Abstract
This study aims to understand rhizosphere effects on soil carbon (C) and nitrogen (N) differences of three annual plants (Crassocephalum crepidioides, Ageratum conyzoides and Bidens pilosa) in different fallow ages: 2 years (FP-2), 5 years (FP-5) and 10 years (FP-10) in Mizoram, northeast India. In 2 and 10 years fallows, dominant annual plants were A. conyzoides and B. pilosa respectively, however, in 5 years fallow; dominance equally shared among three species. The rhizosphere soil nutrients (organic carbon—SOC, total nitrogen—TN, microbial biomass C-MBC and N-MBN, NH4-N, NO3-N, N-mineralization rate—Nmin) were significantly (P < 0.05) greater in longer fallow compared to shorter fallow period. Rhizosphere soil of three annual plants showed 11–25%, 10–24%, 28–53% and 49–103% greater SOC, TN, MBC and MBN, respectively in FP-10 compared to FP-2. Similarly, 118–200%, 50–93%, 100–125% and 58–100% greater NO3-N, NH4-N, nitrification and Nmin rates were recorded in these stands. The concentrations of NH4-N, rates of nitrification and Nmin in rhizosphere soil were greater than bulk soil but the reverse was true in case of NO3-N. Difference between rhizosphere and bulk soil in the studied plants across fallow periods ranged from 17 to 63% in SOC, 27 to 64% in MBC, 21 to 70% in MBN, 52 to 80% in NH4-N and 25 to 67% in Nmin. The magnitude of rhizosphere effect of the three annual plants on soil C and N properties differed with fallow length. These results suggest that the microbial activity in rhizosphere soil is largely affected by the plant species and soil fertility level depending on fallow period. The differences in rhizosphere soil C and N largely depends on the soil fertility levels and the ability of the host plants to exploit resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In northeast India, sustainable agricultural production is threatened by soil degradation due to land use changes, particularly shifting cultivation, a traditional form of agriculture, carried out by tribal populations in tropical moist forest. Previously, this practice was ecologically balanced and economically feasible in northeast India because of prolonged fallow periods (> 20 years) and low population densities (Tanaka et al. 2001; Bruun et al. 2009; Grogan et al. 2012). However, in recent years due to substantial increase in population densities along with inaccessibility of limited land in hilly areas, fallow period has drastically reduced (< 5 years), which has posed a serious problem of soil fertility and food security for the farmers (Grogan et al. 2012). This has led to decrease soil fertility, increase soil erosion and reduce crop yields. Therefore, shifting cultivation system with short fallow periods becomes the most unsustainable farming practice in this region. The implementation of different land use practice like agro-forestry, lengthening the age of fallow would be difficult because of initial monetary investment (Tripathi et al. 2017) and thus a low cost technology to improve soil health and crop productivity would be needed to help farmers in the region.
The cycling of soil carbon (C) and nutrients as affected by the rhizosphere processes have been studied extensively for agricultural crops and grasses grown under controlled conditions (Kuzyakov et al. 2000; Jones et al. 2004). Several studies on rhizosphere nutrient cycling of trees have been conducted on tree seedlings in laboratory microcosms with few under the field conditions (Phillips and Fahey 2006). In previous studies, inorganic N in rhizosphere soil in relation to bulk soils has been reported to increase (De Neergaard and Magid 2001), decrease (Wang et al. 2001; Warembourg et al. 2003), or no change (Yanai et al. 2003). Nutrient cycling in rhizosphere of annual plants may greatly differ from that of trees and tree seedlings due to differences in their physiological characteristics like nutrient requirements, capacity to acquire nutrient, growth periods, and environmental conditions (Gobran et al. 1998; Jones et al. 2009). Studies on the rhizosphere processes in annual plants under different land uses are not available. Therefore, there is a strong need for further studies on rhizosphere nutrient cycling of annual plants under different fallow to understand N cycling properties underlying in rhizosphere soil in shifting agriculture. The main objectives of the present study were: (1) to determine the levels of rhizosphere soil C and N of annual plants (Crassocephalum crepidioides, Ageratum conyzoides and Bidens pilosa) in different fallows and (2) to evaluate differences in rhizosphere soil C and N in relation to bulk soil of dominant annual plants under different fallows i.e. 2 years, 5 years and 10 years (hereafter referred as FP-2, FP-5 and FP-10 respectively) in Muallungthu, Mizoram. We hypothesize that rhizosphere soil C and N in annual plants would increase with the length of fallow periods but the magnitude of rhizosphere effect will vary depending on the fallow periods as well as the plant species. This study will improve our understanding on rhizosphere effect on soil C and N in annual plants under different soil condition as affected by fallow length.
Materials and methods
Site description
The study was conducted in three abandoned fallow lands e.g. FP-2, FP-5 and FP-10 in Muallungthu, Mizoram. The geographic position of the study sites were: 23º36′43″ to 23º36′44″ N and 92º43′11″ to 92º43′14″ E and the altitude ranged from 800 to 850 m amsl. Soil of the study site was light to medium textured (sandy to clay loam), falls under red soil group and belongs to order inceptisol, the slope of land varied from ~35 to 45° (Hauchhum and Tripathi 2018, In press). The study site experienced a warm and wet humid tropical climate with mean annual temperature ranged from 16 to 25 °C with a total annual rainfall of 2350 mm. The annual plant species present in these study sites on the basis of dominance were: Crassocephalum crepidioides, Ageratum conyzoides, Bidens pilosa, Chromolaena odorata, Knoxia corymbosa, Cantella asiatica, Oxalis corniculata, Spilanthes acamella, Phyllanthus urinaria, Mikania cordata, Urena lobata, Melastoma nepalensis, Cassia ocidentalis, Costus speciosa.
Soil sampling
At each site a permanent plot of 1 ha area was selected. Within each permanent plot, six sub-plots of 10 m × 10 m at least 30 m apart from each other were established to carryout intensive sampling of soil and vegetation characteristics. These sub-plots were considered as true replicates. Abundances of early regenerating plants were studied by laying five 1 m × 1 m quadrats in each sub-plot in June, 2014. Three most dominant annual plants of the study sites (Crassocephalum crepidioides, Ageratum conyzoides and Bidens pilosa) were selected for further analysis of their rhizosphere and bulk soils C and N properties. These plants were among the early colonizers of the study area. In July 2014, rhizosphere soil and bulk soils of three dominants species listed above were recovered from 20 cm soil depth from each sub-plot (10 m × 10 m) with the help of soil auger. Rhizosphere soil was collected from soil attached to live roots after gentle shaking and with the help of tweezers. The soil remaining in the core not attached to the roots were considered as bulk soil (Phillips and Fahey 2006). Six soil cores were collected from each sub-plot with a total of 36 soil cores from six replicated sub-plots of each fallow land. The soils composited from two sub-plots were composited by thorough mixing of soils from 12 cores. This way three replicated samples were prepared from each site. Live fine roots, debris and stones were removed from the soil at the site. The soil samples were brought to the laboratory and sieved to pass through 2 mm mesh. The soil samples were divided into two parts, one part was air dried for the analysis of soil pH, soil organic carbon (SOC) and total nitrogen (TN) and the other part was used as fresh and kept in a deep freezer at -5 °C for the determination of soil moisture content (MC), microbial biomass carbon (MBC) and nitrogen (MBN), NH4-N and NO3-N content. MBC and MBN were analyzed within 2 weeks to avoid effect of freezing that may alter the soil microbial properties.
Laboratory analysis
Gravimetric soil moisture content (SMC) was estimated by oven drying the known weight of field moist soil. Soil pH was measured with a glass electrode (1:2.5 soils:water ratio) using digital pH meter. SOC was determined by K2Cr2O7 wet-oxidation method (Walkley 1947) and TN was analyzed using CHN analyzer (CHNS-O Elemental Analyzer EUROEA, 3000). Soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were determined using fresh soil samples (25 g) through chloroform–fumigation–extraction method (Brookes and Joergensen 2006). The fumigated and non-fumigated portions of each soil sample were extracted with 100 ml 0.5 M K2SO4. Soil suspensions were filtered through a Whatman No. 42 filter paper. One part (10 ml) of the filtrate was used for the determination of MBC by the methods described above for SOC and the other part for determination of MBN by Micro-Kjeldahl method (Bremner and Mulvaney 1982). For the determination of MBN samples were digested on heating block (KEL Plus, Pelican Equipment, India) at 360 °C for 2 h followed by distillation in the presence of 50 ml of 40% NaOH using an automatic distillation chamber (Classic DX, Pelican Equipment, India). The difference between fumigated and non-fumigated samples in terms of C and N was determined, and the MBC and MBN were calculated using conversion factors KEC = 0.38 and KEN = 0.45, respectively (Jenkinson et al. 2004). Soil NH4-N and NO3-N were determined by phenate method (Wetzel and Likens 1979) and phenol disulphonic acid method (Jackson 1958), respectively. N-mineralization (Nmin) rate was measured in situ by buried bag technique (Eno 1960) where part of soil sample was sealed in ziplock plastic bag and reburied in field at a depth of 20 cm after the removal of organic debris and roots. After one month, the buried bag was retrieved and the soil samples were analyzed for final NH4-N and NO3-N. Nmin was calculated as the difference in the level of soil mineral N (NH4-N + NO3-N) concentrations before and after the incubation for 30 days. Similarly, the nitrification rate was calculated as the difference in NO3-N concentration before and after the incubation for 30 days.
Computation and statistical analysis
Computation for species composition, relative abundances and importance value index (IVI) were calculated as the procedure outlined by Mueller-Dombois and Ellenberg (1974). Diversity indices by Shannon–Wiener and Margalef were calculated using the following formulae:
where pi is proportion of individuals of species
where R is Margalef index. S is the species number in each quadrat and N is the number of all individual species.
All the results were expressed as means ± 1 standard error (1 SE). Analysis of variance (ANOVA) was performed to test the effect of fallow age and annual plant species on soil variables followed by LSD test to compare the means. All statistical analysis was performed using SPSS software package (Version 20.00 for Windows).
Results
Changes in annual plant diversity and richness with fallow length
Species richness and plant diversity were significantly affected by the length of fallow period. Species richness was significantly less (3.86) in short fallow compared to long fallow (5.13). Consequently, Shannon–Wiener plant diversity was less in FP-2 (1.68) and high in FP-10 (1.91) (Fig. 1a). On the basis of Important Value Index (IVI), Ageratum conyzoides was the dominant species (IVI-97%) followed by Bidens pilosa (IVI-62%) and Crassocephalum crepidioides (IVI-42%) in FP-2 stand. However, in FP-10 stand, the dominant species was B. pilosa (IVI-63%) followed by C. crepidioides (IVI-39%) and A. conyzoides (IVI-28%). In FP-5, dominance was equally distributed among the three annual plants species with IVI ranged from 58 to 61% (Fig. 1b).
The level of rhizosphere soil C and N in three annual plants in different fallow
The amount of SOC, TN, MBC and MBN was significantly influenced by length of fallow but soil pH and SMC was not affected (Table 1). Soil of the study site was acidic in reaction with pH ranged from 5.06 to 5.27 and SMC ranged between 17.1 and 23.4% (Table 2). The amount of SOC and TN in rhizosphere of C. crepidioides and B. pilosa was significantly affected by the length of fallow but the same did not vary in A. conyzoides. The amount of SOC and TN in rhizosphere soil of the annual plants ranged from 2.91 to 3.95 mg g−1 and 0.28 to 0.36 mg g−1, respectively (Table 2). The increase was more marked in the amount of MBC (257–423 mg kg−1) and MBN (21.1–39.4 mg kg−1) in different fallows compared to SOC and TN in three species (Table 2).Variations in MBC/MBN ratio in rhizosphere soil of C. crepidioides and A. conyzoides was not significant due to fallow length. However, the ratio varied significantly due to fallow length in the B. pilosa with higher ratio (14.27) in short fallow compared to long fallow (10.34) (Table 2).
The length of fallow period significantly improved the level of NH4-N and NO3-N, and the rates of nitrification and Nmin in the rhizosphere soil of three dominant plants (Table 1). The concentrations of NH4-N and NO3-N increased significantly with fallow length and the value ranged from 0.13 to 0.45 mg kg−1 and 0.44 to 0.91 mg kg−1, respectively. Similarly, the nitrification and Nmin rates in rhizosphere soil of the studied plants ranged from 0.25 to 0.61 mg kg−1 month−1 and 0.49 to 1.11 mg kg−1 month−1, respectively (Table 3).
Differences in soil C and N in rhizosphere soils of annual plants in relation to bulk soil
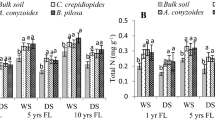
Differences in soil pH, SOC, TN, MBC and MBN in rhizosphere soil compared to bulk soil of three annual plants were presented in Fig. 2. The soil pH significantly decreased in the rhizosphere soil of all plants compared to bulk soil (Fig. 2a). However, the amount of SOC, MBC and MBN significantly differed in rhizosphere soil of annual plants compared to bulk soil. The amount of rhizosphere SOC in C. crepidioides was 33%, 26% and 16.5% greater compared to bulk soil in FP-2, FP-5 and FP-10, respectively. Corresponding level of SOC in A. conyzoides and B. pilosa was: 62.7%, 36%, 27% and 53.5%, 33.5%, 38.5% greater, respectively, in three fallows stands (Fig. 2). Similarly, the amount of TN was also greater in the rhizosphree soil compared to bulk soil with few exception as in case of B. pilosa in FP-2 and A. conyzoides in FP-5 (Fig. 2c). The amount of MBC in the rhizosphere soil was 30–44%, 27–64% and 36–54% greater compared to bulk soil in C. crepidioides, A. conyzoides and B. pilosa, respectively (Fig. 2d). In A. conyzoides, the level of MBN was 69.7%, 37.5% and 20.75% greater in FP-2, FP-5 and FP-10, respectively. Corresponding higher values in the level of MBN in rhizosphere soil of C. crepidioides and B. pilosa was: 44.7%, 40.5% and 22.5%, and 51.3%, 30.5% and 36.25%, respectively in these stands (Fig. 2e).
Changes in (a) soil pH, (b) SOC-soil organic carbon, (c) TN-total nitrogen, (d) MBC-microbial biomass carbon and (e) MBN- microbial biomass nitrogen between bulk soil (BS) and rhizosphere soil of Crassocephalum crepidioides (Cc), Ageratum conyzoides (Ac) and Bidens pilosa (Bp) in three fallow lands (FP-2, 2 years fallow; FP-5, 5 years fallow and FP-10, 10 years fallow)
The concentrations of NH4-N, rate of nitrification and Nmin differed significantly in rhizosphere soil compared to bulk soil (Fig. 3b–d). On the other hand, the concentration of NO3-N was significantly decreased in the rhizosphere soil relative to bulk soil (Fig. 3a). The highest differences in NH4-N between rhizosphere and bulk soil was recorded in FP-2 in A. conyzoides (79.7%) followed by B. pilosa (61.8%) and C. crepidioides (52.5%). Similarly, magnitude of rhizosphere effect in Nmin was greatest in rhizosphere soil of A. conyzoides (25.1–66.7%) followed by C. crepidioides (25.6–51.5%) and B. pilosa (33.5–63.2%). Interestingly, the higher rhizopshere effect was recorded in short fallow compared to long fallow.
Changes in (a) NO3-N, (b) NH4-N, (c) Nitrification rate and (d) N-mineralization rate between bulk soil (BS) and rhizosphere soil of Crassocephalum crepidioides (Cc), Ageratum conyzoides (Ac) and Bidens pilosa (Bp) in three fallow lands (FP-2, 2 years fallow; FP-5, 5 years fallow and FP-10, 10 years fallow)
Discussion
Changes in vegetation composition with fallow periods
The vegetation analysis exhibited the dominance of three annual plants (e.g. A. conyzoides, C. crepidioides and B. pilosa) in bare soil during secondary succession in different fallows (FP-2 to FP-10). This indicates that long-term agriculture activities in the past might have inhibited the proliferation of plants with long-life cycle. On the other hand, annual and bi-annual plants with stable soil seed pools had advantage of accelerating its growth through rapidly exploiting the available soil nutrients and thus, become dominant species by quickly occupying the abandoned land. Further, the dominance of early colonizing plant species (i.e. A. conyzoides and C. crepidioides) in FP-2 and FP-5 was superseded by B. pilosa as fallow period increased (in FP-10) which may be the result of competitive abilities of the species to the altered soil nutrient conditions (Fig. 1b). The increases in plant diversity and species richness with fallow periods were related to increase in soil fertility level and plant biomass in long fallow periods. These findings support that species richness in abandoned land is relatively lower during the early stage of succession that increase till mid-stage and relatively decrease with increasing fallow periods (El-Sheikh 2005; Wang et al. 2009).
Rhizosphere soil C and N differences in different fallow lands
Rhizosphere soil properties of annual plants depend upon fallow periods and soil conditions in which they grow. Lower C and N in rhizosphere soil in short fallows compared to long fallow may be the result of nutrient limited condition (e.g. C and N limitation), however, as a result of increased fallow period, the abiotic stress is benign and utilization of nutrient substrate by microbes is stimulated due to greater root exudation (Zhang et al. 2012). In the present study, greater rhizosphere effects on soil properties (e.g. SOC, N, MBC and MBN) of annual plants in longer fallow may be the result of enhanced microbial metabolic activities due to increased soil nutrient and extensive roots as in FP-10 stand that stimulate the activity of microorganisms in rhizosphere (Hauchhum and Tripathi 2017; Zhang et al. 2012). Increased rhizosphere soil available N (NH4+ –N and NO3− –N) in long fallow compared to short fallow may be the result of enhanced organic matter input and conversion of organic N into mineral N. Rhizosphere priming effect on soil organic matter decomposition have been reported to enhance Nmin and nitrification rate in rhizosphere soil (Colin-Belgrand et al. 2003; Phillips and Fahey 2006). The amount of N in the rhizosphere soil has been reported to be strongly correlated with soil organic matter decomposition and intensely stimulated by root exudates (Jackson et al. 2008). This may also be accountable for greater Nmin rate in longer fallow compared to shorter fallow. Greater microbial biomass C/N ratio in short fallow phase reflects negative effect on nitrification and Nmin rates due to altered rhizosphere microbial composition (higher fungal biomass in relation to bacterial biomass) rather than total microbial biomass (Table 3). This may be considered as an ecosystem level strategy of plants to efficiently utilize the slowly released N from the meager soil organic matter in the short fallow and to avoid its runoff loss on steep slopes. On contrary, decreased microbial biomass C/N ratio in longer fallow indicate dominance of bacterial biomass in relation to fungal biomass. In addition, increase plant species richness during the course of succession (Singh et al. 2015) increases total root exudation in the system that resulted into enhanced rates of Nmin and nitrification in longer fallow.
Rhizosphere soil C and N in relation to bulk soil in three annual plants
Consistent with our hypothesis, variation in rhizosphere effect was observed among the annual plants under different fallows. The present study showed differences in the amount of soil nutrients in their rhizosphere zone which is attributed to differences in microbial composition as well as root exudation rates. In current investigation, differences in the amount of NH4-N, NO3-N, Nmin and nitrification rate in rhizosphere soil with respect to bulk soil were significantly high in dominant species at each stand. This reflected a strong rhizosphere effect of dominant plants like A. conyzoides in FP-2 and B. pilosa in FP-10. Moreover, the extent of nutrient limitation varies among the annual plants studied growing in different fallow length due to differences in their ability to acquire deficient nutrients (Zhao et al. 2010). It shows that during vegetation succession, plants have varying strategy to acquire nutrients (C and N) from soil particularly under nutrient deficient condition in this region for their growth. Changes in rhizosphere effect in Nmin among annual plants were related to variation in quality and quantity of root exudates as well as intrinsic soil characteristics (Jones et al. 2004). It has been hypothesized that plant roots regulate rhizosphere C flow through exudation, recapture of exudes as well as mobilization and acquisition of nutrients from soil and thus rhizopshere effect of different plant species depend on the type and amount of root exudates along with soil properties (Jones et al. 2004). As discussed above, rhizosphere priming effect of soil organic matter may be among the primary factors for differences in the rhizosphere effect in A. conyzoides, C. crepidioides and B. pilosa. Among the dominant species studied B. pilosa rhizosphere exhibit greater soil nutrients than other species. A possible explanation for this result is the low nutrient resources in young fallow for A. conyzoides that probably reduced the microbial utilization of C released by the roots. On the other hand, increased nutrient supplies and better plant growth stimulated the microbial growth and activity in longer fallow. These results imply that soil condition play an important role in altering the microbial community in rhizosphere zone. Pinton et al. (2007) found that soil nutrient condition and root chemistry of the plant have been assumed to be the main factors that regulate the root exudation. Tscherko et al. (2004) also reported that rhizosphere microbiota in early stage of succession is determined by the available resources for microorganisms in the bulk soil rather than by the host plant. Therefore, differences in the amount and type of exudates among annual plants under different soil fertility level in the present study may be principally responsible for C and N dynamic in rhizosphere soil. The current investigation depicts that rhizosphere soil properties primarily depend on soil condition depending on fallow phase rather than the plants.
Conclusions
This study provides information on rhizosphere effects on soil C and N properties of annual plants growing under different fallow periods (FP-2, FP-5 and FP-10) following shifting agriculture in Mizoram, northeast India. The plant species diversity and richness increased from short to long fallow periods. The amount of rhizosphere soil C and N was greater in long fallow compared to short fallow. The magnitude of rhizosphere effect of the annual plants was greater in short fallow compared to long fallow which differ among the plant species suggesting varying capacity of the plants to exploit soil nutrient under limited conditions. This study demonstrates that interaction of rhizosphere microbes changes under different soil fertility levels as affected by the length of fallow. However, rhizosphere processes are rather complicated and need further efforts to identify the interface between plant roots and microbial activity under different soil condition to understand the mechanisms of rhizosphere nutrient cycling.
References
Bremner JM, Mulvaney CS (1982) Nitrogen—total, In: Page AL, Miller RL, Keeny DR (eds) Methods of soil analysis. Part-2 chemical and microbiological properties, 2nd edn. ASA, SSSA, CSSA, Madison, pp 595–613
Brookes PC, Joergensen RG (2006) Microbial biomass measurements by fumigation-extraction. In: Bloem J, Hopkins DW, Benedetti A (eds) Microbiological methods for assessing soil quality. CABI Publishing, Oxfordshire, pp 77–83
Bruun TB, de Neergaard A, Lawrence D, Ziegler AD (2009) Environmental consequences of the demise in swidden cultivation in Southeast Asia: carbon storage and soil quality. Hum Ecol 37:375–388
Colin-Belgrand M, Dambrine E, Bienaimé S, Nys C, Turpault MP (2003) Influence of tree roots on nitrogen mineralization. Scand J Forest Res 18:260–268
De Neergaard A, Magid J (2001) Influence of the rhizosphere on microbial biomass and recently formed organic matter. EurJ Soil Sci 52:377–384
El-Sheikh MA (2005) Plant succession on abandoned fields after 25 years of shifting cultivation in Assuit. Egypt J Arid Environ 61:461–481
Eno CF (1960) Nitrate production in the field by incubating the soil in polyethylene bags. Soil Sci Soc Am 24:277–279
Gobran GR, Clegg S, Courchesne F (1998) Rhizospheric processes influencing the biogeochemistry of forest ecosystems. In: Plant-induced changes: processes and feedbacks, vol 4, pp 107–120
Grogan P, Lalnunmawia F, Tripathi SK (2012) Shifting cultivation in steeply sloped regions: a review of management options and research priorities for Mizoram state, northeast India. Agrofor Syst 64:163–177
Hauchhum R, Tripathi SK (2017) Rhizosphere effects of Melocanna baccifera on soil microbial properties under different fallow phases following shifting cultivation. Int J Plant Soil Sci 17:1–9
Hauchhum R, Tripathi SK (2018) Impact of rhizosphere microbes of three early colonizing annual plants on improving soil fertility during vegetation establishment under different fallow periods following shifting cultivation. Agr Res. https://doi.org/10.1007/s40003-017-0286-2
Jackson ML (1958) Soil chemical analysis. Prentice Hall of India. Pvt. Ltd., New Delhi, pp 1–498
Jackson LE, Burger M, Cavagnaro TR (2008) Roots, nitrogen transformations, and ecosystem services. Annu Rev Plant Biol 59:341–363
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7
Jones DL, Hodge A, Kuzyakov Y (2004) Tansley review: plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil root interface. Plant Soil 321:5–33
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Margalef DR (1958) Information theory in ecology. Gen Syst Yearb 3:36–71
Mueller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley, New York
Phillips RP, Fahey TJ (2006) Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 87:1302–1313
Pinton R, Veranini Z, Nannipieri P (2007) The rhizosphere: biochemistry and organic substances at the soil-plant interface. Taylor & Francis Group LLC, New York
Shannon CE, Weaver WW (1963) The mathematical theory of communication. University Illinois Press, Urbana
Singh SB, Mishra BP, Tripathi SK (2015) Recovery of plant diversity and soil nutrients during stand development in subtropical forests of Mizoram. Northeast India. Biodiversitas 16(2):205–212
Tanaka S, Ando T, Funakawa S, Sukhrun C, Kaewkhongkha T, Sakurai K (2001) Effect of burning on soil organic matter content and N mineralization under shifting cultivation system of Karen people in northern Thailand. Soil Sci Plant Nutr 47:547–558
Tripathi SK, Vanlalfakawma DC, Lalnunmawia F (2017) Shifting cultivation on steep slopes of Mizoram, India: impact of policy reforms. In: Cairns M (ed) Shifting cultivation policies: balancing environmental and social sustainability. CABI International, Wallingford, Oxfordshire, 393–413
Tscherko D, Ute H, Marie-Claude M, Ellen K (2004) Shifts in rhizosphere microbial communities and enzyme activity of Poa alpina across an alpine chronosequence. Soil Biol Biochem 36:1685–1698
Walkley A (1947) Critical examination of rapid method for determining organic carbon in soils, effect of variation in digestion conditions and of inorganic soil constituents. Soil Sci 632:251
Wang ZY, Göttlein A, Bartonek G (2001) Effects of growing roots of Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica L.) on rhizosphere soil solution chemistry. J Plant Nutr Soil Sci 164:35–41
Wang GL, Liu GB, Xu MX (2009) Above and belowground dynamics of plant community succession following abandonment of farmlands on the Loess Plateau, China. Plant Soil 316:227–239
Warembourg FR, Roumet C, Lafont F (2003) Differences in rhizosphere carbon partitioning among plant species of different families. Plant Soil 256:347–357
Wetzel RG, Likens GE (1979) Limnological analyses. W.B. Saunders Company, Philadelphia, pp 915–1156
Yanai RD, Majdi H, Park BB (2003) Measured and modelled differences in nutrient concentrations between rhizosphere and bulk soil in a Norway spruce stand. Plant Soil 257:133–142
Zhang C, Liu G, Xue S, Zhang C (2012) Rhizosphere soil microbial properties on abandoned croplands in the Loess Plateau, China during vegetation succession. Eur J Soil Biol 50:127–136
Zhao Q, Zeng DH, Fan ZP (2010) Nitrogen and phosphorus transformations in the rhizospheres of three tree species in a nutrient-poor sandy soil. Appl Soil Ecol 46:341–346
Acknowledgement
We thank University Grants Commission and Department of Biotechnology, New Delhi for financial support. We also thank farmers for providing lands and their heartfelt co-operation in completion of this work. Department of Forestry, Mizoram University is thankfully acknowledged for providing laboratory facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hauchhum, R., Tripathi, S.K. Carbon and nitrogen differences in rhizosphere soil of annual plants in abandoned lands following shifting agriculture in northeast India. Nutr Cycl Agroecosyst 113, 157–166 (2019). https://doi.org/10.1007/s10705-019-09972-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-019-09972-5