Abstract
This study aimed to evaluate the essential oil of Ocimum gratissimum L. (EOOG) for anesthesia and in the transport of Oreochromis niloticus. Experiment I determined the time of anesthesia induction and recovery during anesthesia of O. niloticus exposed to different concentrations of EOOG (0, 30, 90, 150, and 300 mg L−1). Based on data from Experiment I, Experiment II evaluated the effect of 0, 30, and 90 mg L−1 EOOG on blood parameters and oxidative stress immediately after anesthesia induction and 1 h after recovery. Experiment III evaluated the effect of 0, 5, and 10 mg L−1 EOOG on blood variables immediately after 4.5 h of transport of juveniles. Concentrations between 90 and 150 mg L−1 EOOG were efficient for anesthesia and recovery. The use of 90 mg L−1 of EOOG prevented an increase in plasma glucose. Other changes in blood parameters and oxidative stress are discussed. The use of 10 mg L−1 EOOG in transport increased plasma glucose and decreased hematocrit values immediately after transport. It is concluded that the use of 90 and 150 mg L−1 EOOG causes anesthesia and recovery in O. niloticus within the time intervals considered ideal. The use of 90 mg L−1 EOOG favored stable plasma glucose soon after anesthesia induction and 1 h after recovery, but caused changes in the antioxidant defense system by increasing hepatic and kidney ROS. The transport of 12 g O. niloticus for 4.5 h can be performed with concentration of 5 mg L−1 of EOOG.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The usual handling procedures employed in fish farms trigger stress to animals, which can compromise their productive and reproductive performances (Barton 2002; Gressler et al. 2012). Among the adopted handling procedures, biometry and transport of fish are routine in fish farms. These practices can harm fish, increasing their susceptibility to infectious and pathogenic diseases, besides demanding a greater amount of energy (Gimbo et al. 2008; Goes et al. 2017), which is provided through gluconeogenesis, glycogenolysis, and increased protein turnover (Barton and Iwama 1991; Wendelaar Bonga 1997; Mommsen et al. 1999; Ribeiro et al. 2019). In addition, these handling practices can cause changes in fish metabolism, with oxidative damage through the production of reactive oxygen species (ROS), which often leads to lipid peroxidation (Matés 2000; Velisek et al. 2011; Souza et al. 2018; Baldissera et al. 2020).
In this sense, the use of anesthetics has provided better control for the handling procedures used in fish production (Cunha et al. 2010; Becker et al. 2012; Becker et al. 2017; Rotili et al. 2012; Ribeiro et al. 2013; Ribeiro et al. 2015), by reducing swimming activity and stress of the animals (Ross and Ross 2008; Zahl et al. 2010; Salbego et al. 2017). Anesthetics (at sedative concentrations) also showed positive effects during transport, including reduced waterborne ammonia and greater survival (Becker et al. 2012; Gil et al. 2016; Navarro et al. 2016; Boaventura et al. 2021). However, responses to a given anesthetic may differ among species, and so prior characterization of efficacy is necessary (King 2009).
The plant Ocimum gratissimum L. (Lamiaceae), popularly known as “alfavaca” or basil, is a native species of Africa that is being used in different countries as a condiment, sedative, and stress reducer and to treat headaches in humans (Albuquerque et al. 2007). The essential oil of the leaves of this plant showed safety and efficacy as anesthesia for some species of fish, such as the Brazilian flounder Paralichthys orbignyanus (Benovit et al. 2012), silver catfish Rhamdia quelen (Silva et al. 2012; Silva et al. 2015; Bandeira et al. 2017), matrinxã Brycon amazonicus (Ribeiro et al. 2016), Nile tilapia Oreochromis niloticus (Adewale et al. 2017), cachara Pseudoplatystoma reticulatum (Silva et al. 2020) and pacamã Lophiosilurus alexandri (Boaventura et al. 2020).
Oreochromis niloticus is the fourth most produced fish species worldwide, contributing approximately 4.5 million tons/year (FAO 2018). This high production is attributed to its excellent characteristics that make it favorable to commercial cultivation, as well as its white meat with good organoleptic characteristics and lack of “Y”-shaped spines, which facilitates fillet processing (Simões et al. 2007). Therefore, the evaluation of the anesthetic and sedative effects of this essential oil and its benefits in the transport of juveniles of this species has become important.
Thus, the objective of this study was to evaluate different concentrations of the essential oil of O. gratissimum L. (EOOG) for induction and recovery times of anesthesia, blood and oxidative stress parameters, and its use in the transport of O. niloticus juveniles. Based on previous studies with the extract of this plant in O. niloticus (Adewale et al. 2017) and of the EOOG in other species (Silva et al. 2012; Silva et al. 2015; Ribeiro et al. 2016; Silva et al. 2020; Boaventura et al. 2020; Boaventura et al. 2021), we suppose that this essential oil is effective for anesthesia, reduces the stress of transport, and improves the oxidative status of O. niloticus.
Materials and methods
Animals and environmental acclimation
Juveniles of O. niloticus were grown in rectangular tanks, with a useful volume of 100 L, in a water recirculation system with mechanical and biological filtration. Temperature was maintained at 27.69 ± 0.35 °C and pH at 7.09 ± 0.14 (both measured with a Hanna HI98130 multiparameter probe), dissolved oxygen levels at 6.65 ± 0.28 mg L−1 (measured with an EcoSense® DO200A oximeter), and total ammonia at 0.59 ± 0.03 mg L−1 (determined with an Alfakit Labcon Test colorimetric kit). The photoperiod was 12 L:12D (controlled by Loud Advanced Technology TL63A digital timer). During the lights-on period, the average luminous flux incident on the water surface was 140 lx (measured with Instrutemp ITLD260 luximeter) and 0 lx after lights were turned off. The fish were fed with commercial feed (5 mm in diameter) containing 320 g kg−1 of crude protein, 3200 kcal kg−1 of digestible energy, 70 g kg−1 of ether extract, 130 g kg−1 of mineral matter, 25 g kg−1 of calcium, and 10 g kg−1 of phosphorus, twice a day (8:00 am and 4:00 pm) until apparent satiety. The animals were fasted for 24 h before use; thus, the food was provided until the first meal of the day before each test. A water renewal of 50% of the system volume was performed twice a week.
The essential oil of the leaves of O. gratissimum (EOOG) (73.6% eugenol) was obtained according to Silva et al. (2012). Ethyl alcohol (98.1%) was used in a 1:10 (V/V) proportion in all concentrations to homogenize the EOOG in water. The volume of ethyl alcohol equivalent to the highest concentration of the EOOG was used for the ethanol treatment (0 mg L−1).
Experiment I—Anesthetic effect of EOOG for juvenile O. niloticus
Seventy-two O. niloticus juveniles (weight 45.36 ± 10.70 g; total length 13.16 ± 1.12 cm) were fasted for 24 h prior to use. The animals were distributed in a completely randomized design (n = 12 for each concentration) and exposed to the following EOOG concentrations: 0 (ethanol), 10, 30, 90, 150, or 300 mg L−1. The fish were transferred individually to a 1 L aquarium and exposed to the different concentrations (each fish was used only once). The ethanol group was observed for 10 min (Ferreira et al. 2020), while a digital chronometer (Taksun Ts1809) was used to assess the time of anesthesia induction and recovery for the other concentrations. Fish were considered to have reached deep anesthesia when they completely lost swimming balance and did not respond to external stimuli (Keene et al. 1998; Ross and Ross 2008), at which point they were weighed on an analytical balance (Mars AD5002) and measured with the aid of a ruler. After biometrics, the fish were transferred to 1 L aquaria with clean water (from the culture system itself), for anesthesia recovery. The ventilatory frequency (VF) of the fish was evaluated during anesthesia induction and recovery by counting opercular movements per minute (adapted from Alvarenga and Volpato 1995). The animals were considered recovered when they responded to external stimuli and showed normal swimming balance (Keene et al. 1998; Ross and Ross 2008).
After the experiment, fish of each anesthetic concentration were pooled and kept in 100 L tanks in a recirculating aquaculture system to observe the return to appetite and record their survival after 24 h. During this period, the fish were fed two times a day until apparent satiety.
Experiment II—Hematological, biochemical, and oxidative stress responses of juvenile O. niloticus submitted to different EOOG concentrations
Based on the results of Experiment I, a subsequent test was performed using the concentrations of 0 (ethanol), 30, and 90 mg L−1 EOOG, considering a control and concentrations below the ideal and at the ideal, respectively, for fish anesthesia according to the criteria of Keene et al. (1998) and Ross and Ross (2008). For this test, 72 O. niloticus juveniles (weight 41.36 ± 9.80 g; total length 12.70 ± 1.15 cm, n = 24 per treatment) were fasted for 24 h and then distributed in a completely randomized factorial design. The same procedures as described in Experiment I were adopted. However, in this experiment, blood samples were collected immediately after anesthesia induction and 1 h after anesthesia recovery (n = 12 fish for each concentration after anesthesia and 12 fish for each concentration after recovery). Fish were restrained with a damp cloth and blood (500 μL) was collected by caudal puncture using heparinized syringes (0.2% mg mL−1). The fish were then euthanized through immersion in ice slurry and spinal cord sectioning so that the gills, liver, kidney, and brain could be removed and immediately stored in a − 80 °C freezer for further analysis.
Hematological and biochemical analysis
Blood aliquots were used to quantify hematocrit. Capillary tubes were centrifuged at 10,000 rpm (Microline-Laborline®) for 10 min as described by Goldenfarb et al. (1971). Plasma protein was determined using a Goldberg manual refractometer. Blood was centrifuged at 4000 rpm for 10 min for biochemical analysis. Plasma glucose was determined using a monoreagent glucose commercial kit (K082 Bioclin), followed by reading with a spectrophotometer (Biochrom Libra S21–S22).
Oxidative stress analysis
The liver, gills, kidney, and brain were sampled for evaluation of levels of reactive oxygen species (ROS), superoxide dismutase (SOD), thiobarbituric acid reactive substances (TBARS), non-protein thiols (NPSH), and protein.
Levels of ROS were determined by the DCFH oxidation method described by LeBel et al. (1992). Fluorescence was measured using excitation and emission wavelengths of 485 and 538 nm, respectively. A calibration curve was established with 2′,7′-dichlorofluorescein (DCF) (0.1 nM–1 μM) as the standard, and results were expressed as U DFC per mg of protein.
The activity of SOD was determined according to the auto-oxidation principle of pyrogallol, inhibited in the presence of SOD. The optical density change was determined kinetically for 2 min at 10-s intervals at 420 nm, according to methodology described by Beutler (1984). Activity was expressed as U mg per of protein.
As an index of lipid peroxidation, TBARS formation during an acid-heating reaction was determined as previously described by Ohkawa et al. (1979). A malondialdehyde (MDA) solution was used as a reference standard. TBARS levels were determined by absorbance at 532 nm and were expressed as MDA equivalent nmol MDA per g of tissue.
NPSH levels were determined colorimetrically at 412 nm as previously described by Ellman (1959) and published in detail by Souza et al. (2018). A cysteine solution was used as a reference standard. NPSH levels were expressed as μmol SH per g of tissue.
Protein concentration was determined by the Coomassie Blue method following the methodology described by Read and Northcote (1981) using bovine serum albumin as a standard.
Experiment III—Effect of EOOG on survival rate, feed return, water quality, and blood parameters after transport of juvenile O. niloticus
One hundred and sixty O. niloticus juveniles (weight 12.2 ± 3.4 g; total length 9.5 ± 2.1 cm) were used for Experiment III. The water in the culture system had a temperature of 27.49 ± 0.35 °C, pH of 7.65 ± 1.28 (Hanna HI98130 multiparameter probe), and dissolved oxygen levels of 7.09 ± 1.14 mg L−1 (measured by the EcoSense® DO200A oximeter). Animals were fasted for 24 h before transport. After fasting, blood was collected as previously described from a group of 10 fish (basal group). The fish were distributed in a completely random design in 15 plastic bags of 59.5 × 80.5 cm, with 8 L water, 2/3 dissolved oxygen, and 10 fish bag−1. Three concentrations of EOOG were tested: 0 (ethanol 90 μL L−1), 5, and 10 mg L−1. The animals were transported in a car for 4.5 h and this time was chosen based in an average duration of O. niloticus transport from other studies (Navarro et al. 2016; Hohlenwerger et al. 2017; Ventura et al. 2020); after which, the bags were opened and temperature, dissolved oxygen, pH, and total ammonia were measured and two fish from each bag (n = 10 fish per treatment) were selected for blood collection for the analysis of plasma glucose, hematocrit, and plasma protein, as described in the Experiment II. The remaining fish of each treatment were then placed in three rectangular tanks, with a useful volume of 100 L, in a water recirculation system under a controlled environment, as previously described, to evaluate survival rate and return to feeding up to 96 h after transport.
Statistical analysis
Homogeneity of variances and normality of the data were tested with the Brown-Forsythe and Shapiro-Wilk tests, respectively. The results of anesthesia induction and recovery times and ventilatory frequencies were submitted to one-way ANOVA, followed by regression analyses to better adjust the model (P < 0.05). Two-way ANOVA followed by Tukey’s post-test was used for biochemical, hematological, and oxidative stress variables (P < 0.05). The results of water quality variables were analyzed using the Kruskal-Wallis test (P < 0.05). All analyses were performed using the SigmaPlot program version 12.0.
Results
Experiment I
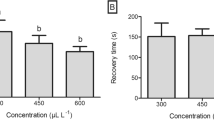
No mortality was observed after 24 h of testing and all fish resumed feeding. Ethanol and 10 mg L−1 EOOG did not induce deep anesthesia. Anesthesia induction time had a quadratic effect (P < 0.05), with a minimum value at 215 mg L−1 (38.79 s) (Fig. 1a). Anesthesia recovery time was directly related to EOOG concentration (P < 0.05) and varied from 149.55 to 568.1 s (Fig. 1b).
Anesthesia induction time (a), anesthesia recovery time (b), ventilatory frequency during induction (c), and ventilatory frequency during recovery (d) (mean ± standard deviations) of Oreochromis niloticus subjected to different concentrations of the essential oil of Ocimum gratissimum (stars represent the minimum value of the equations)
The VF showed a quadratic effect during anesthesia induction (P < 0.05), with a minimum value at 208.33 mg L−1 (11.27 beats min−1) (Fig. 1c). During anesthesia recovery, VF showed an inverse relationship with EOOG concentration (P < 0.05) (Fig. 1d).
Experiment II
Biochemistry and blood hematology
After anesthesia induction, O. niloticus juveniles submitted to 30 mg L−1 EOOG had higher plasma glucose values (P < 0.05) compared to those exposed to 0 mg L−1 (Fig. 2a). One hour after recovery, plasma glucose values were equal among the concentrations evaluated (P > 0.05). However, there was a reduction in plasma glucose levels for the concentration of 30 mg L−1 EOOG after 1 h recovery compared to after anesthesia induction (P < 0.05).
Biochemical and hematological parameters of Oreochromis niloticus measured immediately after anesthesia induction with essential oil of Ocimum gratissimum (EOOG) and 1 h after recovery. Ethanol group: 0 mg L−1 EOOG. a Plasma glucose, b hematocrit and c plasma protein. Values (mean ± standard deviations) (N = 12 animals per treatment). The data were analyzed by two-way ANOVA, followed by Tukey post-test (P < 0.05). Different lowercase letters indicate a significant difference between concentrations at the same time (after induction or 1 h after recovery). Different capital letters indicate a significant difference between times for a given concentration
O. niloticus juveniles exposed to 90 mg L−1 EOOG had higher hematocrit values after anesthesia induction compared to treatments of 0 and 30 mg L−1 (P < 0.05), which were similar to each other (P > 0.05) (Fig. 2b). One hour after recovery, hematocrit was similar between all concentrations evaluated (P > 0.05). There was no significant difference in plasma protein for the tested concentrations between the two evaluated times (P > 0.05). There was also no significant difference among the concentrations assessed during induction and 1 h after recovery, nor between times (Fig. 2c).
Oxidative stress variables
After induction, higher levels of hepatic ROS were observed for fish anesthetized with 30 and 90 mg L−1 EOOG (P < 0.05) (Fig. 3a). These values remained higher than those for fish exposed to 0 mg L−1 EOOG 1 h after recovery, when fish that were submitted to 30 mg L−1 EOOG presented higher levels of hepatic ROS than those recovering from 90 mg L−1 EOOG (P < 0.05). There were no differences in gill ROS between times (P < 0.05) for the three EOOG concentrations assessed. The EOOG concentrations evaluated did not influence the levels of ROS in the gills after anesthesia induction and 1 h after recovery (P > 0.05) (Fig. 3b).
Reactive oxygen species (ROS) of the liver (a), gills (b), kidney (b), and brain (d) of Oreochromis niloticus measured immediately after anesthesia induction with essential oil of Ocimum gratissimum (EOOG) and 1 h after recovery. Ethanol group: 0 mg L−1 EOOG. Values (mean ± standard deviations) (N = 12 animals per treatment). The data were analyzed by two-way ANOVA, followed by Tukey post-test (P < 0.05). Different lowercase letters indicate a significant difference between concentrations at the same time (after induction or 1 h after recovery). Different capital letters indicate a significant difference between times for a given concentration
There were no significant differences among the tested EOOG concentrations for ROS levels in the kidney after anesthesia induction (P > 0.05) (Fig. 3c). However, there was a decrease in the level of ROS in the kidney for fish of the 0 mg L−1 EOOG treatment 1 h after recovery and an increase in those of the 30 mg L−1 EOOG treatment (P < 0.05). The highest kidney ROS levels after recovery were for fish anesthetized with 30 mg L−1 EOOG, followed by those anesthetized with 90 mg L−1 EOOG (P < 0.05).
After anesthesia induction, the highest levels of brain ROS were observed in fish anesthetized with 30 and 90 mg L−1 EOOG (P < 0.05) (Fig. 3d). One hour after recovery, the highest level of brain ROS was found in fish exposed to 30 mg L−1 EOOG, with this value being higher than that observed immediately after anesthesia induction (P < 0.05). The other EOOG concentrations evaluated did not differ significantly between times (P > 0.05).
The highest hepatic SOD activity after anesthetic induction was for fish exposed to 30 mg L−1 EOOG (P < 0.05). One hour after recovery, this group showed the lowest hepatic SOD activity, followed by the group submitted to 90 mg L−1 EOOG (P < 0.05). There was an increase in hepatic SOD activity from induction to 1 h after recovery for fish exposed to 0 mg L−1 EOOG and a decrease in those exposed to 30 mg L−1 EOOG (P < 0.05) (Fig. 4a).
Superoxide dismutase (SOD) of the liver (a), gills (b), kidney (c), and brain (d) of Oreochromis niloticus measured immediately after anesthetic induction with essential oil of Ocimum gratissimum (EOOG) and 1 h after recovery. Values (mean ± standard deviations) (N = 12 animals per treatment). The data were analyzed by two-way ANOVA, followed by Tukey post-test (P < 0.05). Different lowercase letters indicate a significant difference between concentrations at the same time (after induction or 1 h after recovery). Different capital letters indicate a significant difference between times for a given concentration
The lowest SOD activity in the gills after anesthesia induction was for fish anesthetized with 90 mg L−1 EOOG (P < 0.05). One hour after recovery, there were no significant differences among the assessed EOOG concentrations (P > 0.05). However, there was a reduction in SOD activity in the gills in fish exposed to 0 and 30 mg L−1 EOOG 1 h after recovery and an increase in those exposed to 90 mg L−1 EOOG (P < 0.05) (Fig. 4b).
No significant differences were observed for kidney SOD activity after anesthesia induction (P > 0.05). One hour after recovery, the lowest SOD activity was for fish exposed to 30 mg L−1 EOOG and the highest activity for those exposed to 0 mg L−1 EOOG (P < 0.05). There were no significant differences in kidney SOD activity between times for the tested concentrations (P > 0.05) (Fig. 4c).
After anesthesia induction, brain SOD activity was significantly lower for fish exposed to both 30 and 90 mg L−1 EOOG compared those exposed to ethanol (0 mg L−1 EOOG) (P < 0.05). One hour after recovery, the lowest SOD activity was observed in fish exposed to 30 mg L−1 EOOG (P < 0.05). There was a reduction in brain SOD activity between times in fish submitted to 30 mg L−1 EOOG and an increase in those exposed to 90 mg L−1 EOOG (P < 0.05) (Fig. 4d).
After anesthesia induction and 1 h after recovery from EOOG exposure, there were no significant differences in hepatic TBARS levels among the assessed concentrations (P > 0.05). Between times, there was an increase in hepatic TBARS levels in fish exposed to 30 mg L−1 EOOG (P < 0.05) (Fig. 5a).
Thiobarbituric acid reactive substances (TBARS) of the liver (a), gills (b), kidney (c), and brain (d) of Oreochromis niloticus measured immediately after anesthetic induction with essential oil of Ocimum gratissimum (EOOG) and 1 h after recovery. Values (mean ± standard deviations) (N = 12 animals per treatment). The data were analyzed by two-way ANOVA, followed by the Tukey post-test (P < 0.05). Different lowercase letters indicate a significant difference between concentrations at the same time (after induction or 1 h after recovery). Different capital letters indicate a significant difference between times for a given concentration
After anesthesia induction, fish exposed to 30 mg L−1 EOOG had significantly lower TBARS levels in the gills than those exposed to 0 mg L−1 EOOG (P < 0.05). One hour after recovery, there were no significant differences in gill TBARS levels among EOOG concentrations (P > 0.05). Between times, there was a reduction in gill TBARS levels for fish submitted to 0 mg L−1 EOOG and an increase in those exposed to 30 mg L−1 f EOOG (P < 0.05) (Fig. 5b).
After anesthetic induction, no significant differences were observed among the assessed concentrations of EOOG for kidney TBARS levels (P > 0.05). One hour after recovery, higher kidney TBARS levels were observed for fish exposed to 30 and 90 mg L−1 EOOG compared to those exposed to 0 mg L−1 EOOG (P < 0.05). There was an increase in kidney TBARS levels for fish exposed to 30 and 90 mg L−1 EOOG 1 h after recovery compared to after induction (P < 0.05) (Fig. 5c).
Fish exposed to 30 mg L−1 of EOOG had higher brain TBARS levels both after anesthesia induction and 1 h after recovery than those submitted to 0 mg L−1 EOOG (P < 0.05). There were no differences in brain TBARS levels between times for all concentrations of EOOG (P > 0.05) (Fig. 5d).
There were no significant differences in liver NPSH activity among EOOG concentrations after anesthesia induction and 1 h after recovery, and no differences between times for any of the concentrations (P > 0.05) (Fig. 6a). Fish exposed to 30 and 90 mg L−1 of EOOG had significantly lower gill NPSH activity after anesthetic induction compared to in those exposed to 0 mg L−1 EOOG (P < 0.05). However, 1 h after recovery, fish exposed to 30 mg L−1 EOOG had significantly lower gill NPSH activity than did fish exposed to 0 mg −1 EOOG (P < 0.05). Between times, gill NPSH activity increased for fish exposed to 30 mg L−1 of EOOG and decreased for those exposed to 0 mg L−1 EOOG (Fig. 6b).
Non-protein thiols (NPSH) of the liver (a), gills (b), kidney (c), and brain (d) of Oreochromis niloticus measured immediately after anesthetic induction with essential oil of Ocimum gratissimum (EOOG) and 1 h after recovery. Ethanol group: 0 mg L−1 EOOG. Values (mean ± standard deviations) (N = 12 animals per treatment). The data were analyzed by two-way ANOVA, followed by Tukey post-test (P < 0.05). Different lowercase letters indicate a significant difference between concentrations at the same time (after induction or 1 h after recovery). Different capital letters indicate a significant difference between times for a given concentration
No significant differences were observed in kidney NPSH activity after anesthesia induction for all concentrations tested (P > 0.05). However, 1 h after recovery, fish exposed to 30 mg L−1 EOOG had the highest kidney NPSH activity, followed by those exposed to 90 mg L−1 EOOG. Between times, there was only a significant increase in kidney NPSH activity for fish exposed to 30 mg L−1 EOOG (P < 0.05) (Fig. 6c). There were no significant differences in brain NPSH activity among EOOG concentrations assessed after anesthesia induction or between times for any of the concentrations (P > 0.05). However, 1 h after recovery, the highest brain NPSH activity was for fish exposed to 30 mg L−1 EOOG (P < 0.05) (Fig. 6d).
After anesthesia induction, significantly lower and higher hepatic protein levels were observed in fish exposed to 30 and 90 mg L−1 EOOG, respectively, compared to those exposed to 0 mg L−1 EOOG (P < 0.05). One hour after recovery, the highest and lowest hepatic protein levels were for fish exposed to 30 and 0 mg L−1 EOOG, respectively (P < 0.05). Between times, there was an increase in hepatic protein levels for fish exposed to 30 mg L−1 EOOG and a decrease for those exposed to 0 mg L−1 EOOG (P < 0.05) (Fig. 7a).
Protein levels of the liver (a), gills (b), kidney, (c), and brain (d) of Oreochromis niloticus measured immediately after anesthetic induction with essential oil of Ocimum gratissimum (EOOG) and 1 h after recovery. Ethanol group: 0 mg L−1 EOOG. Values (mean ± standard deviations) (N = 12 animals per treatment). The data were analyzed by two-way ANOVA, followed by Tukey post-test (P < 0.05). Different lowercase letters indicate a significant difference between concentrations at the same time (after induction or 1 h after recovery). Different capital letters indicate a significant difference between times for a given concentration
The highest gill protein levels after anesthesia induction was for in fish exposed to 90 mg L−1 EOOG (P < 0.05). One hour after recovery, there were no significant differences in gill protein levels among EOOG concentrations (P > 0.05). Between the times, there was an increase in gill protein levels for fish exposed to 0 and 30 mg L−1 EOOG (P < 0.05) (Fig. 7b).
There were no significant differences among the three EOOG concentrations for kidney protein levels after anesthetic induction (P > 0.05). However, 1 h after recovery, fish exposed to 30 mg L−1 EOOG had the highest kidney protein levels (P < 0.05). Between the times, there was an increase and a decrease in kidney protein levels for 30 and 0 mg L−1 of EOOG, respectively (P < 0.05) (Fig. 7c). Fish exposed to 30 mg L−1 EOOG had the highest brain protein levels, both after anesthesia induction and 1 h after recovery (P < 0.05). Between the times, there was an increase in brain protein level for fish exposed to 30 mg L−1 EOOG (P < 0.05) (Fig. 7d).
Experiment III
Water quality
There were no significant differences (P > 0.05) in water quality variables immediately after the transport of O. niloticus juveniles exposed to different EOOG concentrations (Table 1).
Blood biochemical and hematological parameters
Survival was 100% at 96 h post-transport and all animals resumed feeding within 24 h. After transport, fish transported with 10 mg L−1 EOOG had significantly higher plasma glucose levels and lower hematocrit than the basal group and the other treatments (P < 0.05) (Fig. 8a, b). Plasma protein was not affected by transport for any of the EOOG concentrations (P > 0.05) (Fig. 8c).
Biochemical and hematological parameters of Oreochromis niloticus measured after transport with essential oil of Ocimum gratissimum (EOOG). Basal group: fish not transported. Ethanol group: 0 mg L−1 EOOG. Plasma glucose (a), hematocrit (b), and plasma protein (c). Values (mean ± standard deviations) (N = 12 animals per treatment). The data were analyzed by two-way ANOVA, followed by Tukey post-test (P < 0.05). Different lowercase letters indicate a significant difference between concentrations at the same time (after induction or 1 h after recovery). Different capital letters indicate a significant difference between times for a given concentration. Asterisk indicates a significant difference from the basal group
Discussion
EOOG proved to be efficient and safe as anesthesia and for transport of O. niloticus, with induction and recovery times within acceptable limits for fish and minimal influence on biochemical, hematological, and water quality variables. However, the use of EOOG favored lipid peroxidation in the kidney, which indicates the possible development of oxidative stress.
No mortality was observed during anesthesia and sedation of juvenile O. niloticus. Netto et al. (2017) also did not observe mortality rates for this species after being subjected different concentrations of the essential oils of O. basilicum and Cymbopogum flexuosus for anesthesia and sedation. EOOG also proved to be safe for R. quelen (Silva et al. 2015), Colossoma macropomum (Boijink et al. 2016), B. amazonicus (Ribeiro et al. 2016), and O. niloticus (Adewale et al. 2017). According to Keene et al. (1998) and Ross and Ross (2008), the ideal concentration of an anesthetic should induce anesthesia in fish in less than 180 s and have a recovery time of less than 300 s. Thus, 90 and 150 mg L−1 EOOG would be the most suitable concentrations for juvenile O. niloticus with an average weight of 45 g. Adewale et al. (2017) indicated EOOG concentrations between 60 to 100 mg L−1 for anesthesia of O. niloticus with an average weight of 20 g.
Ventilation frequency is a useful parameter for understanding the physiology of fish when using anesthetics (Alvarenga and Volpato 1995). The juvenile of O. niloticus in the present study had a higher VF during anesthesia induction and recovery with 30 mg L−1 EOOG. Increased fish VF during induction may be related to the disturbance caused by a stressor in the water (anesthetic) (Matthews and Varga 2012). However, after the first minutes of contact with an anesthetic, VF decreases considerably (Becker et al. 2012). Silva et al. (2019) did not establish a direct relationship between anesthetic concentration of the essential oils of L. alba and L. origanoides and VF for C. macropomum, with the exception of the anesthesia stage at 200 μL L−1 L. origanoides EO, which led to a lower VF than did 25 μL L−1, suggesting that this relationship may be species-specific.
Plasma glucose was higher in fish anesthetized with 30 mg L−1 EOOG immediately after induction. Fish in stressful situations, such as biometry and transport, for example, release catecholamines and corticosteroids that activate the processes of gluconeogenesis and glycogenolysis, which mobilize and increase glucose in the bloodstream to deal with the increased energy demand (Barton and Iwama, 1991; Wendelaar Bonga 1997; Pankhurst 2011). However, the hyperglycemia observed with 30 mg L−1 EOOG after induction may be related to increased fish agitation and hypoxia caused during this step. A similar phenomenon was described by Hohlenwerger et al. (2016) during anesthesia of O. niloticus with the essential oil of L. alba, with the glycemia tending to gradually return to normal levels (Teixeira et al. 2017; Santos et al., 2020), as observed after 1 h of recovery in the present study.
The elevation of hematocrit in O. niloticus juveniles anesthetized with 90 mg L−1 EOOG characterizes hemoconcentration caused by osmoregulatory adjustments (Houston et al. 1996; Mcdonald and Milligan 1997); however, all treatments showed similar values at 1 h post-recovery. Increased hematocrit has also been observed for other species during anesthesia with eugenol (Hill and Forster 2004; Pádua et al. 2012; Ribeiro et al. 2019).
Tissue cells maintain a balance between prooxidant and antioxidant agents in situations that do not cause discomfort to animals (Poli et al. 2012). When an imbalance occurs between these agents, the oxidative stress process begins, characterized by the formation of ROS, which cause lipid and protein oxidation (Esterbauer 1996; Baldissera et al. 2020). At the time of anesthesia induction, fish may experience a situation similar to hypoxia due to decreased VF. During recovery from anesthesia, tissue reoxygenation occurs and may affect the synthesis of ROS in tissues (Gressler et al. 2014; Velisek et al. 2011). The handling of fish in water and water with low concentrations of ethanol can affect the prooxidant and antioxidant balance and cause tissue damage (Souza et al. 2018). In the current study, ROS was produced in the liver and kidney 1 h after recovery in O. niloticus anesthetized with EOOG. This increase can be understood as an aversive response of the organism to EOOG. The main compound of EOOG used in this study was eugenol (Silva et al. 2012). Despite its analgesic and antioxidant properties, the concentration of eugenol used can cause cytotoxic effects and increase ROS in tissues (Atsumi et al. 2005; Bezerra et al. 2017).
The increase of antioxidant enzymes in tissues is related to oxidative substances that stimulate the antioxidant defense system (Yu 1994; Łuczaj et al. 2017). SOD is one of the main enzymes involved in the clearance of ROS produced in cells (Cheeseman and Slater 1994). This enzyme functions by converting ROS to H2O2 and H2O, and then convert H2O2 and O2 by the enzymatic action of catalases (CAT) and glutathione peroxidase (GPx) (Li et al. 2009; Velisek et al. 2011). The highest values for hepatic, kidney, and brain SOD were observed 1 h after recovery for O. niloticus juveniles exposed to 0 and 90 mg L−1 EOOG. This increase suggests an attempt by the organism to revert to the synthesis of ROS in tissues after anesthesia with EOOG.
TBARS analysis is commonly used to assess oxidative damage, with lipid peroxidation being responsible for the loss of cell function under oxidative stress (Huang et al. 2003). Higher levels of TBARS in the kidney were observed 1 h after recovery of fish exposed to EOOG. Increased levels of TBARS in the kidney characterize the synthesis of lipid peroxidation, which indicates the possible development of oxidative stress when using EOOG to anesthetize O. niloticus. Recently, Souza et al. (2018) observed higher levels of TBARS in the kidney of R. quelen immediately after anesthesia with L. alba essential oil of the linalool chemotype. However, these authors found that these values were reduced after 8 h of recovery. In addition, the levels of gill, kidney, and brain NPSH and hepatic, kidney, and brain protein remained elevated 1 h after the recovery of O. niloticus exposed to EOOG. This observation demonstrates the protection of cellular proteins against oxidation through the NPSH redox cycle or by directly detoxifying ROS caused by exposure to stressors (Ruas et al. 2008). When evaluating the effects of different chemotypes of the essential oil of L. alba in R. quelen, Souza et al. (2018) observed that the linalool chemotype was able to reduce damage to lipids and proteins in the liver and kidney; however, the same did not occur after 8 h of recovery of fish anesthetized with the citral chemotype, demonstrating that oxidative defenses were not completely capable of reducing ROS synthesis or preventing tissue damage.
The EOOG concentrations used in the present study did not lead to changes in water quality or plasma protein levels of O. niloticus juveniles at the end of transport. Favero et al. (2019) also did not find any differences in water quality parameters and plasma protein values for juvenile Lophiosilurus alexandri transported in water containing eugenol. In addition, the water quality parameters were within acceptable levels for O. niloticus at the end of transport (Shoko et al. 2014).
There was an increase of plasma glucose of O. niloticus immediately after transport with 10 mg L−1 EOOG. This hyperglycemia may be due to the increased energy demand and metabolic responses of fish during stressful situations (Barton and Iwama, 1991; Wendelaar Bonga 1997; Velisek et al. 2011). Juveniles of C. macropomum transported with eugenol also showed an increase in plasma glucose shortly after transport and a reduction to basal levels after 96 h of recovery (Santos et al., 2020). The lower hematocrit of O. niloticus transported with 10 mg L−1 EOOG may also be associated with acute and induced stress (Mcdonald and Milligan 1997; Navarro et al. 2016) or hemodilution through osmoregulatory mechanisms (Houston et al. 1996). The use of eugenol as a sedative and anesthetic for juvenile L. alexandri also decreased hematocrit values (Favero et al. 2019; Ribeiro et al. 2019). However, despite these differences in glucose and hematocrit, survival after 96 h was 100%, and the animals started to feed again within 24 h after the end of the test.
Conclusions
Concentrations of 90 and 150 mg L−1 of essential oil of Ocimum gratissimum (EOOG) are considered ideal for anesthetizing O. niloticus juveniles with an average weight of 41 g. In spite of the use of 90 mg L−1 EOOG prevented elevated plasma glucose, it may have caused lipid damage to the kidneys and changes to the antioxidant defense system by increasing hepatic and brain ROS concentration and reducing brain TBARS activity. Additional studies analyzing the effect of these concentrations on different times of anesthesia and recovery will allow a better understanding of the efficacy of EOOG for O. niloticus. The concentration of 5 mg L−1 EOOG can be indicated for the transport of O. niloticus with an average weight of 12 g for 4.5 h.
Data availability
The data and material that support the findings of this study are available from the corresponding author upon reasonable request.
References
Adewale AY, Adeshina I, Yusuf OY (2017) Anaesthetic effect of Ocimum gratissimum extract on Oreochromis niloticus juveniles. Eur Exp Biol 7(2–7):10.21767/2248-9215.100007
Albuquerque UP, Medeiros PM, Almeida ALS, Monteiro JM, Neto LEMF, Melo JG, Santos JP (2007) Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: a quantitative approach. J Ethnopharmacol 114:325–354. https://doi.org/10.1016/j.jep.2007.08.017
Alvarenga CMD, Volpato GL (1995) Agonistic profile and metabolism in alevins of the Nile tilapia. Physiol Behav 57:75–80. https://doi.org/10.1016/0031-9384(94)00206-K
Atsumi T, Fujisawa S, Tonosaki K (2005) A comparative study of the antioxidant/prooxidant activities of eugenol and isoeugenol with various concentrations and oxidation conditions. Toxicol In Vitro 19:1025–1033. https://doi.org/10.1016/j.tiv.2005.04.012
Baldissera MD, Souza CF, Boaventura TP, Nakayama CL, Baldisserotto B, Luz RK (2020) Involvement of the phosphoryl transfer network in gill bioenergetic imbalance of pacamã (Lophiosilurus alexandri) subjected to hypoxia: notable participation of creatine kinase. Fish Physiol Biochem 46:405–416. https://doi.org/10.1007/s10695-019-00728-0
Bandeira JG, Pês TS, Saccol EM, Sutili FJ, Rossi JW, Murari AL, Baldisserotto B (2017) Potential uses of Ocimum gratissimum and Hesperozygis ringens essential oils in aquaculture. Ind Crop Prod 97:484–491. https://doi.org/10.1016/j.indcrop.2016.12.040
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525. https://doi.org/10.1093/icb/42.3.517
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26. https://doi.org/10.1016/0959-8030(91)90019-G
Becker AG, Parodi TV, Heldwein CG, Zeppenfeld CC, Heinzmann BM, Baldisserotto B (2012) Transportation of silver catfish, Rhamdia quelen, in water with eugenol and the essential oil of Lippia alba. Fish Physiol Biochem 38:789–796. https://doi.org/10.1007/s10695-011-9562-4
Becker AG, Luz RK, Mattioli CC, Nakayama CL, Silva WDS, Leme FDOP, Baldisserotto B (2017) Can the essential oil of Aloysia triphylla have anesthetic effect and improve the physiological parameters of the carnivorous freshwater catfish Lophiosilurus alexandri after transport? Aquaculture 481:184–190. https://doi.org/10.1016/j.aquaculture.2017.09.007
Benovit SC, Gressler LT, Lima SL, Oliveira GL, Okamoto MH, Santos PJ, Baldisserotto B (2012) Anesthesia and transport of Brazilian flounder, Paralichthys orbignyanus, with essential oils of Aloysia gratissima and Ocimum gratissimum. J World Aquacult Soc 43:896–900. https://doi.org/10.1111/j.1749-7345.2012.00604.x
Beutler E (1984) Superoxide dismutase. In: Beutler E (ed) Red cell metabolism. A manual of biochemical rmethods. Grune and Stratton, Philadelphia, pp 83–85
Bezerra DP, Militão GCG, Morais MC, Sousa DP (2017) The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients 9:1–15. https://doi.org/10.3390/nu9121367
Boaventura TP, Souza CF, Ferreira AL, Favero GC, Baldissera MD, Heinzmann BM, Baldisserotto B, Luz RK (2020) Essential oil of Ocimum gratissimum (Linnaeus, 1753) as anesthetic for Lophiosilurus alexandri: induction, recovery, hematology, biochemistry and oxidative stress. Aquaculture 529:735676. https://doi.org/10.1016/j.aquaculture.2020.735676
Boaventura TP, Souza CF, Ferreira AL, Favero GC, Baldissera MD, Heinzmann BM, Luz RK (2021) The use of Ocimum gratissimum L. essential oil during the transport of Lophiosilurus alexandri: water quality, hematology, blood biochemistry and oxidative stress. Aquaculture 531:735964. https://doi.org/10.1016/j.aquaculture.2020.735964
Boijink CL, Queiroz CA, Chagas EC, Chaves FCM, Inoue LAKA (2016) Anesthetic and anthelminthic effects of clove basil (Ocimum gratissimum) essential oil for tambaqui (Colossoma macropomum). Aquaculture 457:24–28. https://doi.org/10.1016/j.aquaculture.2016.02.010
Cheeseman KH, Slater TF (1994) An introduction to free radicals biochemistry. Br Med Bull 31:31–2692. https://doi.org/10.5860/choice.31-2692
Cunha MA, Barros FMC, Garcia LO, Veeck APL, Heinzmann BM, Loro VL, Emanuelli T, Baldisserotto B (2010) Essential oil of Lippia alba: a new anesthetic for silver catfish, Rhamdia quelen. Aquaculture 306:403–406. https://doi.org/10.1016/j.aquaculture.2010.06.014
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Esterbauer H (1996) Estimation of peroxidative damage. A critical review. Pathol Biol 44:25–28
FAO (2018) The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome
Favero GC, Silva WDS, Boaventura TP, Leme FDOP, Luz RK (2019) Eugenol or salt to mitigate stress during the transport of juvenile Lophiosilurus alexandri, a Neotropical carnivorous freshwater catfish. Aquaculture 512:734321. https://doi.org/10.1016/j.aquaculture.2019.734321
Ferreira AL, Silva WDS, Neves CL, Ferreira NS, Takata R, Luz RK (2020) Benzocaine and menthol as anesthetics for the African cichlid Aulonocara nyassae. Aquac Int 28:1837–1846. https://doi.org/10.1007/s10499-020-00561-w
Gil HW, Ko MG, Lee TH, Park IS, Kim DS (2016) Anesthetic effect and physiological response in olive flounder (Paralichthys olivaceus) to clove oil in a simulated transport experiment. Dev Reprod 20:255–266. https://doi.org/10.12717/DR.2016.20.3.255
Gimbo RY, Saita MV, Gonçalves AFN, Takahashi LS (2008) Diferentes concentrações de benzocaína na indução anestésica do lambari-do-rabo- amarelo (Astyanax altiparanae). Rev Bras Saúde Prod Anim 9:350–357
Goes GA, Oliva RA, Ronqui RG, Queiroz TR, Satolo EG (2017) Descrição do sistema logístico de transporte: uma análise conceitual envolvendo piscicultura. S Am Dev Soc J 1:100–115
Goldenfarb PB, Bowyer FP, Hall E, Brosius E (1971) Reproducibility in the hematology laboratory: the microhematocrit determinations. Amer J Clin Pathol 56:35–39. https://doi.org/10.1093/ajcp/56.1.35
Gressler LT, Parodi TV, Riffel APK, Costa ST, Baldisserotto B (2012) Immersion anaesthesia with tricaine methanesulphonate or propofol on different sizes and strains of silver cat fish Rhamdia quelen. J Fish Biol 81:1436–1445. https://doi.org/10.1111/j.1095-8649.2012.03409.x
Gressler LT, Riffel APK, Parodi TV, Saccol EMH, Koakoski G, Costa ST, Pavanato MA, Heinzmann BM, Caron B, Schmidt D, Llesuy SF, Barcellos LJG, Baldisserotto B (2014) Silver catfish Rhamdia quelen immersion anaesthesia with essential oil of Aloysia triphylla (L’Hérit) Britton or tricaine methanesulfonate: effect on stress response and antioxidant status. Aquac Res 45:1061–1072. https://doi.org/10.1111/are.12043
Hill JV, Forster ME (2004) Cardiovascular responses of Chinook salmon (Oncorhynchus tshawytscha) during rapid anaesthetic induction and recovery. Comp Biochem Physiol C Toxicol Pharmacol 137:167–177. https://doi.org/10.1016/j.cca.2004.01.002
Hohlenwerger JC, Copatti EC, Sena CA, Couto DR, Baldisserotto B, Heinzmann BM, Schmidt D (2016) Could the essential oil of Lippia albaprovide a readily available and cost-effective anaesthetic for Nile tilapia (Oreochromis niloticus)? Mar Freshw Behav Physiol 49:119–126. https://doi.org/10.1080/10236244.2015.1123869
Hohlenwerger JC, Baldisserotto B, Couto RD, Heinzmann BM, Silva DTD, Caron BO, Copatti CE (2017) Essential oil of Lippia alba in the transport of Nile tilapia. Cienc Rural 47:e20160040. https://doi.org/10.1590/0103-8478cr20160040
Houston AH, Dobric N, Kahurananga R (1996) The nature of hematological response in fish. Studies on rainbow trout Oncorhyncus mykiss exposed to stimulated winter, spring and summer conditions. Fish Physiol Biochem 15:339–347. https://doi.org/10.1007/bf02112561
Huang CH, Chang RJ, Huang SL, Chen WL (2003) Dietary vitamin E supplementation affects tissue lipid peroxidation of hybrid tilapia, Oreochromis niloticus × Oreochromis aureus. Comp Biochem Physiol B 134:265–270. https://doi.org/10.1016/S1096-4959(02)00256-7
Keene JI, Noakes DIG, Moccia RD, Soto GC (1998) The efficacy of clove oil as an anesthetic for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 29:89–101. https://doi.org/10.1046/j.1365-2109.1998.00927.x
King HR (2009) Fish transport in the aquaculture sector: an overview of the road transport of Atlantic salmon in Tasmania. J Vet Behav 4:163–168. https://doi.org/10.1016/j.jveb.2008.09.034
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231. https://doi.org/10.1021/tx00026a012
Li ZH, Xie S, Wang JX, Sales J, Li P, Chen DQ (2009) Effect of intermittent starvation on growth and some antioxidant indexes of Macrobrachium nipponense (De Haan). Aquac Res 40:526–532. https://doi.org/10.1111/j.1365-2109.2008.02123.x
Łuczaj W, Gęgotek A, Skrzydlewska E (2017) Antioxidants and HNE in redox homeostasis. Free Radic Biol Med 111:87–101. https://doi.org/10.1016/j.freeradbiomed.2016.11.033
Matés JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104. https://doi.org/10.1016/S0300-483X(00)00306-1
Matthews M, Varga ZM (2012) Anesthesia and euthanasia in zebrafish. ILAR J 53:192–204. https://doi.org/10.1093/ilar.53.2.192
Mcdonald G, Milligan L (1997) Ionic, osmotic and acid-base regulation in stress. In: Iwana GW, Pickering AD, Sumpter JP, Schreck CB (eds) Fish stress and health in aquaculture. University Press, Cambridge, pp 119–144
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Navarro RD, França PR, Paludo GR, Bizarro SYW, Silva FR, Pereira S, Navarro FK (2016) Physiological and hematological responses of Nile tilápia (Oreochromis niloticus) to different anesthesics during simulated transport conditions. Acta Sci 38:301–306. https://doi.org/10.4025/actascitechnol.v38i3.28377
Netto JDL, Oliveira RS, Copatti CE (2017) Efficiency of essential oils of Ocimum basilicum and Cymbopogum flexuosus in the sedation and anaesthesia of Nile tilapia juveniles. An Acad Bras Cienc 89:2971–2974. https://doi.org/10.1590/0001-3765201720170001
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pádua SB, Ventura AS, Satake F, Ishikawa MM, Hisano H, Rotta MA, Arantes FC (2012) Hematological responses in tuvira after clove oil anesthesia at different concentrations. Bol Inst Pesca 38:181–188
Pankhurst NW (2011) The endocrinology of stress in fish: an environmental perspective. Gen Comp Endocrinol 170:265–275. https://doi.org/10.1016/j.ygcen.2010.07.017
Poli G, Leonarduzzi G, Biasi F, Chiarpotto E (2012) Oxidative stress and cell signalling. Curr Med Chem 11:1163–1182. https://doi.org/10.2174/0929867043365323
Read SM, Northcote DH (1981) Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem 116:53–64. https://doi.org/10.1016/0003-2697(81)90321-3
Ribeiro PAP, Miranda Filho KC, Melillo Filho R, Santos AEH, Souza WDS, Rodrigues LA, Luz RK (2013) Efeito anestésico do eugenol em juvenis de pacamã. Pesq Agropec Bras 48:1136–1139. https://doi.org/10.1590/S0100-204X2013000800048
Ribeiro PA, Miranda Filho KC, Melo DCD, Luz RK (2015) Efficiency of eugenol as anesthetic for the early life stages of Nile tilapia (Oreochromis niloticus). An Acad Bras Cienc 87:529–535. https://doi.org/10.1590/0001-3765201520140024
Ribeiro AS, Batista EDS, Dairiki JK, Chaves FCM, Inoue LAKA (2016) Anesthetic properties of Ocimum gratissimum essential oil for juvenile matrinxã. Acta Sci Anim Sci 38:1–7. https://doi.org/10.4025/actascianimsci.v38i1.28787
Ribeiro PAP, Hoyos MDC, Oliveira CG, Flora DMAL, Luz RK (2019) Eugenol and benzocaine as anesthetics for Lophiosilurus alexandri juvenile, a freshwater carnivorous catfish. Aquac Int 27:313–321. https://doi.org/10.1007/s10499-018-0326-3
Ross LG, Ross B (2008) Anaesthetic and sedative techniques for aquatic animals, 3rd edn. Blackwell Science, United Kingdom, Oxford
Rotili DA, Devens AM, Diemer O, Lorenz KE, Lazzari R, Boscolo RW (2012) Uso de eugenol Como anestésico em pacu. Pesqui Agropecu Trop 42:288–294. https://doi.org/10.1590/S1983-40632012000300013
Ruas CBG, Carvalho CS, Araújo HSS, Espíndola ELG, Fernandes MN (2008) Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal contaminated river. Ecotoxicol Environ Saf 71:86–93. https://doi.org/10.1016/j.ecoenv.2007.08.018
Salbego J, Toni C, Becker AG, Zeppenfeld CC, Menezes CC, Loro VL, Baldisserotto B (2017) Biochemical parameters of silver catfish (Rhamdia quelen) after transport with eugenol or essential oil of Lippia alba added to the water. Braz J Biol 77:696–702. https://doi.org/10.1590/1519-6984.16515
Santos ELR, Rezende FP, Moron SE (2020) Stress-related physiological and histological responses of tambaqui (Colossoma macropomum) to transportation in water with tea tree and clove essential oil anesthetics. Aquaculture 523:735164. https://doi.org/10.1016/j.aquaculture.2020.735164
Shoko AP, Limbu SM, Mrosso HD, Mgaya YD (2014) A comparison of diurnal dynamics of water quality parameters in Nile tilapia (Oreochromis niloticus, Linnaeus, 1758) monoculture and polyculture with African sharp tooth catfish (Clarias gariepinus, Burchell, 1822) in earthen ponds. Int Aquatic Res 6:56–63. https://doi.org/10.1007/s40071-014-0056-8
Silva LL, Parodi TV, Reckziegel P, Oliveira GV, Bürger ME, Baldisserotto B, Heinzmann BM (2012) Essential oil of Ocimum gratissimum L.: anesthetic effects, mechanism of action and tolerance in silver catfish, Rhamdia quelen. Aquaculture 350:91–97. https://doi.org/10.1016/j.aquaculture.2012.04.012
Silva LL, Garlet QI, Koakoski G, Oliveira TA, Barcellos LJG, Baldisserotto B, Heinzmann BM (2015) Effects of anesthesia with the essential oil of Ocimum gratissimum L. in parameters of fish stress. Rev Bras Plantas Med 17:215–223. https://doi.org/10.1590/1983-084X/13_034
Silva HNPD, Carvalho BCFD, Maia JLDS, Becker AG, Baldisserotto B, Heinzmann BM, Silva LVFD (2019) Anesthetic potential of the essential oils of Lippia alba and Lippia origanoides in Tambaqui juveniles. Cien Rural 49:e20181059. https://doi.org/10.1590/0103-8478cr20181059
Silva LA, Martins MA, Santo FE, Oliveira FC, Chaves FCM, Chagas EC, Martins LM, Campos CM (2020) Essential oils of Ocimum gratissimum and Zingiber officinale as anesthetics of the south American catfish Pseudoplatystoma reticulatum. Aquaculture 528:735595
Simões RM, Ribeiro AFC, Ribeiro ACS, Park JK, Murr XEF (2007) Composição físico-química, microbiológica e rendimento do filé de tilápia tailandesa (Oreochromis niloticus). Cienc Tecnol Aliment 27:608–613. https://doi.org/10.1590/s0101-20612007000300028
Souza CF, Baldissera MD, Bianchini AE, Silva EG, Mourão RHV, Silva LVF, Baldisserotto B (2018) Citral and linalool chemotypes of Lippia alba essential oil as anesthetics for fish: a detailed physiological analysis of side effects during anesthetic recovery in silver catfish (Rhamdia quelen). Fish Physiol Biochem 44:21–34. https://doi.org/10.1007/s10695-017-0410-z
Teixeira RR, Souza RC, Sena AC, Baldisserotto B, Heinzmann BM, Couto RD, Copatti CE (2017) Essential oil of Aloysia triphylla in Nile tilapia: anaesthesia, stress parameters and sensory evaluation of fillets. Aquac Res 48:3383–3392. https://doi.org/10.1111/are.13165
Velisek J, Stara A, Li ZH, Silovska S, Turek J (2011) Comparison of the effects of four anaesthetics on blood biochemical profiles and oxidative stress biomarkers in rainbow trout. Aquaculture 310:369–375. https://doi.org/10.1016/j.aquaculture.2010.11.010
Ventura AS, Jerônimo GT, Oliveira SN, Araújo GAM, Cardoso CAL, Teodoro GC, Povh JA (2020) Natural anesthetics in the transport of Nile tilapia: hematological and biochemical responses and residual concentration in the fillet. Aquaculture 526:735365. https://doi.org/10.1016/j.aquaculture.2020.735365
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625. https://doi.org/10.1152/physrev.1997.77.3.591
Yu BP (1994) Cellular defenses against damage from reactive oxygen species. Physiol Rev 74:139–162. https://doi.org/10.1152/physrev.1994.74.1.139
Zahl IH, Kiessling A, Samuelsen OB, Olsen RE (2010) Anesthesia induces stress in Atlantic salmon (Salmo salar), Atlantic cod (Gadus morhua) and Atlantic halibut (Hippoglossus hippoglossus). Fish Physiol Biochem 36:719–730. https://doi.org/10.1007/s10695-009-9346-2
Funding
The present research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-Brazil). R.K. Luz and B. Baldisserotto received research fellowships from CNPq (CNPq No. 308547/2018-7 and 301225/2017-6, respectively).
Author information
Authors and Affiliations
Contributions
Andre Lima Ferreira: Conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, writing—reviewing and editing, visualization.
Gisele Cristina Favero: Conceptualization, methodology, validation, formal analysis, investigation, final approval of the version to be submitted.
Tulio Pacheco Boaventura: Conceptualization, methodology, validation, formal analysis, investigation, final approval of the version to be submitted.
Carine de Freitas Souza: Conceptualization, methodology, validation, formal analysis, investigation.
Nathália Soares Ferreira: Conceptualization, methodology, validation, formal analysis, investigation.
Sharine Nunes Descovi: Conceptualization, methodology, validation, formal analysis, investigation.
Bernardo Baldisserotto: Conceptualization, methodology, validation, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, final approval of the version to be submitted, funding acquisition.
Berta Maria Heinzmann: Conceptualization, methodology, validation, analysis and interpretation of data, final approval of the version to be submitted.
Ronald Kennedy Luz: Conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, investigation, writing—original draft, writing—reviewing and editing, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Three experiments were carried out at the Laboratório de Aquacultura (LAQUA) of the Escola de Veterinária (EV) of the Universidade Federal de Minas Gerais (UFMG), with approval of the Comissão de Ética no Uso de Animais (CEUA) of this institution (registration numbers 324/2018 and 42/2020).
Consent to participle
All names in the author list have been involved in various stages of experimentation or writing.
Consent to publication
All authors agree to submit the paper for publication in the Journal of Fish Physiology and Biochemistry.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, A.L., Favero, G.C., Boaventura, T.P. et al. Essential oil of Ocimum gratissimum (Linnaeus, 1753): efficacy for anesthesia and transport of Oreochromis niloticus. Fish Physiol Biochem 47, 135–152 (2021). https://doi.org/10.1007/s10695-020-00900-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00900-x