Abstract
The seasonal fluxes of CO2 and its characteristics with relation to environmental variables were investigated under tropical lowland flooded rice paddies employing the open path eddy covariance technique. The seasonal net ecosystem carbon budget was quantified by empirical modelling approach. The integrated net ecosystem exchange (NEE), gross primary production (GPP) and ecosystem respiration (RE) in the flooded rice field was −448, 811 and 363 g C m−2 in wet season. Diurnal variations of mean NEE values during the season varied from +3.99 to −18.50 μmol CO2 m−2 s−1. The daily average NEE over the cropping season varied from +2.73 to −7.74 g C m−2 day−1. The net ecosystem CO2 exchange reached its maximum in heading to flowering stage of rice with an average value of −5.67 g C m−2 day−1. On daily basis the flooded rice field acted as a net sink for CO2 during most of the times in growing season except few days at maturity when it became a net CO2 source. The rate of CO2 uptake by rice as observed from negative NEE values increased proportionally with air temperature up to 34 °C. The carbon distribution in different component of soil-plant system namely, soil organic carbon, dissolved organic carbon, methane emission, rhizodeposition, carbon in algal biomass, crop harvest and residues were quantified and carbon balance sheet was prepared for the wet season in tropical rice. Carbon balance sheet for tropical rice revealed 7.12 Mg C ha−1 was cycled in the system in wet season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quantification of greenhouse gas exchanges between the ecosystem and the atmosphere is one of the key issues to assess the global budget of greenhouse gases. Rice is the major food crop in Asia and about 80 % of it is grown under flooded conditions (Towprayoon et al. 2005). Rice is grown in different environments ranging from tropical to temperate regions with varying climatic, edaphic and biological conditions and under different agricultural management practices which naturally affect the rates of CH4 and CO2 emissions. According to the Food and Agricultural Organization of the United Nations (FAO), the area of rice paddies in Asia is about 87 % of the world’s total rice cultivated area (Pakoktom et al. 2009). Rice paddies in tropical low land flooded soil play a crucial role in the global budget of greenhouse gases such as CO2 and CH4 (IPCC 2007). Many of the factors controlling gas exchanges between rice paddies and the atmosphere are different from dry land agriculture and other ecosystems because rice is flooded during most of its cultivation period. Therefore, field studies to measure net CO2 fluxes from flooded soil and to improve the understanding of the factors controlling the fluxes are important.
The eddy covariance (EC) technique is widely employed as the standard micrometeorological method to monitor fluxes of CO2, water vapour and heat, which are bases to determine CO2 and heat balances of land surfaces (Aubinet et al. 2000). Long-term observations of CO2 flux have been carried out in various ecosystems in the world. These observation sites are mostly located in forest ecosystems (Saigusa et al. 2002; Carrara et al. 2003). On the other hand, some studies in non-forest ecosystems, viz. grasslands, wetlands and agricultural fields have also been observed because of their contribution to regional and global CO2 budgets (Saito et al. 2005; Tsai et al. 2006). In Asia, EC flux measurements were conducted in Japan (Miyata et al. 2000, 2005; Saito et al. 2005), Thailand (Pakoktom et al. 2009), China (XiuE et al. 2007; Hossen et al. 2010), South Korea (Moon et al. 2003), Bangladesh (Hossen et al. 2007; Hossen et al. 2011), Philippines (Alberto et al. 2009) and Taiwan (Tseng et al. 2010) to monitor seasonal, annual and inter-annual variations in CO2 fluxes in irrigated and aerobic rice fields. Most of the above studies were limited to the temporal variation of CO2 exchange in relation to meteorological parameters and crop harvest. But understanding the processes and components of net ecosystem carbon budget (NECB) in lowland flooded rice paddy is essential to know whether the system is behaving as net C sink (net carbon accumulation in the system) or C source (net carbon depletion or loss from the system). Agricultural activities involving the addition of C inputs in the form of organic manures and other associated phenomena (rhizodeposition, aquatic biomass, CO2–C fixation in the form of NEP, decayed roots and stubbles left in the field) and C output through crop harvest and gaseous C emission (CH4 and CO2). It is very important to quantify the balance between these two processes in order to get a clear insight of C cycling under lowland flooded rice paddy ecosystem for maintaining ecosystem health and sustainability.
To address these issues, the present study was undertaken to understand the season-long net ecosystem exchange (NEE), gross primary production (GPP) and ecosystem respiration (RE) and its characteristics in relation to environmental variables and to quantify the carbon budget of the ecosystem by considering the possible inflow and outflow of carbon in tropical lowland flooded rice paddies.
Materials and methods
Site description
The study was conducted at the experimental farm of the Central Rice Research Institute (CRRI), Cuttack, India (85°56′25″ E, 20°27′6″ N, and 24 m above mean sea level). Mean annual highest and lowest temperatures were 39.2 and 22.5 °C, respectively and mean annual temperature is 27.7 °C. Average annual rainfall is 1,500 mm, of which 75–80 % is received during June to September. The difference between mean summer and mean winter soil temperature is more than 5 °C, thus qualifying as a hypothermic temperature class. The soil is an Aeric Endoaquept with sandy clay loam texture (25.9 % clay, 21.6 % slit, 52.5 % sand), bulk density 1.41 Mg m−3, pH (1:2.5 soil: solution ratio) 6.2, electrical conductivity 0.40 dSm−1, total carbon (TC) 1.12 % and total nitrogen (TN) 0.08 %.
Crop establishment
The study was conducted during wet season (July to October) in rice-fallow-rice cropping sequence. Flooded rice field (2.25 ha (150 m × 150 m)) was uniformly planted with low land rice variety. Thirty days old seedlings were transplanted on 8th July, 2010 with a spacing of 15 cm × 15 cm. The field was remained flooded with 12 ± 3 cm water depth throughout the growing season up to 2 weeks before harvest. Three split doses of N (40–20–20 kg ha−1) fertilizer were applied at basal, maximum tillering (MT) and panicle initiation (PI) stages. Compost was applied to the experimental plot at a rate of 5 t ha−1 during field preparation. After harvest the field was kept fallow for 2 months allowing the ratoons to grow on the field till mid January just prior to the next dry season’s field preparation. The site was flat and had enough fetch for micrometeorological flux measurements which is required for foot print of the measured fluxes.
Eddy covariance flux measurement, quality control, gap-filling of NEE data and flux partitioning
The eddy covariance system was installed in the middle of a 2.25 ha lowland flooded rice field. Flux densities of CO2 over the rice canopy were measured by the open path eddy covariance technique. A sonic anemometer (CSAT3, Campbell Scientific. Corp., Canada) measured three-dimensional wind speed and sonic temperature. To measure the fluctuations in CO2 and water vapour densities, an open path infrared gas analyzer (LI-7500, LICOR Inc., USA) was used. Both the sensors, CSAT3 and LI-7500 were installed at 3.0 m height on a tripod aluminium mast. The LI-7500 was set back from CSAT3 to minimize flow distortions and the head was tilted about 12° from vertical to minimize the amount of precipitation that accumulated on the window. The prevalent wind was blowing from south west direction to the flux measurement sensors. Net ecosystem CO2 exchange (NEE) was calculated as the sum of eddy CO2 flux (Fc) and CO2 storage change (Fs) within air space below flux-measuring height. The Fs was neglected for the NEE calculation because canopy height was relatively low at less than 1.3 m. The net gain or loss of carbon from an ecosystem is defined as net ecosystem production (NEP) and results from the gain of carbon from autotrophic organisms (gross primary production, GPP) minus its loss from autotrophic (Ra) and heterotrophic (Rh) respiration:
Eddy covariance measurements and meteorological data were recorded for the entire 24-h throughout the season. The mean vertical flux density of CO2 was obtained as the 30 min covariance between vertical fluctuations (ω′) and the CO2 mixing ratio (C′) (Baldocchi 2003):
where ρa refers to air density, the overbars denote time averaging and the primes represent fluctuations of average value. A positive covariance between ω′ and C′ indicates net CO2 transfer into the atmosphere and a negative value indicates net CO2 absorption by the vegetation. The 30 min average of NEE was used for calculation (Massman and Lee 2002).
The footprint analysis was done to confirm and ensuring that the measured fluxes were representative of the plots of interest. The model of Schuepp et al. (1990) was used which measured the cumulative normalized contribution to the surface flux from the upwind location. The eddy flux in the flooded field came from an average of 141 m during daytime and extended up to an average of 145 m during night.
The NEE were sampled at 10 Hz using a data logger programme performed all the processing online and in real time. It applied cross products (second moments) required for offline coordinate rotation following Kaimal and Finnigan (1994) and Tanner and Thurtell (1969) as well as Webb et al. (1980) term for CO2 flux. Quality checks of eddy covariance flux data were done to eliminate the badly affected data by rainfall, instrument malfunction and inappropriate meteorological conditions. The spike detection and storage correction (Papale et al. 2006), U* filtering (Reichstein et al. 2005; Papale et al. 2006) were performed on the datasets. The friction velocity (U*) threshold for night time data was 0.1 m s−1. Gap-filling of missing and or discarded data were done by “look-up” table approach (Falge et al. 2001) in order to estimate seasonal NEE.
For flux partitioning (i.e., NEE into GPP and RE) of eddy data, rectangular hyperbola (Ruimy et al. 1995) method was used which depended on α (apparent ecosystem quantum yield), β (NEE at light saturation maximum CO2 uptake rate), γ (estimate of ecosystem respiration, RE), and Q (PAR, photosynthetically active radiation) with the help of following equation:
The GPP was calculated by using the equation
Meteorological, soil and plant parameters
Air temperature and relative humidity were measured at 3 m height with temperature-humidity sensors (HMP45C, Campbell Scientific Corp., Canada) on half-hourly basis. The net radiation was measured with the help of net radiometer (NR LITE, KIPP and ZONEN, the Netherlands) installed at 2 m height above the soil surface. Soil temperature, soil heat flux and volumetric water content at 15 cm soil depth were measured on a half-hourly basis using soil and water temperature probe (107 B), heat flux probe (HFT3 transducer) and water content reflectometer (CS 616-L) (Campbell Scientific Corp., Canada), respectively. Vapour pressure deficit (VPD) was estimated from the difference between saturated vapour pressure and actual vapour pressure which were monitored (LI-7500) on half-hourly basis. Leaf area index (LAI) was periodically measured by digital LAI meter (M/S Delta T devices, UK) at different crop growth stages.
Estimation of parameters for net ecosystem carbon budgeting
The net ecosystem carbon budget (NECB) was estimated by partitioning organic carbon into several components in flooded rice paddy ecologies to quantify carbon accumulation and loss from the ecosystem using following equation (Smith et al. 2010):
where, NEP was net ecosystem production, D was the carbon loss as dissolved organic carbon, F was the loss of carbon by fire, H was the carbon loss by harvest, VOC was the carbon loss by volatile organic compound, methane (CH4) was the carbon loss as microbially produced methane, E was the carbon loss by erosion and eluviation, I was the addition of carbon from organic manure and other sources (compost, rice roots and stubbles of previous season, rhizodeposition and algal biomass of present season).
Carbon inputs
The NEP was estimated through eddy covariance system (described in previous section). Total carbon content of the added compost on dry weight basis was measured by using the organic elemental analyzer (Thermo Scientific, Flash 2000). Carbon input from rhizodeposition was estimated by using conversion factor of 15 % of total above ground biomass at harvest (Mandal et al. 2008). The rice roots and stubble left over in field of previous season, and plant biomass (root, shoot and grain) of present season were measured from ten replications (1 m2 area each), randomly selected from fetch area of eddy covariance system. The default value of algal C load i.e. 0.70 Mg C ha−1 (Mandal et al. 2008) in tropical rice was used for carbon balance calculation.
Carbon output
To estimate dissolved organic carbon (DOC), the soil-water solution were collected at 3–6 days intervals coinciding the date of CH4 estimation throughout the cropping season in ten replications. The samples were passed through 0.45 μm filter and air dried. The total C of the samples was measured by organic elemental analyzer (Thermo Scientific, Flash 2000) (Zsolnay 2003).
Methane fluxes were measured by manual closed chambers at close periodical intervals of 3–6 days from study site. Samplings for CH4 flux measurements (3 replications) were done from flooded rice field in the morning (09:00–09:30 h) and afternoon (15:00–15:30 h), and the average of the morning and afternoon fluxes was used as the flux for the day. The samplings were done at 6, 11, 16, 20, 25, 31, 37, 41, 46, 52, 57, 61, 67, 73, 79, 85, 91, 97, 103, 108, 112, and 116 days after transplanting (DATs) of the rice crop encompassing the all growth phase of the crop. For measuring CH4 emissions, six rice hills were covered with a locally fabricated Perspex chamber (53 cm × 37 cm × 51 cm, length × width × height from seedling to tillering, and 53 cm × 37 cm × 71 cm, length × width × height from maximum tillering to maturity stages). A battery-operated air circulation pump with an air displacement of 1.5 L min−1 (M/s Aerovironment Inc., Monrovia, CA, USA) and connected to polyethylene tubing was used to mix the air inside the chamber and draw the air samples into Tedlar gas-sampling bags (M/s Aerovironment Inc.) at fixed intervals of 0, 15 and 30 min. Methane concentrations of gas samples from the sampling bags were analyzed by gas chromatography (Chemito, CERES 800 plus, M/s Thermo Scientific) equipped with a flame ionization detector (FID) and Porapak Q column (6 feet long, 1/8 inch outer diameter, 80/100 mesh size, stainless steel column). The temperature of the injector, column and detector was maintained at 150, 50 and 230 °C, respectively. The carrier gas (nitrogen) flow was maintained at 15 mL min−1. The gas chromatograph was calibrated before and after each set of measurements by using 1.2 and 1.8 μL CH4 L−1 in N2 (M/s Chemtron Science Laboratories, India) as the primary standard. Fluxes of CH4 were calculated by successive linear interpolation of the average emissions on the sampling days, assuming that the emissions followed a linear trend during the periods when no sampling was done (Datta et al. 2009; Bhattacharyya et al. 2012a, b). Cumulative CH4 emissions for the entire cropping period were computed by plotting the flux values against the days of sampling and were expressed as Mg CH4–C ha−1.
Total harvest C removal was estimated from shoot and grain yield after harvest (mentioned in ‘carbon input’ section). Eluviation, erosion and leaching component were not estimated it was derived from net ecosystem C budget equation (Eq. 4).
Total organic carbon of soil
Total organic carbon in soil (TOC) before and after the season were measured by taking composite soil samples (ten replications in the study area) by a sample probe (8 cm diameter) from a depth of 0–15 cm. Immediately after sampling, excess water was allowed to drain off, visible root fragments and stones were removed manually and transferred to the laboratory for analyses. The fresh soil was air-dried until constant weight reached, sieved through a 2 mm mesh, mixed and stored in sealed plastic jars for analyses. The TOC of the soil samples were measured by dry combustion method using organic elemental analyzer (Thermo Scientific, Flash 2000).
Results and discussion
Diurnal and seasonal variation of NEE, GPP and RE
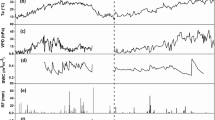
Over the season, diurnal variations of mean NEE varied from +3.99 to −18.50 μ mol CO2 m−2 s−1, where, positive sign indicated net CO2 emission into the atmosphere and negative sign denote net CO2 assimilation or uptake by the crop. The rice paddy ecosystem was behaving as a CO2 source during night hours and a CO2 sink during the day. Almost over the entire season, on daily basis, rice crop behaved as net CO2 sink except few days during the maturity period. Throughout the cropping season on the basis of average diurnal NEE, maximum CO2 assimilation or uptake by rice crop was found at 11.00 h and maximum emission was observed at 4.00 h. The daily average NEE over the cropping season varied from +2.63 to −7.47 μ mol CO2 m−2 s−1, i.e., +2.73 to −7.74 g C m−2 day−1. The NEE was lower in stage I (vegetative stage, average −2.36 g C m−2 day−1), then gradually decreased in stage II (tillering to panicle initiation stage, average −3.98 g C m−2 day−1) and III (reproductive stage, average −5.46 g C m−2 day−1) and reached its maximum in the stage IV (heading to flowering stage, average −5.67 g C m−2 day−1), and then started to increase in stage V (ripening stage, average −4.00 g C m−2 day−1) and VI (harvest stage, average −3.15 g C m−2 day−1) (Fig. 1a–f). The eddy covariance based CO2 flux monitoring was done for 111 consecutive days (6–116 days after transplanting) and the integrated season-long NEE value was–448 g C m−2. The GPP and RE varied from −5.74 to −8.99 g C m−2 day−1 and 2.89 to 3.86 g C m−2 day−1, respectively at different growth stages. The highest GPP (−8.99 g C m−2 day−1) and RE (3.86 g C m−2 day−1) were recorded at the reproductive stage and maximum tillering to panicle initiation stage, respectively (Fig. 1a–f).
It was evident from the study that the amplitude of the daily variation in NEP increased as leaf area index (LAI) at different growth stages increased (vegetative stage LAI 1.2, NEP +2.36 g C m−2 day−1; tillering to panicle initiation stage LAI 3.5, NEP +3.98 g C m−2 day−1; reproductive stage LAI 4.8, NEP +5.46 g C m−2 day−1; heading to flowering stage LAI 5.5, NEP +5.67 g C m−2 day−1; ripening stage LAI 4.6, NEP +4.00 g C m−2 day−1 and harvest stage LAI 2.9, NEP +3.15 g C m−2 day−1) and reached its peak around the heading to flowering stage and, then on, decreased gradually till maturity due to leaf senescence or reduction in leaf greenness during maturity. This is supported by the study of Pakoktom et al. (2009) and Patel et al. (2011). Net exchanges of CO2 between rice paddies and the atmosphere are controlled by several biological and physical processes. During the day time, NEE is the difference between the CO2 uptake from plant photosynthesis and the CO2 emitted through respiration of plant and soil. Respiration at night led to an efflux of CO2 to the atmosphere in absence of photosynthesis. Same type of results were found by Alberto et al. (2009) in Philippines, Tseng et al. (2010) in Taiwan and Miyata et al. (2000) in Japan in rice ecologies. CO2 uptake declined through the afternoon hours due to reduction in leaf level gas exchange (Larcher 1995; Goulden et al. 2004). Afternoon depression might be due to stomatal response to vapour pressure deficit, low leaf water potential or retarding photosynthesis due to relatively increased temperature (Jones 1992; Larcher 1995).
Effect of net radiation, PAR on GPP
During the study period diurnal mean net radiation varied from −36.89 (19.00 h) to 448.98 W m−2 (11.00 h) (mean value 106.67 W m−2) (Fig. 2a). After sun rise, due to incoming solar radiation it gradually started to increase and reached its peak at 11.00 h and then started to decline gradually due to inclined radiation. Significantly (p < 0.01) positive correlation (r = 0.69**, 0.61**) was found between net radiation and PAR with GPP (Table 1a). As a whole, the NEE becomes more negative during the day because of increasing CO2 uptake through photosynthesis (increase in GPP) as net radiation increases (Alberto et al. 2009; Saito et al. 2005).
Effect of vapour pressure deficit on GPP
Vapour pressure deficit significantly influenced the GPP during the study. The daily average VPD in the study area was 0.722 kPa (varied between 0.234 to 1.136 kPa) (Fig. 2c). The lowland rice field was flooded with 12–15 cm of standing water that increased the vapour pressure in the canopy-soil system of rice (Fig. 2b), which in turn, influenced VPD and consequently the GPP. Increasing VPD caused partial stomatal closure which resulted in reduction in photosynthesis and thereby reducing GPP (Saitoh and Ishihara 1987; Mahrt and Vickers 2002; Pakoktom et al. 2009). On the other hand, VPD increases air temperature that leads to increase in respiration and thereby reduces the NEE and GPP (Alberto et al. 2009).
Effect of soil moisture on GPP
Volumetric soil moisture content was not significantly correlated with GPP as there was no moisture stress most of the time of crop growing season (Table 1a). Soil CO2 efflux might have been enhanced due to microbial decomposition of soil organic matter in submerged soils. The magnitude and duration of CO2 efflux depends on the amount and duration of precipitation (Huxman et al. 2004; Sponseller 2007), during moisture stress condition, which in our study was only limited to last 15 days before harvest of the crop. It actually depend on antecedent soil moisture conditions and reflects trade-offs between autotrophic (net CO2 uptake) and heterotrophic (net CO2 release) contributions (Potts et al. 2006). In this study, as the rice fields remained submerged most of the times during the crop season, the magnitude of the CO2 efflux strongly depended on the ratio of activated autotrophic to heterotrophic soil organisms and on the actual wetting depth.
Effect of soil temperature on RE
The diurnal mean soil temperature at 15 cm soil depth varied between 28.9 to 30.6 °C (Fig. 2d) depending on the intensity of the incoming solar radiation, soil characteristics and moisture conditions. Standing water of 12–15 cm was maintained in the field most of the times during the cropping season except few days during maturity. Due to standing water in the lowland (flooded) rice fields, the soil temperature did not vary significantly throughout the cropping season up to 14 days before harvest as standing soil water acted as an insulator (Mowjood et al. 1997). Significant relationship (r = 0.05**) was existed between soil temperature and RE (Table 1b).
Effect of soil heat flux on RE
Diurnal variation of average soil heat flux at 15 cm soil depth showed a negative trend from 21.00 h to 11.30 h and positive trend from 12.00 h to 20.30 h and the average value ranged from −8.53 W m−2 (7.30 h) to +12.04 W m−2 (15.30 h) (Fig. 2e). In tropical flooded rice ecology the heat flux became important as high temperatures prevailed with high relative humidity (71–98 %, in the study site) at most of the growth stages during kharif. However, in this study non-significant correlation was found between soil heat flux and RE (Table 1b).
Effect of air temperature on NEE and RE
During the study period diurnal mean air temperature was in the range of 28.4–32.1 °C (Fig. 2f). Whereas, the daily mean values of air temperature varied from 24.6–32.2 °C (data not presented). Maximum CO2 uptake was observed between 22 and 34 °C and its uptake gradually decreased beyond 34 °C (Fig. 3). The increase of the air temperature above 34 °C increased NEE (less negative value) by decreasing photosynthetic rate in flooded rice ecology (Fig. 3). Decline in photosynthesis due to onset of stress triggering into circadian rhythm or stomatal response to higher temperature (>34 °C) and increased latent heat. Air temperature showed significant positive correlation (r = 0.12**) with RE during the course of study (Table 1b).
Photosynthesis is particularly sensitive to heat stress. The sensitivity of photosynthesis to heat stress is associated with the inactivation of the enzyme Rubisco involved in the Calvin cycle. The enzyme Rubisco catalyzes the first major step of carbon fixation and is temperature sensitive (Crafts-Brandner and Salvucci 2000). Warm temperature stimulates photorespiration and ordinary respiration in plants. Thus, most plants drop their photosynthetic activity at higher temperature. Decline in CO2 uptake may also be due to stomatal closure in response to evaporative demand or a change in photosynthetic biochemistry in response to increased temperature or a circadian rhythm.
The regression model between selected environmental variables on GPP and RE
The environmental factors primarily affecting GPP are PAR, VPD, temperature and soil moisture, whereas, RE is influenced by air or soil temperature, soil moisture and soil heat flux. The observed results showed that the GPP and RE varied in diurnal and seasonal time scales due to leaf gas exchange and pattern of light interception by the canopy (Ruimy et al. 1995; Jarvis and Leverenz 1983). The analysis of the observed results of GPP, RE and environmental factors over flooded rice paddy ecosystem in the growing season revealed that CO2 absorption in the daytime (morning) was higher than that of in the afternoon hours. Plant photosynthesis during the afternoon hours was inhibited by the higher temperatures (resulting in low enzyme activity) and vapour pressure deficit (causing stomatal closure) and thus carbon absorption was restrained during afternoon. Mid-day photosynthesis inhibition is a self-protection mechanism of plants adapting to drought stress (Matos et al. 1998).
The regression models of GPP and RE with the environmental variables like PAR, VPD, air temperature (AT), soil moisture (SM) and soil heat flux (SHF) were:
The model implies that GPP was positively correlated with AT and PAR, whereas, it was negatively correlated with VPD. However, RE was positively correlated with AT and SM but negatively correlated with SHF.
Partitioning of carbon cycling and budgeting in rice-ecosystem
The net ecosystem production estimated during the season was in the order of +4.48 Mg C ha−1. The NEP is equal but opposite in sign to NEE (NEE = −NEP; Schmid et al. 2000; Barker and Griffis 2005; Black et al. 2007). As plants exchanged most of their carbon as CO2, so eddy flux-derived NEP was an ideal variable for C budgeting as well as C cycling in flooded rice paddy ecosystem (Baldocchi 2003). Therefore, NEP (4.48 Mg C ha−1) after completion of wet season was carbon input in the system. Addition of carbon inputs from stubble and root biomass (residue left in the field of the previous season), rhizodeposition, algal biomass, compost addition and NEP were 0.17, 0.87, 0.68, 0.70, 0.22, 4.48 Mg C ha−1, respectively totalling of about 7.12 Mg C ha−1 (Fig. 4). But, the stored carbon was released and lost from the system due to several farm practices and crop biochemical and physiological processes. Carbon removal after harvest through rice grain, straw, stubble and root was 5.76 Mg C ha−1 (Fig. 4). The cumulative CH4-C and DOC losses of the season were in the order of 0.08, 0.93 Mg C ha−1 (Fig. 5). Therefore, C output (as grain and biomass yield plus CH4–C and DOC) from the system during the study period was 6.77 Mg C ha−1. As crop residue, stubble burning was not done, the gaseous carbon loss due to fire was not included during balancing the input and output components in NECB model (Eq. 5). Volatile organic carbon (VOC) losses from lowland flooded rice ecosystem were assumed to be negligible (Smith et al. 2010). As, total organic carbon content in the soil (upper 15 cm surface) was not changed significantly before and after the experiment (data not presented), the balancing between the carbon input and output [7.12 − (5.76 + 0.93 + 0.08) = 0.35 Mg C ha−1] of the system were done by considering the losses from erosion and eluviations (leached from upper layer of profile to lower layer) (Smith et al. 2010).
Conclusion
The season-long study revealed that the low land rice fields have the capacity to sequester carbon from the atmosphere. NEE exhibited a clear diurnal pattern throughout the season. It showed day time uptake and night time release of carbon dioxide by the rice canopy. It is evident that the flooded rice paddy ecosystem behaved as net CO2 sinks. Carbon distribution in different soil-plant system revealed that 63, 9.5 and 10 % of total carbon entered into the system through net ecosystem production, rhizodeposition and algal biomass, respectively. Whereas, 81 and 14 % of total C removal from the system through crop harvest and combined process of dissolved organic carbon leaching and methane emission, respectively. Whereas, most of the studies in Bangladesh, Philippines, Japan, India and other Asian countries concentrated on temporal and spatial variation of GPP, NEE, RE in relation to meteorological variable, the present study gave emphasis on system based C balancing incorporating NEP as a major component. However, a long term study incorporating different cultivars and exhaustive soil and gaseous C component analysis could give clearer picture of net ecosystem carbon budget.
References
Alberto RCM, Wassmann R, Hirano T, Miyata A, Kumar A, Padre A, Amante M (2009) CO2/heat fluxes in rice fields: comparative assessment of flooded and non-flooded fields in the Philippines. Agric For Meteorol 149:1737–1750
Aubinet M, Grelle A, Ibrom A, Rannik U, Moncrieff J, Foken T, Kowalski AS, Martin PH, Berbigier P, Bernhofer CH, Clement R, Elbers J, Granier A, Grunwald T, Morgenstern K, Pilegaard K, Rebmann C, Snijders W, Valentini R, Vesala T (2000) Estimates of the annual net carbon and water exchange of forests: the EUROFLUX methodology. Adv Ecol Res 102:113–175
Baldocchi DD (2003) Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystem: past, present and future. Glob Change Biol 9:479–492
Barker JM, Griffis TJ (2005) Examining strategies to improve the carbon balance of corn/soyabean agriculture is using eddy covariance and mass balance techniques. Agric For Meteorol 128:163–177
Bhattacharyya P, Roy KS, Neogi S, Adhya TK, Rao KS, Manna MC (2012a) Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil Till Res 124:119–130
Bhattacharyya P, Roy KS, Neogi S, Chakravorti SP, Behera KS, Das KM, Bardhan S, Rao KS (2012b) Effect of long term application of organic amendment on C storage in relation to global warming potential and biological activities in tropical flooded soil planted to rice. Nutr Cyc Agroecosyst. doi:10.1007/s10705-012-9540-y
Black K, Bolger T, Darvis P, Nieuwenhuis M, Reidy B, Saiz G, Tobin B, Osborne B (2007) Inventory and eddy covariance-based estimates of annual carbon sequestration in a Sitka spruce (Picea sitchensis (Bong.) Carr.) forest ecosystem. Eur J For Res 126:167–178
Carrara A, Kowalsk AS, Neirynck J, Janssens IA, Yuste JC, Ceulemans R (2003) Net ecosystem CO2 exchange of mixed forest in Belgium over 5 years. Agric For Meteorol 119:209–227. doi:10.1016/S0168-1923(03).00120-5
Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA 97(24):13430–13435
Datta A, Nayak DR, Sinhababu DP, Adhya TK (2009) Methane and nitrous oxide emissions from an integrated rainfed rice-fish farming system of Eastern India. Agric Ecosyst Environ 129:228–237
Falge E, Baldochhi D, Olso R (2001) Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric For Meteorol 107:43–69
Goulden ML, Miller SD, da Rocha HR, Menton MC, de Freitas HC, Figueira AMS, de Sousa CAD (2004) Diel and seasonal patterns of tropical forest CO2 exchange. Ecol Appl 14(4):S42–S54
Hossen MS, Baten MA, Khatun R, Khan MB, Mano M, Ono K, Miyata A (2007) Establishment of a flux study site in Bangladesh with its preliminary observation result. AsiaFlux Newsletter (special issue)
Hossen MS, Hiyama T, Tanaka H (2010) Estimation of nighttime ecosystem respiration over a paddy field in China. Biogeosci Discuss 7:1201–1232
Hossen MS, Mano M, Miyata A, Baten MA, Hiyama T (2011) Seasonality of ecosystem respiration in a double-cropping paddy field in Bangladesh. Biogeosci Discuss 8:8693–8721.
Huxman TE, Cable JM, Ignance DD, Eilts JA, English NB, Weltzin J, Williams DG (2004) Response of net ecosystem gas exchange to a simulated precipitation pulse in semi-arid grassland: the role of native versus non-native grasses and soil texture. Oecologia 141:295–305
Intergovernmental Panel on Climate Change (IPCC) (2007) Climate change: the scientific basis. Cambridge University Press, Cambridge
Jarvis PG, Leverenz JW (1983) Productivity of temperate, deciduous and evergreen forests. In: Land OL, Nobel PS, Osmond CB, Ziegler H (eds) Ecosystem processes: mineral cycling, productivity and man's influence. Physiological plant ecology: new series, vol 12D. Springer, New York, pp. 233–280
Jones HG (1992) Plants and microclimate: a quantitative approach to environmental plant physiology, 2nd edn. Cambridge University Press, Cambridge
Kaimal JC, Finnigan JJ (1994) Atmospheric boundary layer flows. Oxford University Press, New York, p 289
Larcher W (1995) Physiological plant ecology: ecophysiology and stress physiology of functional groups, 3rd edn. Springer, Berlin
Mahrt L, Vickers D (2002) Relationship of area-averaged carbon dioxide and water vapour fluxes to atmospheric variables. Agric For Meteorol 112:195–202. doi:10.1016/S0168-1923(02)00076-5
Mandal B, Majumdar B, Adhya TK, Bandyopadhyay PK, Gangopadhyay A, Sarkar D, Kundu MC, Choudhyry SG, Hazra GC, Kundu S, Samantaray RN, Mishra AK (2008) Potential of double-cropped rice ecology to conserve organic carbon under subtropical climate. Glob Change Biol 14:2139–2151
Massman WJ, Lee X (2002) Eddy covariance flux corrections and uncertainties in long-term studies of carbon and energy exchanges. Agric For Meteorol 113:121–144
Matos MC, Matos AA, Mantas A, Cordeiro V, Vieira DA, Silva JB (1998) Diurnal and seasonal changes in Prunus amygdalus gas exchanges. Photosynthetica 35:517–524
Miyata A, Leuning R, Denmead OW, Kim J, Harazano Y (2000) Carbon dioxide and methane fluxes from an intermittently flooded paddy field. Agric For Meteorol 102:287–303
Miyata A, Iwata T, Nagai H, Yamada T, Yoshikoshi H, Mano M, Ono K, Han GH, Harazano Y, Ohtaki E, Baten MA, Inohara S, Takimoto T, Saito M (2005) Seasonal variation of carbon dioxide and methane fluxes at single cropping paddy fields in Central and Western Japan. Phyton (Austria) 45:89–97
Moon BK, Hong J, Lee BR, Yun JI, Park EW, Kim J (2003) CO2 and energy exchange in rice paddy for the growing season of 2002 in Hari, Korea. K J Agric For Meteorol 5:51–60
Mowjood MIM, Ishiguro K, Kasubuchi T (1997) Effect of convection in ponded water on the thermal regime of a paddy field. Soil Sci 162(8):583–587
Pakoktom T, Aoki M, Kasemsap P, Boonyawat S, Attarod P (2009) CO2 and H2O fluxes ratio in paddy fields of Thailand and Japan. Hydrol Res Lett 3:10–13
Papale D, Reichstein M, Aubinet M, Canfora E, Bernhofer C, Kutsch W, Longdoz B, Rambal S, Valentini R, Vesala T, Yakir D (2006) Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: algorithms and uncertainty estimation. Biogeosciences 3:571–583
Patel NR, Dadhwal VK, Saha SK (2011) Measurement and scaling of Carbon Dioxide (CO2) exchanges in wheat using flux-tower and remote sensing. J Ind Soc Remote Sensing. doi:10.1007/s12524-011-0107-1
Potts DL, Huxman TE, Cable JM (2006) Antecedent moisture and seasonal precipitation influence the response of canopy-scale carbon and water exchange to rainfall pulses in semi-arid grassland. J Ecol 94:23–30
Reichstein M, Falge E, Baldocchi D, Papale D, Aubinet M, Berbigier P, Bernhofer C, Buchmann N, Gilmaov T, Granier A, Grunwald T, Havrankova K, Ilvesniemi H, Janous D, Knohl A, Laurila T, Lohila A, Loustau D, Matteucci G, Meyers T, Miglietta F, Ourcival JM, Pumpanen J, Rambal S, Rotenberg E, Sanz M, Tenhunen J, Seufert G, Vaccari F, Vesala T, Yakir D, Valentini R (2005) On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob Change Biol 11:1424–1439
Ruimy A, Jarvis PG, Baldocchi DD, Sauiger B (1995) CO2 fluxes over plant canopies and solar radiation: a review. Adv Ecol Res 26:1–81
Saigusa N, Yamamoto S, Muruyama S, Kondo H, Nishimura H (2002) Gross primary production and net ecosystem exchange of a cool-temperate deciduous forest estimated by the eddy covariance method. Agric For Meteorol 112:203–215. doi:10.1016/S0168-1923(02)00082-5
Saito M, Miyata A, Nagai H, Yamada T (2005) Seasonal variation of carbon dioxide exchange in rice paddy field in Japan. Agric For Meteorol 135:93–109
Saitoh K, Ishihara K (1987) Effect of vapor pressure deficit on photosynthesis of rice leaves with reference to light and CO2 utilization efficiency. Jpn J Crop Sci 56(2):163–170 (in Japanese with English abstract)
Schmid HP, Grimmond BSC, Cropley F, Offcrle B, Su HB (2000) Measurements of CO2 and energy fluxes over a mixed hardwood forest in the mid-western United States. Agric For Meteorol 103:357–374
Schuepp SH, Leclerc MY, Macpherson JI, Desjardins RL (1990) Footprint prediction of scalar fluxes from analytical solutions of the diffusion equation. Boundary Layer Meteorol 42:167–180
Smith P, Lanigan G, Kutsch WL, Buchmann N, Eugster W, Aubinet M, Ceschia E, Beziat P, Yeluripati JB, Osborne B, Moors EJ, Brut A, Wattenbach M, Saunders M, Jones M (2010) Measurements necessary for assessing the net ecosystem carbon budget of croplands. Agric Ecosyst Environ 139:302–315. doi:10.1016/j.agee.2010.04.004
Sponseller RA (2007) Precipitation pulses and soil CO2 flux in a Sonoran desert ecosystem. Glob Change Biol 13:426–436
Tanner CB, Thurtell GW (1969) Anemoclinometer measurements of reynolds stress and heat transport in the atmospheric surface layer. University of Wisconsin Tech. Rep ECOM-66-G22-F, pp 82
Towprayoon S, Smakgahn K, Poonkaew S (2005) Mitigation of methane and nitrous oxide emissions from drained irrigated rice fields. Chemosphere 59:1547–1556
Tsai JL, Tsuang BJ, Lu PS, Yao MH, Hsieh HY (2006) Surface energy components, CO2 flux and canopy resistance from rice paddy in Taiwan. The 17th symposium on boundary layers and turbulence, 27th conference on agricultural and meteorology, and the 17th conference on biometeorology and aerobiology, 21–25, San Deigo, CA, USA
Tseng HK, Tsai LJ, Alagesan A, Tsuang JB, Yao HM, Kuo HP (2010) Determination of methane and carbon dioxide fluxes during the rice maturity period in Taiwan by combining profile and eddy covariance measurements. Agric For Meteorol 150:852–859
Webb EK, Pearman GI, Leuning R (1980) Correction of flux measurements for density effects due to heat and water vapour transfer. Q J Royal Meteorol Soc 106:85–100
XiuE R, QinXue W, ChengLi T, JinShui W, KeLin W, YongLi Z, ZeJian L, Masataka W, GuoYoung T (2007) Estimation of soil respiration in a paddy ecosystem in the subtropical region of China. Chi Sci Bull 52:2722–2730
Zsolnay A (2003) Dissolved organic matter: artefacts, definitions, and functions. Geoderma 113:187–210
Acknowledgments
The work has been partially supported by the grant of ICAR-NAIP, Component-4 (2031), project entitled “Soil organic carbon dynamics vis-à-vis anticipatory climatic changes and crop adaptation strategies”, ICAR-NICRA and CRRI. The suggestion given by Dr. D. C. Uprety, Dr. S. N. Singh, Dr. V. R. Rao and Dr. T. K. Adhya is gratefully acknowledged. Part of the result is the PhD work of Mr. Suvadip Neogi. Technical support was provided by the technical staffs of the Division of Crop Production, CRRI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharyya, P., Neogi, S., Roy, K.S. et al. Net ecosystem CO2 exchange and carbon cycling in tropical lowland flooded rice ecosystem. Nutr Cycl Agroecosyst 95, 133–144 (2013). https://doi.org/10.1007/s10705-013-9553-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-013-9553-1