant), phaseolin type and mode of germination, could be classified as runner beans (P. coccineus) or as hybrids between common and runner beans. Finally, the larger cluster (C) included the Andean genotypes (Jalo EEP558 and Midas) together with all the Italian varieties and 70 Calabrian landraces. On the basis of Nei’s (1973) genetic distances, this latter large cluster could be further subdivided in 5 sub-clusters, C1, C2, C3, C4 and C5 (Fig. 4a). In the first sub-cluster (C1) a large number of landraces (11) had come from the border areas between Cosenza and Catanzaro provinces, and the “Paulitana” landrace (as a zipper between sub-clusters C1 and C2) were present. The sub-cluster C3 included four out of the five Italian varieties, while 40 % of the landraces from Reggio Calabria were included in sub-cluster C4 (Fig. 4a). In summary, the Calabrian landraces were distributed between all the clusters identified, which meant that the area of sampling was not distinguishable by clustering.
Structure of the genetic groups
To ascertain the likely number of genetic groups (K) within the collection, STRUCTURE software was utilized. The distribution of all genotypes across different groups was determined on the assumption that (i) the Mesoamerican genotype BAT93 (Colombia) and the wild accessions G12783 (Morelos, Mexico) belonged to group I and (ii) the Andean genotypes Jalo EEP558 (Brazil) and Midas (USA) were included in group II.
The number of genetic groups (K) showed a clear peak at 6, where 6 main groups (I, II, III, IV, V and VI) were distinguished, including all Calabrian landraces. Group I consisted of 11 individuals, group II-14, group III-25, group IV-16, group V-14 and group VI-7 (Table 4, Table S2). Group I was considered representative of the putative Mesoamerican gene pool, groups II to V represented the putative Andean gene pool, while group VI could represent the genetic structure of the landraces derived from putative hybridizations between common (P. vulgaris) and runner bean (P. coccineus). The landraces were colored based on the STRUCTURE assignments at K = 6 (Fig. 4b). These results closely mirrored the pattern of diversity described in the UPGMA dendrogram (Fig. 4a).
Based on their mixed genetic structure (Fig. 4b), some landraces of Andean gene pool origin seemed to be derived from hybridization events. Furthermore, rare events of hybridization between landraces from both Andean and Mesoamerican gene pools were observed in “Cocò gialla” (sub-cluster C1), “Suraca larga”, “Posa di montagna” (sub-cluster C3) and “Sangue di porco” (sub-cluster C4) (Fig. 4b). Finally, analysis using the STRUCTURE software also inferred a potential natural hybridization between P. coccineus and P. vulgaris from the Andean gene pool found in group VI, in particular the landrace “Ciota Serpiata” (cluster B).
Discussion
Genetic population structure and the domestication events of P. vulgaris were broadly analyzed based on different types of markers such as morphological traits (Singh et al. 1991a, b; Gepts and Debouck 1991); seed proteins (Gepts et al. 1986; Gepts and Bliss 1986; Logozzo et al. 2007); allozymes (Koenig and Gepts 1989; Singh et al. 1991c); RFLPs (Freyre et al. 1998); RAPDs (Freyre et al. 1996); AFLPs (Tohme et al. 1996; Papa and Gepts 2003; Pallottini et al. 2004); and SSRs (Yu et al. 2000; Blair et al. 2003; Masi et al. 2003; Blair et al. 2006; Kwak and Gepts 2009, Burle et al. 2010). In particular, the last technique, first applied to the common bean by Yu et al. (2000), has been successfully used in recent years to characterize large bean germplasm collections from both America and Europe (Blair et al. 2009; Angioi et al. 2010). Furthermore, the analysis of genetic diversity based on SSRs could allow inferences on relationships among different groups of germplasm and the rate of intra- and interspecific hybridization in the bean worldwide collections.
In the present study, different markers, such as SSRs, morphological seed traits, phaseolin and 100-seed weight, have been combined with different statistical data analyzes in order to characterize the Calabrian collection of 87 P. vulgaris landraces. The aim was to increase understanding of the patterns of bean genetic diversity in this Italian region and to confirm the evolutionary origins of bean germplasm in Southern Europe. In general, this approach is of crucial importance in estimating the levels of germplasm diversity, to avoid its loss, and to allow its potential utilization.
Twelve SSRs, chosen among the most informative in bean and considered to characterize different worldwide core collections (Kwak and Gepts 2009; Blair et al. 2009), were used. However, with four of them it was not possible to distinguish among Calabrian landraces, although they were reported as informative SSR in previous studies (Blair et al. 2007; Zhang et al. 2008). The SSR ability to distinguish among landraces (PIC) was highly variable as reported in previous studies (Kwak and Gepts 2009; Blair et al. 2009). Conversely, the overall loci probability of identity (PI) was very low, confirming the informativeness of the SSR panel in the Calabrian germplasm. According to Kwak and Gepts (2009), BM160 appeared to be the best SSR for discriminating among Calabrian landraces, due to its higher PIC and lower PI values. The percentage of homozygosity (Hom) varied among loci, but was rather high on average, as would be expected in an autogamous species.
According to Nei’s index (average H e = 0.595, Nei 1987), a high level of genetic diversity was found in the Calabrian germplasm collection, which may reflect the large number of private alleles (19) and the allele richness (AR = 2.5). H e was higher than that reported in previous studies on Sardinian (Italy) (Angioi et al. 2009) and worldwide bean (Blair et al. 2009) collections. The observed heterozygosity (H o ) was low as expected in an autogamous species (Wells et al. 1988; Ibarra-Perez et al. 1997), in which cross hybridizations, due to the close together cultivation of different landraces, have been frequently observed.
According to a number of Italian reports (reviewed in Piergiovanni and Lioi 2010), cluster analysis on the Calabrian common bean revealed the presence of both gene pools but with a large prevalence of Andean origin landraces (79.3 %). This result was also confirmed by STRUCTURE analysis which revealed that 39 landraces (44.8 %) were present in groups II and III and 30 (34.5 %) in groups IV and V when the Andean genotype testers were included. In contrast, the Mesoamerican gene pool contribution to Calabrian germplasm appeared to be consistent (12.6 %) as shown in group I; smaller than that reported in the Marche region collection (Sicard et al. 2005) but greater than was observed in Sardinia (Angioi et al. 2009). However, the high level of genetic diversity of the Calabrian landraces, in contrast with their prevalent Andean origin (>80 %), could be due to: (1) the high diversity of Andean germplasm; and (2) the cross hybridization phenomena between the Andean and Mesoamerican germplasm, prominent in several areas of Calabria over the years, according to STRUCTURE analysis.
Thus, a three-step process could have occurred in Calabria: (1) a substantial introduction of germplasm from the Andean gene pool; (2) high diversity within this Andean germplasm, in contrast with that recently reported in Sardinia (Angioi et al. 2009); and (3) rare but significant cross hybridization events between gene pools or bean species.
According to STRUCTURE analysis, the cross hybridization between the 2 gene pools was identified in four landraces found in sub-clusters C1, C3 and C4. In addition, the same suspect of cross hybridization event was observed between common and runner bean, confirmed by the genetic structure in “Ciota Serpiata”, and by the 100-seed weight and germination results. In particular, the average 100-seed weight of the landraces putatively derived from runner bean was higher (85 g 100-seed weight−1) than Mesoamerican gene pool small-medium seed (<25–40 g 100-seed weight−1) or the large seeded (40–60 g 100-seed weight−1) landraces of Andean origin (Gepts et al. 1986; Gepts 1988; Singh et al. 1991a). In addition, “Ciota Serpiata”, “Favarula nera”, “A fava”, “Quarantino” and “Piani corona” landraces showed clear hypogeal germination, which is typical of runner bean, such as red color flowers observed in “Ciota Serpiata” and “Favarula nera”. These events of interspecific hybridization could be expected since runner and common beans are often found in sympatry (Papa et al. 2006; Spataro et al. 2011).
The contribution of Andean and Mesoamerican gene pools in Calabria was also clarified by seed storage analysis. Indeed, phaseolin pattern, very useful for gene pool identification, confirmed that the Calabrian germplasm was of Andean origin, although the Mesoamerican gene pool contribution was still considerable. These results were in accordance with phaseolin data from other Italian regions such as Basilicata and Abruzzo (Piergiovanni et al. 2000a, b). Furthermore, the phaseolin analysis supported the hypothesis of a cross hybridization between the gene pool by a presence of C type in a landrace (“Bianco”) of Mesoamerican origin. Hybridization events between the common (Andean gene pool) and runner beans are also suspected, since the “Ciota Serpiata” landrace, together with a genetic structure similar to runner bean, harbored a phaseolin type (T), typical of the Andean gene pool. Further analysis of the “Ciota Serpiata” landrace and the other members of cluster B-group VI is needed to confirm this rare natural cross hybridization event.
Finally, in Calabria, as in other regions, the local name could represent a ‘label’ of genetic divergence underlining a primary difference among landraces. Indeed, local names often refer to the seed traits (e.g., “Fagiolo a ughia” = nail bean; “Fagiolo ciuncu” = truncated bean; “Fagiolo bianco piccolo” = little white bean; “Russa janca” = red and white bean), possible geographical origins (“Paulitana” = bean from Paola; “Piani corona” = bean from an area near Reggio Calabria), cultivation system (“Azzicca” = “Azzicca grande” = sticking to a support because of a climbing growth habit, “Fasolu vasciu” = low bean with dwarf growth habit), or to the shape and origin simultaneously (“Poverello di Mormanno” = little bean from Mormanno). A similar approach to classification has been previously proposed (Jarvis et al. 2008; Angioi et al. 2009). Thus, the local name or ‘label’, together with the areas of origin, could allow a stratified sampling strategy able to recover rather all the genetic diversity, included landraces with the same name but from different areas, harboring different genetic profiles (homonymies).
In conclusion, the genetic resources of the common bean in a Mediterranean region have been described. More knowledge about bean evolution in areas far from their center of origin has been obtained. These results shed light on the genetic relationships between common beans and further demonstrated that the large genetic diversity of Phaseolus vulgaris L. is still unexploited in Calabria. Cross hybridizations between the 2 gene pools and 2 cultivated bean species are presumably present in the Calabrian germplasm, underlining that interspecific hybridization could represent an important source for common bean breeding (Beaver and Osorno 2009). Moreover, the present results will be useful in defining how bean genetic resources are managed in Calabria and for establishing a core collection.
References
Acampora A, Ciaffi M, De Pace C, Paolacci AR, Tanzarella OA (2007) Pattern of variation for seed size traits and molecular markers in Italian germplasm of Phaseolus coccineus L. Euphytica 157:69–82
Angioi SA, Rau D, Rodriguez M, Logozzo G, Desiderio F, Papa R, Attene G (2009) Nuclear and chloroplast microsatellite diversity in Phaseolus vulgaris L. from Sardinia (Italy). Mol Breed 23:413–429
Angioi SA, Rau D, Attene G, Nanni L, Bellucci E, Logozzo G, Negri V, Spagnoletti Zeuli PL, Papa R (2010) Beans in Europe: origin and structure of the European landraces of Phaseolus vulgaris L. Theor Appl Genet 121:829–843
Beaver JS, Osorno JM (2009) Achievements and limitations of contemporary common bean breeding using conventional and molecular approaches. Euphytica 168(2):145–175
Beebe S, Skroch P, Tohme J, Duque MC, Pedraza F, Nienhuis J (2000) Structure of genetic diversity among common bean landraces of Middle American origin based on correspondence analysis of RAPD. Crop Sci 40:264–273
Beebe S, Renjifo J, Gaitán-Solís E, Duque MC, Tohme J (2001) Diversity and origin of andean landraces of common bean. Crop Sci 41:854–862
Blair MW, Pedraza F, Buendia HF, Gaitán-Solís E, Beebe SE, Gepts P, Tohme J (2003) Development of a genome-wide anchored microsatellite map for common bean (Phaseolus vulgaris L.). Theor Appl Genet 107:1362–1374
Blair MW, Giraldo MC, Buendía HF, Tovar E, Duque MC, Beebe SE (2006) Microsatellite marker diversity in common bean (Phaseolus vulgaris L.). Theor Appl Genet 113:100–109
Blair MW, Diaz JM, Hidalgo R, Diaz LM, Duque MC (2007) Microsatellite characterization of andean races of common bean (Phaseolus vulgaris L.). Theor Appl Genet 116:29–43
Blair MW, Díaz LM, Buendía HF, Duque MC (2009) Genetic diversity, seed size associations and population structure of a core collection of common beans (Phaseolus vulgaris L.). Theor Appl Genet 119:955–972
Broughton WJ, Hernandez G, Blair M, Beebe S, Gepts P, Vanderleyden J (2003) Beans (Phaseolus spp.)—model food legumes. Plant Soil 252:55–128
Burle ML, Fonseca JR, Kami JA, Gepts P (2010) Microsatellite diversity and genetic structure among common bean (Phaseolus vulgaris L.) landraces in Brazil, a secondary center of diversity. Theor Appl Genet 121:801–813
CIAT (centro international de agricoltura tropical) (1986) The cultivated species of Phaseolus. Scientific content: Hidalgo R, Song L, Gepts P. Production: Fernando Fernandez O, Cali, Colombia, series: 04EB09.02
Escribano MR, Santalla M, Casquero PA, Ron AM, De Ron AM (1998) Patterns of genetic diversity in landraces of common bean (Phaseolus vulgaris L.) from Galicia. Plant Breed 117:49–56
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
FAOSTAT (2009) ProdSTAT: crops. FAO statistical databases (FAOSTAT), food and agriculture organization of the United Nations (FAO), http://faostat.fao.org
Freyre R, Rios R, Guzman L, Debouck D, Gepts P (1996) Ecogeographic distribution of Phaseolus spp. (Fabaceae) in Bolivia. Econ Bot 50:195–215
Freyre R, Skroch P, Geffroy V, Adam-Blondon AF, Shirmohamadali A, Johnson W, Llaca V, Nodari R, Pereira P, Tsai SM, Tohme J, Dron M, Nienhuis J, Vallejos C, Gepts P (1998) Towards an integrated linkage map of common bean. 4. development of a core map and alignment of RFLP maps. Theor Appl Genet 97:847–856
Gaitán-Solís E, Duque MC, Edwards KJ, Tohme J (2002) Microsatellite repeats in common bean (Phaseolus vulgaris): isolation, characterization, and cross-species amplification in Phaseolus ssp. Crop Sci 42:2128–2136
Gepts P (1988) Phaseolin as an evolutionary marker. Resources of Phaseolus beans. Kluwer, Dordrecht, In, pp 215–241
Gepts P, Bliss FA (1986) Phaseolin variability among wild and cultivated common beans (Phaseolus vulgaris) from Colombia. Econ Bot 40:469–478
Gepts P, Debouck DG (1991) Origin, domestication and evolution of the common bean, Phaseolus vulgaris. In: Voysest O, Van Schoonhoven A (eds) Common Beans: research for crop improvement, 2nd edn. CAB, Oxford, pp 7–53
Gepts P, Osborn TC, Rashka K, Bliss FA (1986) Phaseolin-protein variability in wild forms and landraces of the common bean (Phaseolus vulgaris): evidence for multiple centers of domestication. Econ Bot 40:451–468
Goudet J (2002) FSTAT: a program to estimate and test gene diversities and fixation indices. Version 2.9.3.2
Guerra-Sanz JM (2004) New SSR markers of Phaseolus vulgaris from sequence databases. Plant Breed 123:87–89
Ibarra-Perez F, Ehadaie B, Waines G (1997) Estimation of outcrossing rate in common bean. Crop Sci 37:60–65
IBPGR(IPGRI) (1982) Phaseolus vulgaris descriptors. Rome, p 32
Jarvis D, Brown AHD, Cuong PH, Panduro CL, Moreno LL, Gyawali S, Tanto T, Sawadogo M, Mar I, Sadiki M, Hue NTN, Reyes AL, Balma D, Bajracharya J, Castillo F, Rijal D, Belqadi L, Rana R, Seddik S, Ouedraogo J, Zangre R, Rhrib K, Chavez JL, Schoen D, Sthapit B, De Santis P, Fadda C, Hodgking T (2008) A global perspective of the richness and evenness of traditional crop-variety diversity maintained by farming communities. PNAS 105:5326–5331
Koenig R, Gepts P (1989) Allozyme diversity in wild Phaseolus vulgaris: further evidence for two major centers of diversity. Theor Appl Genet 78:809–817
Kwak M, Gepts P (2009) Structure of genetic diversity in the two major gene pools of common bean (Phaseolus vulgaris L., Fabaceae). Theor Appl Genet 118:979–992
Limongelli G, Laghetti G, Perrino P, Piergiovanni AR (1996) Variation of seed storage protein in landraces of common bean (Phaseolus vulgaris L.) from Basilicata, southern Italy. Euphytica 92:393–399
Liu K, Muse SV (2005) Powermarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep 12:6–13
Logozzo G, Donnoli R, Macaluso L, Papa R, Knüpffer H, Spagnoletti Zeuli P (2007) Analysis of the contribution of Mesoamerican and andean gene pools to European common bean (Phaseolus vulgaris L.) germplasm and strategies to establish a core collection. Genet Resour Crop Evol 54:1763–1779
Ma Y, Bliss F (1978) Seed proteins of common bean. Crop Sci 18:431–437
Masi P, Spagnoletti Zeuli PL, Donini P (2003) Development and analysis of multiplex microsatellite markers sets in common bean (Phaseolus vulgaris L.). Mol Breed 11:303–313
Nei M (1973) The theory and estimation of genetic distance p45–54 in genetic structure of populations, edited by Morton NE. University Press of Hawaii, Honolulu
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Paetkau D, Calvert W, Stirling I, Stroberk C (1995) Microsatellite analysis of population structure in Canadian polar bears. Mol Ecol 4:347–354
Page RDM (1996) Treeview: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Pallottini L, Garcia E, Kami J, Barcaccia G, Gepts P (2004) The genetic anatomy of a patented yellow bean. Crop Sci 44:968–977
Papa R, Gepts P (2003) Asymmetry of gene flow and differential geographical structure of molecular diversity in wild and domesticated common bean (Phaseolus vulgaris L.) from Mesoamerica. Theor Appl Genet 106:239–250
Papa R, Nanni L, Sicard D, Rau D, Attene G (2006) The evolution of genetic diversity in Phaseolus vulgaris L. In: Motley TJ, Zerega N, Cross H (eds) Darwin’s harvest: new approaches to the origins, evolution, and conservation of crop. Columbia University Press, New York, pp 121–142
Peakall R, Smouse PE (2006) GenAlEx6: genetic analysis in excel. population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Piergiovanni AR, Lioi L (2010) Italian common bean landraces: history, genetic diversity and seed quality. Diversity 2:837–862
Piergiovanni AR, Cerbino D, Brandi M (2000a) The common bean populations from Basilicata (southern Italy). an evaluation of their variation. Genet Resour Crop Evol 47:489–495
Piergiovanni AR, Taranto G, Pignone D (2000b) Diversity among common bean populations from the Abruzzo region (central Italy): a preliminary inquiry. Genet Resour Crop Evol 47:467–470
Piergiovanni AR, Taranto G, Losavio FP, Pignone D (2006) Common bean (Phaseolus vulgaris L.) landraces from Abruzzo and Lazio regions (Central Italy). Genet Resour Crop Evol 53:313–322
Pritchard JK, Wen W (2003) Documentation for STRUCTURE Software Version 2. http://pritch.bsd.uchicago.edu
Santalla M, Monteagudo AB, Gonzalez AM, Lema M, De la Fuente M, De Ron AM (2004) Agronomic potential value of great northern recombinant lines and breeding implications in common bean. Annu Rep Bean Improv Coop 47:147–148
Sicard D, Nanni L, Porfiri O, Bulfon D, Papa R (2005) Genetic diversity of Phaseolus vulgaris L and P. coccineus L. landraces in central Italy. Plant Breed 124:464–472
Singh SP, Gepts P, Debouck DG (1991a) Races of common bean (Phaseolus vulgaris L., Fabaceae). Econ Bot 45:379–396
Singh SP, Gutierrez JA, Molina A, Urrea C, Gepts P (1991b) Genetic diversity in cultivated common bean: II. marker-based analysis of morphological and agronomic traits. Crop Sci 31:23–29
Singh SP, Nodari R, Gepts P (1991c) Genetic diversity in cultivated common bean. I Allozymes Crop Sci 31:19–23
Spataro G, Tiranti B, Arcaleni P, Bellucci E, Attene G, Papa R, Spagnoletti Zeuli P, Negri V (2011) Genetic diversity and structure of a worldwide collection of Phaseolus coccineus L. Theor Appl Genet 122:1281–1291
Tohme J, Gonzalez DO, Beebe S, Duque MC (1996) AFLP analysis of gene pools of a wild bean core collection. Crop Sci 36:1375–1384
Wagner HW, Sefc KM (1999) IDENTITY 1.0. Centre for Applied Genetics, University of Agricultural Sciences, Vienna
Weber JL (1990) Informativeness of human (dC-dA)n-(dGdT)n polymorphisms. Genomics 7:524–530
Wells WC, Isom WH, Waines JG (1988) Outcrossing rates of six common bean lines. Crop Sci 28:177–178
Yu K, Park J, Poysa V, Gepts P (2000) Integration of simple sequence repeat (SSR) markers into a molecular linkage map of common bean (Phaseolus vulgaris L.). Am Genet Assoc 91:429–434
Zhang X, Blair MW, Wang S (2008) Genetic diversity of Chinese common bean (Phaseolus vulgaris L.) landraces assessed with simple sequence repeat markers. Theor Appl Genet 117:629–640
Acknowledgments
This study was supported by a grant from “Agenzia Regionale Sperimentazione Servizi in Agricoltura (ARSSA–Calabria)” in the frame of the projects ‘‘Programma Interregionale–P.O. Proteine Vegetali’’. The Authors would like to thank the referents from ARSSA farm of Rogliano (CS), M. Turco, M. Convertino, M. Bruno and F. Filella, for their attempts against the loss of biodiversity and in obtaining the seed collection.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mercati, F., Leone, M., Lupini, A. et al. Genetic diversity and population structure of a common bean (Phaseolus vulgaris L.) collection from Calabria (Italy). Genet Resour Crop Evol 60, 839–852 (2013). https://doi.org/10.1007/s10722-012-9879-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-012-9879-6