Abstract
The present study aimed to characterize the glucan from C. mollissima Blume fruits and its selenium derivative, then investigate their antitumor activity in vitro. A glucan, designated as CPA, was firstly isolated from the fruits of C. mollissima Blume. Structure analysis indicated that CPA was a linear 1,6-α-D-glucan with the average molecular weight about 2.0 × 103 kDa. The selenylation modification derivative of CPA (sCPA), exhibited a stronger antiproliferative effect on tumor cells than CPA in vitro. CPA and sCPA could induce HeLa cells apoptosis and decrease mitochondrial membrane potential. sCPA could also arrest HeLa cells in S phase, promote reactive oxygen species generation and activate caspase-3 activity in HeLa cells. These results manifest that CPA and sCPA inhibit the proliferation of HeLa cells via different mechanisms, which is meaningful for their potential use as antitumor drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer has become a global health problem in recent years. Chemotherapy, as one of conventional cancer treatments, causes severe side effects. Therefore, searching for new anticancer drugs with high efficacy and low toxicity is essential. Natural products have been considered to be a valuable source of anticancer drugs [1]. Polysaccharides are a class of natural biopolymers that exhibit the biological activities of antitumor, immunoregulatant and antioxidant effects. Natural polysaccharides, especially in some foods are a potential source of bioactive compounds with low side effects for cancer chemoprevention and chemotherapy [2, 3]. For example, polysaccharides from Pleurotus nebrodensis [4] and porcine cartilage [5] could not only inhibit the proliferation of cancer cells, but also induce cancer cells apoptosis.
Chestnut belongs to the Fagaceae family and distributed in Asia, Europe and America. Chestnut fruits contain carbohydrates, proteins, vitamins, minerals, etc. [6, 7]. According to the statistical data from Food and Agriculture Organization of the United Nations, China accounted for about 83% of total world chestnut production [8]. Chinese chestnut (C. mollissima Blume) fruits have been used for thousands of years as traditional medicine and food, they can improve the function of kidneys according to the ancient encyclopedia of China [9]. Xylans and glucuronoxylan extracted from Spanish chestnut (C. sativa) could inhibit the proliferation as well as the migration and invasion capability of human epidermoid carcinoma cells (A431) [10, 11], but few references were reported about the antitumor activity of polysaccharides from Chinese chestnut (C. mollissima Blume).
Selenium (Se) is an essential trace element for human beings, and it is a key constituent of selenoproteins, e.g. glutathione peroxidase (Gpx), thioredoxin reductase (TrxR) and formate dehydrogenase [12, 13]. Lack of Se could cause the diseases of cardiovascular sclerosis, cancer, diabetes [14–16]. Organic selenium can be easily absorbed and has less toxicity by comparison with inorganic selenium [17]. Selenylation modification polysaccharides, as an organic selenium compound, could enhance the antitumor [18, 19], antioxidant [20, 21], antibacterial [22], immunoregulatory [23, 24] effects of polysaccharides. However, studies aimed at the antitumor effect of chemical synthetic selenium polysaccharides are limited. In this text, a glucan was firstly extracted from C. mollissima Blume fruits, and its selenized derivative was synthesized with the commonly used nitric acid-sodium selenite method. The physicochemical properties, structure characteristics and antitumor activity of the polysaccharide and its selenylation modification derivative were investigated in vitro.
Materials and methods
Experimental materials and chemicals
C. mollissima Blume fruits were purchased from Qianxi County, Hebei province of China. Q Sepharose Fast Flow and Sephacryl S-400/HR were purchased from GE Healthcare Life Sciences (USA). Standard dextrans (Mw: 2350, 1220, 708, 344, 200, 107 and 47.1 kDa) were purchased from Shodex (Tokyo, Japan). D-glucose, L-rhamnose, D-xylose, L-arabinose, D-mannose, D-galactose, D-glucuronic acid, D-galacturonic acid were purchased from Sigma (St. Louis, MO, USA). Human cervical carcinoma cell line (HeLa), human breast cancer cell line (MCF-7) and rat liver cell line (BRL-3A) were obtained from Shanghai Institute of Cell Biology (Shanghai, China). Other reagents used were analytical grade without further purification.
Extraction of polysaccharides from C. mollissima Blume
C. mollissima Blume fruits were hulled, dried at room temperature, ground into powder of 200 mesh size, and stored at −20 °C. The powder was dipped into distilled water (1 : 20, w/v) and stirred with a mechanical agitator at room temperature for 2 h. The extract was centrifuged at 4000 rpm for 10 min, and the residue was extracted repeatedly for two times. The supernatants were combined and concentrated in a rotary evaporator under reduced pressure at 40 °C. Then the concentrated solution was precipitated by adding threefold volume of 95% (v/v) cold ethanol and kept at 4 °C for 12 h. The precipitate was collected, dialyzed against distilled water and freeze-dried to acquire the crude polysaccharides.
Isolation and purification of the crude polysaccharides from C. mollissima Blume
The crude polysaccharides (CP) were dissolved in distilled water and fractionated with a Q Sepharose Fast Flow anion exchange column (QFF, 50 × 30 cm). The column was equilibrated with distilled water in advance and eluted with a stepwise gradient of NaCl. The carbohydrate content in the fractions was determined using the phenol-sulfuric acid method [25]. The main polysaccharide fraction eluted with 0 mol/L NaCl was pooled, concentrated and freeze-dried to obtain the polysaccharide CP0. CP0 was further purified on a gel filtration Sephacryl S-400 chromatography column (26 × 100 cm). The eluent was 0.2 mol/L NH4HCO3 at a flow rate of 0.35 mL/min. The major polysaccharide fractions were pooled according to the detection curve of the phenol-sulfuric acid method [25] and lyophilized to acquire the polysaccharides CPA.
Physicochemical properties
Total sugar content was determined at 490 nm by the phenol-sulfuric acid method using glucose as the standard [25]. Uronic acid content was assayed at 530 nm according to the carbazole-sulfuric acid method using glucuronic acid as the standard [26]. Protein was estimated by Folin-phenol assay at 750 nm using BSA as the standard [27].
Homogeneity and molecular weight analysis
High performance gel permeation chromatography (HPGPC) instrument (Agilent 1260, USA) was used to determine the homogeneity and molecular weight of the polysaccharides. Samples were dissolved and detected on a Shodex OHpak SB-805 HQ column (Tokyo, Japan) by a refractive index detector G1362A. The column was eluted with 0.1 mol/L Na2SO4 at 0.8 mL/min. The molecular weight was calculated according to the calibration curve of Log Mw (molecular weight) of molecular standards against their retention time.
Monosaccharide composition analysis
The monosaccharide composition was measured by a high performance liquid chromatography (HPLC, Agilent, USA) system after precolumn derivatization. The sample was hydrolyzed with 2 mol/L trifluoroacetic acid (TFA) at 105 °C for 6 h. After removing TFA, the hydrolysis product was derivatized with 1-phenyl-3-methyl-5-pyrazolone (PMP) according to the literature [28]. The derivative was detected on an Agilent Zorbax SB-C18 column (5 μm, 4.6 × 250 mm, USA) fitted with an UV detector at 245 nm at 30 °C. The column was eluted with 0.1 mol/L KH2PO4 (pH 6.7)-acetonitrile (83 : 17) at 1.0 mL/min. Monosaccharide composition was identified by comparing the retention time of standards with the samples. The molar ratio was calculated according to the peak area detected by HPLC chromatography spectrum.
Selenylation modification of CPA
The polysaccharide CPA was selenized by the nitric acid-sodium selenite method previously reported with minor modification [24, 29]. 100 mg CPA was completely dissolved with 0.5% nitric acid (v/v), then 100 mg sodium selenite and 50 mg barium chloride was added. After stirring at 65 °C for 10 h, the mixture was cooled to room temperature by adjusting the pH to 7–8 with sodium hydroxide. Then the mixture was concentrated and dialyzed against distilled water for three successive days. The dialysis residue was concentrated and lyophilized to yield the selenium polysaccharide sCPA. Inductively coupled plasma optical emission spectroscopy (ICP-OES, Thermo iCAP 6300) was applied to determine the Se content in the polysaccharide sCPA.
Methylation analysis
Methylation of the polysaccharides was carried out according to the method of Hakomori with minor modification [30]. Samples (1 mg) were dissolved in dimethyl sulfoxide by ultrasonic assisted dissolution, 100 mg sodium hydride was added and stirred for 1.5 h at room temperature. 1 mL iodomethane was added and incubated in the darkness for another 1.5 h, then the reaction was terminated by adding 2 mL distilled water. The methylated polysaccharides were extracted by dichloromethane. After hydrolysis with 2 mol/L TFA at 105 °C for 6 h, the methylated sugar residues were reduced by sodium borohydride, followed by acetylation with acetic anhydride. The derivatized sugar residues were dried and dissolved in dichloromethane. A DB 225 fused silica capillary column was used to analyze the glycosidic linkages coupled to a HP6890II gas chromatography–mass spectrometry (Agilent, USA). The temperature was increased from 100 to 220 °C at 5 °C/min, then maintained at 220 °C for 15 min. Identification of partially methylated alditol acetates was carried out on the basis of the retention time (tR) and its mass fragmentation pattern by comparing to Complex Carbohydrate Research Center Database (http://www.ccrc.uga.edu/).
Spectroscopy analysis
Samples were grounded with KBr powder and pressed into pellets. FT-IR spectrum was measured in the range of 4000–400 cm−1 on a FT-IR spectrometer (Bruker Vertex 70, Germany). For NMR analysis, samples were dissolved in 0.5 mL D2O (99.98%). 1H (40 °C), 13C, DEPT (135°), 1H-1H COSY, 1H-1H NOESY, 1H-13C HMQC and 1H-13C HMBC experiments were carried out at 23 °C on a Bruker 600 MHz spectrometer (Germany). Acetone was used as the internal standard (1H: δ 2.225; 13C: δ 31.07).
Antitumor activity analysis
Cell proliferation assay
The effect of CPA and sCPA on cancer cells proliferation in vitro was measured using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) colorimetry [31]. HeLa, MCF-7 and BRL-3A cells were cultured in Dulbecco’s modified Eagel’s medium (DMEM, Gibco, USA) solution supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells with a concentration of 2 × 104 cells/mL were plated into 96-well microplate (90 μL/well) and incubated in a humidified atmosphere of 5% CO2 at 37 °C for 12 h to acquire adherent cells. Then, 10 μL of CPA and sCPA at a final concentration of 5, 10, 25, 50 or 100 μg/mL was added into each well. 5-Fu (10 μg/mL) was used as a positive control. After incubation for 24 and 48 h, 10 μL of MTT (5 mg/mL) was added to each well, and cells were incubated for 4 h at 37 °C. Afterward, 100 μL of DMSO was added to each well. Absorbance was measured at 570 nm by an enzyme-linked immunosorbent analyzer (SpectraMax M4, USA). The inhibition rate was calculated according to the following formula: Inhibition rate (%) = (1 − Asample)/Acontrol × 100, where Asample and Acontrol are the absorbance of treated cells and untreated cells, respectively.
Morphology analysis
HeLa cells (1 × 105 cells/mL) were seeded in 6-well plate and treated with CPA and sCPA at a final concentration of 50 μg/mL for 48 h. After washing with PBS and fixing with 4% paraformaldehyde solution for 10 min, cells were suspended with PBS and incubated by adding two drops 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Life Technologies, USA) for 5 min at room temperature in the darkness. The cell nuclear morphology was captured using a fluorescence microscope (EVOS FL Auto, Life Technologies, USA).
Apoptosis analysis
The apoptosis of HeLa cells was detected with the Annexin V-FITC/PI double labeling method. Briefly, HeLa cells (1 × 105 cells/mL) were seeded in 6-well plate and allowed to attach overnight. After incubating with CPA and sCPA at 50 μg/mL for 48 h, cells were harvested and centrifuged at 1500 rpm for 5 min. Then cells were resuspended in 1 × binding buffer and stained with Annexin V-FITC and PI per the manufacturer’s instructions of Apoptosis Detection Kit (Life Technologies, USA) at 37 °C in the darkness for 15 min. The stained cells were analyzed using a flow cytometer (BD FACS, USA) within 1 h.
Cell cycle analysis
For cell cycle distribution analysis, HeLa cells (1 × 105 cells/mL) were seeded into 6-well plate and incubated overnight. After treating with CPA and sCPA at a final concentration of 50 μg/mL for 24 h, HeLa cells were collected and fixed with 70% cold ethanol at 4 °C overnight. Then cells were washed with PBS and resuspended in PBS by adding 30 U/mL RNase and 50 μg/mL propidum iodide (PI, Life Technologies, USA) in the darkness for 15 min. The flow cytometry (BD FACS, USA) was used to analyze the cellular DNA content.
Reactive oxygen species analysis
The level of reactive oxygen species (ROS) in HeLa cells was measured with DCFH-DA (2’,7’-dichlorodihydrofluorescein diacetate) fluorescent probe (Life Technologies, USA). Briefly, HeLa cells (1 × 105 cells/mL) were seeded in 6-well plate, cultured overnight, and incubated with CPA and sCPA at 50 μg/mL for 48 h. Then cells were collected and stained with DCFH-DA at 37 °C for 30 min. The fluorescence intensity was determined by flow cytometry (BD FACS, USA).
Mitochondrial membrane potential analysis
Changes of mitochondrial membrane potential was measured using the lipophilic cationic probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine iodide (JC-1, Beyotime, China). In brief, HeLa cells (1 × 105 cells/mL) were cultured in 6-well plate for 12 h prior exposure to CPA and sCPA at 50 μg/mL for 48 h, then cells were washed with PBS, digested with trypsin, and centrifuged at 1500 rpm for 5 min, the pellets were suspended in PBS and stained per the manufacturer’s instructions of JC-1 detection kit. Aggregates and monomers of JC-1 were detected in the FL2 channel (red fluorescence) and FL1 channel (green fluorescence) respectively by flow cytometry (BD FACS, USA).
Besides that, the mitochondrial membrane potential was further studied by a fluorescence microscope (EVOS FL Auto, Life Technologies, USA). HeLa cells (1 × 105 cells/mL) were seeded in 6-well plate and cultured overnight. After cells were incubated with CPA and sCPA at 50 μg/mL for 48 h, cells were washed with PBS and stained with the JC-1 detection kit. Images of fluorescence were captured with a fluorescence microscope (EVOS FL Auto, Life Technologies, USA).
Caspase-3 activity analysis
Caspase-3 activity was assessed using PE active caspase-3 apoptosis kit (BD Pharmingen, USA) per the manufacturer’s instruction. Briefly, HeLa cells (1 × 105 cells/mL) were seeded in 6-well plates for 12 h. After incubating with CPA and sCPA at 50 μg/mL for 48 h, Cells were collected and wash with cold PBS for two times. Then cells were incubated with 1 × cytofix/cytoperm solution on ice for 20 min, washed with 1 × perm/wash buffer for two times and stained with 20 μL PE rabbit anti-active casepase-3 for 30 min. The stained cells were analyzed by flow cytometry (BD FACS, USA).
Statistical analysis
All the results were collected from at least three separate experiments and expressed as mean±S.D. P-values of less than 0.05 were considered to be statistical differences.
Results and discussion
Preparation and physicochemical properties of the polysaccharides
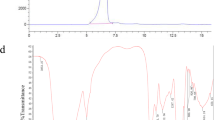
The fruits of C. mollissima Blume were ground and extracted with distilled water (1:20, m/v) for three times. After centrifugation, the supernatants were combined, concentrated and deposited by ethanol to acquire the crude polysaccharides CP. The yield of the crude polysaccharides was about 8.62%. The crude polysaccharides were then isolated by a QFF column eluted using a step gradient of 0, 0.1, 0.2 and 0.3 mol/L NaCl (Fig. 1a). The major component eluted at the 0 mol/L NaCl step was concentrated and further purified on a Sephacryl S-400 column. The main fraction, named as CPA, was acquired. HPGPC chromatography analysis demonstrated that CPA was a homogeneous polysaccharide (Fig. 1b) and its average molecular weight was about 2.0 × 103 kDa. Total sugar content of CPA was 98.3%. No uronic acid or protein was detected in CPA. Monosaccharide composition analysis indicated that CPA was mainly composed of glucose (Fig. S1). CPA was a homogeneous glucan.
Structure analysis of CPA
Methylation analysis indicated that CPA was mainly composed of [→6)-Glcp(1→], it could be assigned from the presence of 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl-glucitol.
In the 1H and 13C NMR spectra of CPA (Fig. 2a, b), there were one anomeric proton and one carbon at δ 4.98 and δ 98.5, respectively. The downfield shift of C-6 at δ 66.4 in the 13C NMR spectrum (Fig. 2b) indicated that CPA was [→6)-α-D-Glcp(1→], as the C-6 of glucose with free hydroxyl group should be around δ 62.0 [32]. In the 1H-13C HMQC spectrum (Fig. 2c), the anomeric proton at δ 4.98 correlated with the anomeric carbon at δ 98.5, and it was assigned to the H-1 and C-1 of [→6)-α-D-Glcp(1→][32, 33]. The assignment of proton and carbon signals of CPA is listed in Table 1.
Glucans with different glycosidic linkages, such as β-(1 → 3)-, β-(1 → 6)-, α-(1 → 3)-, α-(1 → 4)- and α-(1 → 6)-glucans have been found in nature [34]. Most of these glucans are branched with various side chains attached to different positions, and some are bound with proteins or peptides as polysaccharide complexes, but it is rare to find α-(1 → 6)-D-glucan in plants. As reported by the literature, only several species such as Cistanche deserticola [35], Ipomoea batatas [36], Panax ginseng [37], Pueraria lobata [38] and Longan [32] have been detected to have (1 → 6)-α-D-glucan. They are all important ingredients in modern functional food formula.
Characterization of the selenium polysaccharide sCPA
After selenylation modification of CPA, the Se content in sCPA was 573.9 μg/g as determined by ICP-OES. Compared with the IR spectra of CPA and sCPA, their structures were similar and there was a peak at 636 cm−1 in the spectrum of sCPA (Fig. S2). This peak was attributed to an asymmetrical Se-O-C stretching vibration that indicated the successful selenylation modification of CPA [14, 21]. In the DEPT (135°) spectrum of sCPA (Fig. S3), the peaks became narrow and there were some small new peaks at δ 100.1, 75.0, 73.9 and 72.6, which indicated the selenylation modification of C-1, C-2, C-3 and C-4 position in sCPA, respectively. The selenylation modification ratio of sCPA was lower than that reported by the literature with the same nitric acid-sodium selenite method [24, 29], but higher than that reported by Chen et al. [18]. As reported by the literature [20, 29], the non-selective selenylation modification of polysaccharides was easily happened at C-6 position, but due to that CPA was a linear (1 → 6)-glucan, selenylation modification at C-6 position was hard. At the same time, the high molecular weight and space steric effect also resulted in the lower selenylation modification ratio of sCPA.
Assessment of the antiproliferative effect
(1 → 6)-α-D-glucan has been reported to have the immune activity. Three active (1 → 6)-α-glucan from Cistanche deserticola could stimulate the incrementation of mice splenocytes [37]. The (1 → 6)-α-D-glucan from Panax ginseng C. A. Meyer could significantly induce lymphocyte proliferation and increase NO production [36]. (1 → 6)-α-D-glucan with branches at C-3 position of every four residues showed potent stimulating effects on murine lymphocyte proliferation as well as on splenocyte antibody production [39]. The (1 → 6)-α-D-glucan isolated from Longan had an antiproliferative effect on the growth of HepG2 cells and also could improve the immune system [32], but few literatures were reported about the antitumor activity of (1 → 6)-α-D-glucan.
The antiproliferative effect of CPA and sCPA was investigated on HeLa and MCF-7 cells by the MTT assay in vitro. As shown in Fig. 3, CPA and sCPA both exhibited the antiproliferative effects on HeLa and MCF-7 cells in a dose and time-dependent manner. When cells were incubated with the polysaccharides at 100 μg/mL for 48 h, the inhibition rate of CPA against HeLa and MCF-7 cells was 31.6% and 29.8%, respectively, and for sCPA, the inhibition rate on HeLa and MCF-7 cells was 54.8% and 47.4%, respectively. At the same incubated concentration and time, sCPA showed a stronger antiproliferative effects than CPA on cancer cells. BRL-3A cells were used to assess the cytotoxicity of the polysaccharides in vitro. After incubating with CPA and sCPA for 48 h, the cell viability was higher than 80% at 100 μg/mL. These results manifested that selenylation modification of CPA could significantly enhance its antiproliferative effect on cancer cells and have low cytotoxicity to normal cells.
Glucans as an antitumor agent have been shown in the literature. For example, the linear (1 → 6)-α-D-glucan (Mw 3.26 × 105 Da) from Polygonum multiflorum inhibited HepG2 and BGC-823 cells growth by 53.3% and 38.6% at 400 μg/mL for 24 h [40]. The polysaccharides from Agaricus bisporus (Ab) and Lactarius rufus (Lr) fruiting bodies were composed of a linear (1 → 6)-linked and a branched (1 → 3), (1 → 6)-linked backbone, respectively. Lr and Ab decreased the viability of HepG2 cells by ~30% and ~70% at 400 μg/mL for 24 h [41]. A glucan (SHPSA, Mw 5.78 × 105 Da) with β-(1 → 6) side chains linked to a β-(1 → 3) backbone was obtained from Sargassum horneri. The inhibition rate on DLD cells reached 74.82 ± 2.53% at 100 μg/mL [42]. The α-(1 → 4)-glucan (CSPS-1) with 1,6-glycosidic branch was isolated from Cyclina sinensis. After sulfation modification of CSPS-1, the sulfated derivatives showed much higher inhibitory activity against BGC-823 cells [43]. A hyper branched (1 → 4)-α-D-glucan (RPS3) isolated from Rhizoma Panacis Japonici have no specific bioactivity against H-22 tumor cells at 6.25–400 μg/mL for 24 h. But after sulfated (S-), phosphated (P-) and carboxymethylated (CM-) modification of RPS3, S-RPS3 and P-RPS3 could significantly inhibit H-22 cells growth [44]. Glucans, especially after chemical modification, have a significantly enhancement of antiproliferative activity on cancer cells, this may be helpful for the potential medical application of gluans.
Analysis of HeLa cells apoptosis
To investigate whether the antiproliferative effect of CPA and sCPA on HeLa cells was caused by apoptosis, both DAPI and Annexin V FITC/PI double staining method were performed with fluorescence microscopic and flow cytometric analysis. As shown in Fig. 4a, both CPA and sCPA could change the nucleus morphology of HeLa cells, the apoptotic nucleus became brighter fluorescence than control. HeLa cells treated with sCPA showed more significant morphological characteristics of apoptosis than CPA at 50 μg/mL for 48 h, including nuclear pyknosis and nuclear fragmentation. Flow cytometry analysis indicated that the ratios of apoptotic cells (early and later apoptotic cells) treated with CPA and sCPA at 50 μg/mL for 48 h were 13.7% and 50.2%, respectively (Fig. 4b). The result suggested that both CPA and sCPA could trigger apoptotis in HeLa cells, but the effect of sCPA was much more stronger than CPA.
HeLa cells were exposed to CPA and sCPA at 50 μg/mL for 48 h. (a) The morphology of HeLa cells captured by fluorescence microscopy (Bar = 200 μm); (b) The apoptosis of HeLa cells detected by flow cytometry; (c) Cell cycle distribution of HeLa cells detected by flow cytometry. ***p ≤ 0.001 compared with control group
Analysis of cell cycle
In order to determine the mechanisms of the antiproliferative effect, the DNA contents were investigated using PI staining assay with a flow cytometer. As shown in Fig. 4c, CPA (50 μg/mL for 24 h) had no significant impact on cell cycle distribution of HeLa cells, and sCPA (50 μg/mL for 24 h) induced a significant cell population in S phase, while the percentage of cells in G0/G1 phase was declined. The result indicated that sCPA could inhibit HeLa cells proliferation through the blockage of S checkpoint.
Measurement of mitochondrial membrane potential
Mitochondria are the most important sensor for cells apoptosis. The mitochondrial membrane potential was assessed using a cationic dye that shifts from red to green fluorescence depending on the functional status of mitochondria. Treatment of HeLa cells with 50 μg/mL CPA and sCPA for 48 h resulted in a noticeable reduction in the red fluorescence compared to the untreated cells (Fig. 5a). The Red/Green fluorescence ratio decreased after being treated with CPA and sCPA, which indicated the dysfunction of mitochondrial membrane potential (Fig. 5b).
Measurement of intracellular reactive oxygen species
Generation of intracellular ROS can cause cancer cells cytotoxicity and apoptosis. To clarify the mechanism of CPA and sCPA inducing HeLa cells apoptosis, intracellular ROS levels were detected with DCFH-DA probe. As determined by the flow cytometry (Fig. 6a), ROS production in HeLa cells was remarkably increased after treating by sCPA for 48 h at 50 μg/mL, but the ROS level in CPA treated cells had no significant difference by comparison with the control group. The result suggested that sCPA could induce HeLa cells apoptosis through ROS generation.
Analysis of caspase-3 activity
To elucidate the molecular mechanism responsible for HeLa cells apoptosis, the activity of caspase-3 was investigated with PE active caspase-3 apoptosis kit by the flow cytometry. After treating HeLa cells with CPA and sCPA at 50 μg/mL for 48 h (Fig. 6b), sCPA significantly stimulated the caspase-3 activity compared to CPA. Therefore, sCPA could induce HeLa cells apoptosis by caspase-3 dependent apoptotic process.
The linear (1 → 6)-α-D-glucan is rarely found in nature. The antitumor activity of (1 → 6)-α-D-glucan has been confirmed, but its antitumor mechanism is not known yet. Se as an effective cancer preventive and therapeutic agent has been investigated vastly either in the form of Se nanoparticles or Se compounds [45, 46]. Selenylation modification can enhance the biological activities of the native polysaccharides [18, 21, 24, 39], but the mechanisms are not clear, so we attempt to investigate the antitumor mechanisms of the glucan and its selenylation modification derivative in vitro.
Apoptosis and cell cycle arrest are two of the main mechanisms for inhibiting cells proliferation [4]. Apoptosis is characterized by nucleus morphological changes, such as condensation of chromatin, appearance of apoptotic bodies, which were observed in CPA and sCPA treated HeLa cells. Also the early and later apoptotic cells had been detected using Annexin V FITC/PI double staining method by flow cytometry. Cell cycle arrest is one of the key mechanisms for anticancer drugs to exert their effect on cancer cells. sCPA could arrest HeLa cells in S phase. Mitochondria are the cell main energy producers and essential for cellular functions. The mitochondrial pathway is one of the most pivotal mechanisms in initiating cells apoptosis. Loss of mitochondrial membrane potential is an early event in apoptosis [4]. The results showed the significant decrease of mitochondrial membrane potential after HeLa cells were treated with CPA and sCPA, which indicated the dysfunction of mitochondrial membrane potential. The free radical reactive oxygen species (ROS) is a byproduct of oxidative phosphorylation. High levels of ROS may elicit dysfunction of mitochondria and cause cells apoptosis. According to our results, sCPA could significantly increase ROS levels in HeLa cells, while the levels of ROS in CPA treated cells did not change greatly. The caspase cascade triggered by caspase family members plays a key role in apoptosis. caspase-3 is considered to be the most important executioner of apoptosis [4, 47]. A remarkable increase in the caspase-3 levels was observed in sCPA treated HeLa cells, but CPA could not significantly change the activity of caspase-3 in HeLa cells compared to the control group.
Both CPA and sCPA had antiproliferative effect on HeLa and MCF-7 cells in vitro. By comparison of the mechanisms of CPA and sCPA inducing HeLa cells apoptosis, both CPA and sCPA decreased the mitochondrial membrane potential. The selenium-polysaccharide sCPA could also significantly arrest HeLa cells in S phase, stimulate the intracellular ROS generation and increase intracellular caspase-3 activity, but the native polysaccharide CPA had no significant impact on cell cycle distribution, ROS and caspase-3 level in HeLa cells. The different apoptotic mechanisms of CPA and sCPA may be caused by the selenylation modification of glucan. The detailed apoptotic mechanism for the native polysaccharide and its selenium derivative still need to be further investigated.
Conclusion
A natural linear (1 → 6)-α-D-glucan was firstly isolated from the fruits of C. mollissima Blume. CPA could inhibit the proliferation of HeLa and MCF-7 cells, induce HeLa cells apoptosis and decrease mitochondrial membrane potential. The selenylation modification polysaccharide sCPA exhibited a stronger antitumor activity than CPA. sCPA could induce HeLa cells apoptosis by arresting the cell cycle, losing mitochondrial membrane potential, increasing ROS levels and activating caspase-3. Therefore, the results indicated that selenylation modification of glucan from C. mollissima Blume was an effective way to enhance its antitumor activity. Consequently, CPA and sCPA were much more potent antitumor agents to be worth further investigated.
References
Cragg, G.M., Newman, D.J.: Nature: a vital source of leads for anticancer drug development. Phytochem. Rev. 8, 313–331 (2009)
Huang, F., Zhang, R.F., Dong, L.H., Guo, J.X., Deng, Y.Y., Yi, Y., Zhang, M.W.: Antioxidant and antiproliferative activities of polysaccharide fractions from litchi pulp. Food Funct. 6, 2598–2606 (2015)
Guo, M., Ding, G.B., Guo, S.J., Li, Z.Y., Zhao, L.Q., Li, K., Guo, X.R.: Isolation and antitumor efficacy evaluation of a polysaccharide from Nostoc commune Vauch. Food Funct. 6, 3035–3044 (2015)
Cui, H.Y., Wu, S.F., Sun, Y.P., Wang, T.T., Li, Z.J., Chen, M.H., Wang, C.L.: Polysaccharide from Pleurotus nebrodensis induces apoptosis via a mitochondrial pathway in HepG2 cells. Food Funct. 7, 455–463 (2016)
Song, W., Hu, P.P., Shan, Y.J., Du, M., Liu, A.J., Ye, R.: Cartilage polysaccharide induces apoptosis in K562 cells through a reactive oxygen species-mediated caspase pathway. Food Funct. 5, 2486–2493 (2014)
Vasconcelos, M.C.D., Bennett, R.N., Rosa, E.A., Ferreira-Cardoso, J.V.: Composition of European chestnut (Castanea sativa Mill) and association with health effects: fresh and processed products. J. Sci. Food Agric. 90, 1578–1589 (2010)
Yang, Y., Pan, X., Wang, G.: China food composition, vol. 80, 2nd edn. Peking University Medical Press, Beijing (2009)
FAOSTAT. Country rank in the world by commodity: China. (http://faostat.fao.org/site/339/default.aspx; 2013.10.3)
Li, Q., Shi, X.H., Zhao, Q.J., Cui, Y.H., Ouyang, J., Xu, F.: Effect of cooking methods on nutritional quality and volatile compounds of Chinese chestnut (Castanea mollissima Blume). Food Chem. 201, 80–86 (2016)
Barbat, A., Gloaguen, V., Moine, C., Sainte-Catherine, O., Kraemer, M., Rogniaux, H., Ropartz, D., Krausz, P.: Structural characterization and cytotoxic properties of a 4-O-methylglucuronoxylan from Castanea sativa. 2. evidence of a structure-activity relationship. J. Nat. Prod. 71, 1404–1409 (2008)
Moine, C., Krausz, P., Chaleix, V., Sainte-Catherine, O., Kraemer, M., Gloaguen, V.: Structural characterization and cytotoxic properties of a 4-O-methylglucuronoxylan from Castanea sativa. J. Nat. Prod. 70, 60–66 (2007)
Malinowska, E., Krzyczkowski, W., Herold, F., £apienis, G., Ślusarczyk, J., Suchocki, P., Kuraś, M., Tur£o, J.: Biosynthesis of selenium-containing polysaccharides with antioxidant activity in liquid culture of Hericium erinaceum. Enzym Microb. Technol 44, 334–343 (2009)
Potapov, V.A., Musalov, M.V., Amosova, S.V.: Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 52, 4606–4610 (2011)
Qin, T., Chen, J., Wang, D.Y., Hu, Y.L., Zhang, J., Nguyen, T.L., Liu, C., Liu, X.: Optinization of selenylation conditions for Chinese angelica polysaccharide based on immune-enhancing activity. Carbohydr. Polym. 92, 645–650 (2013)
Rayman, M.P.: Selenium and human health. Lancet 379, 1256–1268 (2012)
Duntas, L.H., Benvenga, S.: Selenium: an element for life. Endocrine 48, 756–775 (2015)
Rayman, M.P.: The importance of selenium to human health. Lancet 356, 233–241 (2000)
Chen, W.X., Chen, J.Y., Wu, H.M., Guo, Y.Q., Hu, F.D., Liu, L.J., Gao, X., Zhang, P.: Optimization of selenylation conditions for a pectic polysaccharide and its structural characteristic. Int. J. Biol. Macromol. 69, 244–251 (2014)
Decker, C., Bianchi, C., Decker, D., Morel, F.: Photoinitiated polymerization of vinyl ether-based systems. Prog. Org. Coat. 42, 253–266 (2001)
Wang, J.L., Zhao, B.T., Wang, X.F., Yao, J., Zhang, J.: Synthesis of selenium-containing polysaccharides and evaluation of antioxidant activity in vitro. Int. J. Biol. Macromol. 51, 987–991 (2012)
Zhao, B.T., Zhang, J., Yao, J., Song, S., Yin, Z.X., Gao, Q.Y.: Selenylation modification can enhance antioxidant activity of Potentilla anserina L. polysaccharide. Int. J. Biol. Macromol. 58, 320–328 (2013)
Lü, H.T., Gao, Y.J., Shan, H., Lin, Y.T.: Preparation and antibacterial activity studies of degraded polysaccharide selenide from Enteromorpha prolifera. Carbohydr. Polym. 107, 98–102 (2014)
Liu, J., Chen, X., Yue, C.J., Hou, R.R., Chen, J., Lu, Y., Li, X.P., Li, R.J., Liu, C., Gao, Z.Z., Li, E.T., Li, Y.Y., Wang, H., Yan, Y., Li, H.Q., Hu, Y.L.: Effect of selenylation modification on immune-enhancing activity of Atractylodes macrophala polysaccharide. Int. J. Biol. Macromol. 72, 1435–1440 (2015)
Gao, Z.Z., Chen, J., Qiu, S.L., Li, Y.Y., Wang, D.Y., Liu, C., Li, X.P., Hou, R.R., Yue, C.J., Liu, J., Li, H.Q., Hu, Y.L.: Optimization of selenylation modification for garlic polysaccharide based on immune-enhancing activity. Carbohydr. Polym. 136, 560–569 (2016)
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
Bitter, T., Muir, H.M.: A modified uronic acid carbazole reaction. Anal. Biochem. 4, 330–334 (1962)
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
Liu, X., Sun, Z.L., Zhang, M.S., Meng, X.M., Xia, X.K., Yuan, W.P., Xue, F., Liu, C.H.: Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr. Polym. 90, 1664–1670 (2012)
Wei, D.F., Chen, T., Yan, M.F., Zhao, W.H., Li, F., Cheng, W.D., Yuan, L.X.: Synthesis, characterization, antioxidant activity and neuroprotective effects of selenium polysaccharide from Radix hedysari. Carbohydr. Polym. 125, 161–168 (2015)
Hakomori, S.: A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 55, 205–208 (1964)
Mosman, T.: Rapid colorimetric assay for cellular growth and survival: application to proliferation and antitumor activity assays. J. Immunol. Methods 65, 55–63 (1983)
Zhu, Q.Q., Jiang, Y.M., Lin, S., Wen, L.R., Wu, D., Zhao, M.M., Chen, F., Jia, Y.X., Yang, B.: Structural identification of (1 → 6)-α-D-Glucan, a key responsible for the health benefits of Longan, and evaluation of anticancer activity. Biomacromolecules 14, 1999–2003 (2013)
Liu, J., Wen, X.Y., Kan, J., Jin, C.H.: Structural characterization of two water-soluble polysaccharides from Black Soybean (Glycine max (L.) Merr.). J Agric Food chem 63, 225–234 (2015)
Huang, X.J., Nie, S.P.: The structure of mushroom polysaccharides and their beneficial role in health. Food Funct. 6, 3205–3217 (2015)
Cui, H.X., Liu, Q., Tao, Y.Z., Zhang, H.F., Zhang, L.N., Ding, K.: Structure and chain conformation of a (1 → 6)-α-D-glucan from the root of Pueraria lobata (Willd.) Ohwi and the antioxidant activity of its sulfated derivative. Carbohydr Polym 74, 71–778 (2008)
Sun, L., Peng, X.X., Sun, P., Shi, J.H., Yuan, X.W., Zhu, J.J., Tai, G.H., Zhou, Y.F.: Structural characterization and immunostimulatory activity of a novel linear α-(1 → 6)-D-glucan isolated from Panax ginseng C. A. Meyer. Glycoconj J. 29, 357–364 (2012)
Wu, X.M., Tu, P.F.: Isolation and characterization of α-(1 → 6)-glucans from Cistanche deserticola. J. Asian Nat. Prod. Res. 7, 823–828 (2005)
Zhao, G.H., Kan, J.Q., Li, Z.X., Chen, Z.D.: Characterizaiton and immunostimulatory activity of an (1 → 6)-α-D-glucan from the root of Ipomoea batatas. Int. Immunopharmacol. 5, 1436–1445 (2005)
Zhao, C., Li, M., Luo, Y.F., Wu, W.K.: Isolation and structural characterization of an immunostimulating polysaccharide from fuzi, Axonitum carmichaeli. Carbohydr. Res. 341, 485–491 (2006)
Zhu, W.L., Xue, X.P., Zhang, Z.J.: Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 91, 132–142 (2016)
Pires, A.R.A., Ruthes, A.C., Cadena, S.M.S.C., Acco, A., Gorin, P.A.J., Iacomini, M.: Cytotoxic effect of Agaricus bisporus and Lactarius rufus β-D-glucans on HepG2 cells. Int. J. Biol. Macromol. 58, 95–103 (2013)
Shao, P., Liu, J., Chen, X.X., Fang, Z.X., Sun, P.L.: Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int. J. Biol. Macromol. 73, 124–130 (2015)
Jiang, C.X., Xiong, Q.P., Li, S.L., Zhao, X.R., Zeng, X.X.: Structural characterization, sulfation and antitumor activity of a polysaccharide fraction from Cyclina sinensis. Carbohydr. Polym. 115, 200–206 (2015)
Chen, C., Wu, W.H., Xu, X.J., Zhang, L.N., Liu, Y., Wang, K.P.: Chain conformation and anti-tumor activity of derivatives of polysaccharide from Rhizoma Panacis Japonici. Carbohydr. Polym. 105, 308–316 (2014)
Fernandes, A.P., Gandin, V.: Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 1850, 1642–1660 (2015)
Jiang, W.T., Fu, Y.T., Yang, F., Yang, Y.F., Liu, T., Zheng, W.J., Zeng, L.L., Chen, T.F.: Gracilaria lemanneiformis polysaccharide as integrin-targeting surface detector of selenium nanoparticles to achieve enhanced anticancer efficacy. ACS Appl. Mater. Interfaces 6, 13738–13748 (2014)
Zhang, Z.Z., Liu, W., Zheng, Y., Jin, L., Yao, W.B., Gao, X.D.: SGP-2, an acidic polysaccharide from Sarcandra glabra, inhibits proliferation and migration of human osteosarcoma cells. Food Funct. 15, 167–175 (2014)
Acknowledgements
This work was supported by National Natural Science Foundations of China (21271059 and 31470961), Natural Science Foundation of Hebei province in China (B2015201069; B2015201213), Youth Fund Project of Hebei Education Department (QN2014131), Bureau of Baoding city science and technology project (14ZF083).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 190 kb)
Rights and permissions
About this article
Cite this article
Li, H., Wang, Y., Wang, C. et al. Extraction, selenylation modification and antitumor activity of the glucan from Castanea mollissima Blume. Glycoconj J 34, 207–217 (2017). https://doi.org/10.1007/s10719-016-9753-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9753-4