Abstract
The objective of this study was to investigate the effects of benzocaine and menthol on the anesthesia of Aulonocara nyassae juveniles. Two size classes of fish were used in trials with benzocaine: Juveniles I—70 fish of 0.74 ± 0.31 g (39.41 ± 7.48 mm); and Juveniles II—70 fish of 3.80 ± 0.92 g (76.58 ± 9.83 mm). The fish used for trials with menthol were as follows: Juveniles I—70 fish of 1.01 ± 0.39 g (50.39 ± 12.75 mm) and Juveniles II—70 fish of 3.73 ± 0.78 g (64.94 ± 8.98 mm). Seven concentrations—0, 12.5, 25.0, 50.0, 75.0, 100.0, and 125.0 mg L−1—of each anesthetic (benzocaine and menthol) were tested on each size class of fish (n = 10 fish per size class and anesthetic concentration). Thus, anesthesia induction time and recovery were evaluated. Concentrations of 12.5 and 25 mg L−1 of benzocaine did not lead to a deep stage of anesthesia in the animals; however, the other concentrations presented anesthetic effect. For menthol, the concentration of 12.5 mg L−1 also did not anesthetize the fish. However, the other doses were effective and safe. The recommended anesthetic concentrations provided induction and recovery times within the limits considered ideal for fish. Concentrations between 75 and 125 mg L−1 of benzocaine for Juvenile I and 50 to 125 mg L−1 for Juvenile II are recommended. For menthol, concentrations between 50 and 125 mg L−1 can be used for both size classes of A. nyassae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Handling and transport are stressful procedures involved in the management and trading of ornamental fish (Pickering 1981; Barton 2002; Romaneli et al. 2018; Oliveira et al. 2019). Exposure to stressors triggers physiological changes that may compromise fish health and survival (Wendelaar Bonga 1997). Blood glucose and cortisol concentrations are often employed as stress indicators (Morgan and Iwama 1997; Barton 2002) and observations of ventilation frequency can provide indications of changes in metabolic rate following exposure to a stressor (Summerfelt and Smith 1990; Alvarenga and Volpato 1995; Toni et al. 2014; Becker et al. 2018).
The use of anesthetics in effective doses promotes safety for both fish and the handler during different management practices adopted in pisciculture (Ross and Ross 2008; Weber 2011; Ribeiro et al. 2015), and can promote animal welfare and increased survival of fish. The concentration and efficacy required for anesthetic induction by a drug are related to fish size and species (Woody et al. 2002; Ross and Ross 2008; Tarkhani et al. 2017), which makes it necessary to validate an anesthetic before its use (King 2009). The choice of a particular anesthetic is associated with its legal implications, economic viability, ease of application, and low risk to animals and handlers (Padua et al. 2012). Ideally, anesthetic induction time should not exceed 180 s, while recovery time should not exceed 300 s (Keene et al. 1998; Ross and Ross 2008).
Benzocaine is a synthetic anesthetic that is widely used because it is easily accessible and inexpensive, has no mutagenic action and can be rapidly metabolized by fish (Woody et al. 2002; Gontijo et al. 2003). This product can be used frequently without affecting the productive and reproductive performance of fish and is environmentally friendly (Okamoto et al. 2009; Okamura et al. 2010).
Menthol, which is an essential oil extracted from plants of the genus Mentha (Patel et al. 2007), is another alternative anesthetic that has been used effectively for many fish species, such as rainbow trout, Oncorhynchus mykiss (Teta and Kaiser 2019); curimba, Prochilodus lineatus (Junior et al. 2018); and the angelfish Pterophyllum scalare (Romaneli et al. 2018). Menthol is easily found in pharmacies, is inexpensive (Facanha and Gomes 2005), and is a safe and natural product (Yadegarinia et al. 2006).
Species of the genus Aulonocara are the most popular ornamental fish among African cichlids and have had great international commercialization in recent years (Schwalbe et al. 2012). The Blue cichlid Aulonocara nyassae originates from Lake Malawi and is of ornamental interest due to its “blue orchid” coloration that catches the attention of aquarists. Nonetheless, no data are available on the effects of benzocaine and menthol as anesthetics for the species.
Therefore, the present study aimed to investigate the effects of benzocaine and menthol on anesthesia of A. nyassae juveniles, as well as on ventilation frequency during anesthetic induction and recovery.
Material and methods
The experiments were carried out at Laboratório de Aquacultura of the Escola de Veterinária, Universidade Federal de Minas Gerais, under registration number 326/2019 of Comissão de Ética no Uso de Animais.
Fish of A. nyassae were produced and cultivated in six rectangular tanks with 42-L useful volume, at a density 50 fish tank−1, in a water recirculation system with temperature maintained at 27.21 ± 0.09 °C, pH around 7.08 ± 0.06 (measured by Hanna HI98130 multiparameter probe), dissolved oxygen of 6.81 ± 0.58 mg L−1, (Water Quality Meter AK87 oximeter), and total ammonia of 0.53 ± 0.02 mg L−1 (Alfakit Labcon Test colorimetric kit). The animals were fed 1.7-mm-diameter commercial feed containing 460.0 g kg−1 crude protein, 80 g kg−1 ether extract, 140 g kg−1 mineral matter, 20 g kg−1 calcium, and 15 g kg−1, as reported by the manufacturer, three times a day (09:00, 12:00, and 15:00 h) until apparent satiety. The water of the system was changed once a week with 50% renewal of its useful volume. Fish were fasted for 24 h prior to testing.
The anesthetics tested were benzocaine (ethyl 4-aminobenzoate 99%, Sigma-Aldrich®, SLBH1225V) and menthol (99% CRQ-1006310100). Two size classes of fish were used in trials with benzocaine: Juveniles I—70 fish of 0.74 ± 0.31 g (39.41 ± 7.48 mm); and Juveniles II—70 fish of 3.80 ± 0.92 g (76.58 ± 9.83 mm). The fish used for trials with menthol were as follows: Juveniles I—70 fish of 1.01 ± 0.39 g (50.39 ± 12.75 mm) and Juveniles II—70 fish of 3.73 ± 0.78 g (64.94 ± 8.98 mm). Seven concentrations—0 (control), 12.5, 25.0, 50.0, 75.0, 100.0, and 125.0 mg L−1—of each anesthetic (benzocaine and menthol) were tested on each size class of fish. Trials consisted of 10 replicates for each size class and concentration of anesthetic, with individual fish acting as independent replicates. The experiments were conducted independently for each anesthetic and size class of fish, using a completely randomized design. Anesthetic concentrations were prepared by dilution in 5 mL ethyl alcohol (98.1% purity), and the control consisted of ethyl alcohol without anesthetic (Ribeiro et al. 2013).
For analysis of anesthesia induction and recovery, fish were randomly captured one at a time and placed in a 1-L beaker containing the concentration of anesthetic to be tested. Control animals were observed for 10 min. Anesthetic induction time was evaluated using a digital timer (Taksun Ts1809). The behavioral characteristics of the deep anesthesia used in this study followed the recommendations of Ross and Ross (2008) and were basically the loss of equilibrium and absence of swimming. Throughout the anesthetic induction process, the ventilation frequency (VF) of the animals was evaluated by counting the number of opercular beats per minute adapted from Alvarenga and Volpato (1995). Once fish reached deep anesthesia, they were weighed on an analytical balance (Marte AD5002) and measured for length using a digital caliper (Starret® 799). The fish were then transferred individually to a 1-L beaker with clean water (from the cultivation system itself) for anesthetic recovery, which was timed and VF measured. The animals were considered recovered when they presented reflexes to external stimuli and normal swimming balance (Ross and Ross 2008).
After the experiments, fish of each size class and anesthetic were pooled and kept in 28-L tanks in a recirculating aquaculture system to observe the return to appetite and record survival after 24 h. The fish were fed three times a day until apparent satiety.
Data were tested for normality by the Shapiro-Wilk test and homoscedasticity of variances by the Leven test, followed by ANOVA. Regression analyses were performed to better fit the model (P < 0.05). Data were analyzed using R software version 3.5.2.
Results
Benzocaine
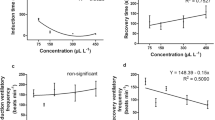
Benzocaine concentrations of 0, 12.5, and 25.0 mg L−1 did not induce deep anesthesia in the fish of either size class. For the other tested concentrations, induction time had a quadratic effect (P < 0.05) for Juvenile I (Fig. 1a), with an estimated inflection point at 108 mg L−1 (41.87 s). Recovery time for Juvenile I also presented a quadratic effect (P < 0.05) among the studied concentrations with a maximum point at 68 mg L−1 (118.93 s) (Fig. 1b).
A quadratic effect (P < 0.05) was also observed for induction time for the Juvenile II size class, with the estimated minimum point being at 112.85 mg L−1, while induction time ranged from 48.20 to 76.40 s (Fig. 1c). Recovery time also had a quadratic effect (P < 0.05) among concentrations for these fish, with the maximum point at 121.67 mg L−1 (101.41 s) (Fig. 1d). Increasing doses of benzocaine were accompanied by increasing recovery times for the Juvenile II size class.
Ventilation frequency during induction of Juvenile I presented a quadratic effect (P < 0.05) among the studied concentrations, with the estimated minimum point being at 110 mg L−1 (50.63 beats min−1) (Fig. 2a). During recovery, the VF for Juvenile I also had a quadratic effect (P < 0.05) among evaluated concentrations, with a minimum point at 66.67 mg L−1 and a range of 72.11–83.53 beats min−1 (Fig. 2b).
Ventilation frequency during induction of Juvenile II presented a quadratic effect among the evaluated concentrations (P < 0.05) with the minimum point at 65 mg L−1 (79.94 beats min−1) (Fig. 2c). During recovery, the VF for Juvenile II had a direct linear effect (P < 0.05) among the studied concentrations and a range of 94.47–112.75 beats min−1 (Fig. 2d).
Survival for Juvenile I was > 90% at 24 h after the end of the experiment and presented a linear effect (P < 0.05) among the evaluated concentrations with the estimated equation Y = 109.0–0.16x, R2 = 0.80, and all surviving animals feeding normally. Survival for Juvenile II was 100% 24 h after the end of the experiment for all benzocaine concentrations, with all animals resuming feeding.
Menthol
Menthol concentrations of 0 and 12.5 mg L−1 did not induce deep anesthesia in the fish of either size class. For the other concentrations, induction time for Juvenile I had a quadratic effect (P < 0.05), with an estimated inflection point of 122.25 mg L−1 (11.78 s) (Fig. 3a). Recovery time for Juvenile I also had a quadratic effect (P < 0.05) with a maximum point of 40 mg L−1 (91.98 s) (Fig. 3b).
The induction time for Juvenile II also had a quadratic effect (P < 0.05) among the different menthol concentrations, with the minimum at 93.83 mg L−1 (58.02 s) (Fig. 3c). The recovery time for Juvenile II had a quadratic effect (P < 0.05) among the studied concentrations with a maximum point of 25 mg L−1 and a range of 50.90–86.70 s (Fig. 3d).
Ventilation frequency for Juvenile I during induction had a direct linear effect (P < 0.05) among the concentrations and ranged 44.52–169.78 beats min−1 (Fig. 4a). For the same size class, VF during recovery had a quadratic effect (P < 0.05) among the concentrations with a minimum point of 47.50 mg L−1 (70.20 beats min−1) (Fig. 4b).
Ventilation frequency for Juvenile II during induction had a direct linear effect (P < 0.05) among concentrations ranging 48.13–110.70 beats min−1 (Fig. 4c). For the same size class, VF during recovery also had direct linear effect (P < 0.05) among concentrations (Fig. 4d).
Survival for Juvenile I and Juvenile II size classes was 100% at all concentrations evaluated after 24 h of recovery, and all animals resumed feeding.
Discussion
Benzocaine and menthol proved to be efficient for anesthesia of A. nyassae. Concentrations were found with induction times of less than 180 s and recovery times less than 300 s, as recommended by Keene et al. (1998) and Ross and Ross (2008).
Benzocaine and menthol are anesthetics that require dilution in ethanol; however, this solvent has no induction effect and does not cause mortality in fish at low concentration (Junior et al. 2018; Ribeiro et al. 2019; Teta and Kaiser 2019), as also observed for fish in the control group of the present study.
In the benzocaine experiment, anesthetic efficiency for the Juvenile I size class was between the concentrations of 75 and 125 mg L−1, while for Juvenile II, it was between 50 and 125 mg L−1; then, providing induction and recovery times within the limits considered ideal for fish (Keene et al. 1998; Ross and Ross 2008). During the process of anesthesia, and until a complete loss of swimming balance is achieved, anesthetic-related corticosteroids and catecholamines are released. Therefore, the faster that immobilization can occur, the less of these metabolites will be released in the organism (Rothwell et al. 2005). Thus, benzocaine concentrations of 108 and 112.85 mg L−1 were calculated for Juvenile I and Juvenile II size classes, respectively. Values close to the concentrations of benzocaine indicated in this study have been reported for some species. Concentrations of 87.5 and 100 mg L−1 were recommended for common carp, Cyprinus carpio, weighing an average 1.9 g (Bittencourt et al. 2013), whereas concentrations ranging 110–160 mg L−1 were recommended for angelfish, P. scalare, weighing an average of 16 g (Romaneli et al. 2018). Ideal concentrations of benzocaine for juvenile pacamã, Lophiosilurus alexandri, with an average weight of 214 g, were found to range 60–120 mg L−1 (Ribeiro et al. 2019). This variation in the concentration of a given anesthetic is related to fish size and species (Ross and Ross 2008; Fernandes et al. 2016; Tarkhani et al. 2017), as observed in the present study.
High survival (> 90%) was observed for the Juvenile I size class 24 h after the benzocaine experiment. In addition, the surviving fish resumed feeding during this period. The recorded mortality was not associated with anesthetic toxicity, but rather with fights (bites) observed among animals in experimental units. Survival was 100% for the Juvenile II size class, and all animals fed normally after 24 h. Benzocaine is a safe synthetic anesthetic for fish and can be rapidly metabolized (Gontijo et al. 2003; Okamoto et al. 2009; Okamura et al. 2010), which may explain the high survival rates observed after its use. Junior et al. (2019) evaluated different concentrations of benzocaine for juvenile curimba, P. lineatus, and observed a survival rate of > 90%, 96h after the end of the study. However, for juvenile pacamã, L. alexandri, benzocaine concentrations of 60, 120, 240, and 480 mg L−1 also did not cause mortality in fish 24 h after the test (Ribeiro et al. 2019), demonstrating its efficiency for different species.
The present study demonstrated that the concentration of 25 mg L−1 of menthol promoted deep anesthesia for Juvenile I and Juvenile II size classes, but it took longer than 180 s. For the other concentrations evaluated, however, induction was reached within 180 s, and recovery times were less than 300 s, as suggested by Keene et al. (1998) and Ross and Ross (2008), with good anesthetic efficiency at concentrations of 122.25 mg L−1 and 93.83 mg L−1 for Juvenile I and Juvenile II, respectively. Hoshiba et al. (2015) evaluated the anesthetic effect of menthol for juveniles of the platy Xiphophorus maculatus weighing an average of 0.168 g and found that concentrations between 100 and 250 mg L−1 had a safe effect. Similarly, menthol concentrations of 150 to 250 mg L−1 for the angelfish, P. scalare, weighing 16 g (Romaneli et al. 2018) and of 50 to 125 mg L−1 for lambari, Oligosarcus argenteus, weighing an average of 11.3 g (Uehara et al. 2019), were also found to be suitable for anesthesia management, which are close to the concentrations found in the present study.
In the menthol experiment, survival was 100% after 24 h for both size classes of A. nyassae and all concentrations evaluated, with all animals resuming feeding. This high survival may be related to the product because it is natural and safe (Yadegarinia et al. 2006). Menthol concentrations of 50, 100, 150, 200, and 250 mg L−1 did not cause mortality for the platy X. maculatus (Hoshiba et al. 2015). Evaluating different concentrations of menthol for juvenile curimba, P. lineatus, Junior et al. (2018) observed high survival (> 90%) after 96 h of anesthetic recovery with all animals resuming feeding.
The present study was not able to establish a direct relationship between increasing benzocaine and menthol concentrations and reduced VF for the Juvenile I and Juvenile II size classes of A. nyassae, as was also observed by Silva et al. (2019). Ventilation frequency is a useful parameter for understanding how fish physiology responds to anesthetics (Alvarenga and Volpato 1995; Becker et al. 2012). According to Toni et al. (2014), increased VF was observed for the catfish Rhamdia quelen after some minutes of exposure to essential oils of Hesperozygis ringens and Lippia alba. This hyperventilation is common in fish and is associated with increased oxygen consumption during anesthesia (Summerfelt and Smith 1990). Generally, after initial contact with an anesthetic, VF values decrease considerably (Becker et al. 2012). In contrast, Becker et al. (2018) did not observe any differences in VF values for the catfish R. quelen exposed to essential oils of L. alba and L. origanoides compared to the control group. According to these authors, difficulty in establishing a general increase or decrease response of VF when using anesthetics can be explained by species-specific responses, which is also apparent from the present study.
Conclusion
Benzocaine and menthol proved to be effective anesthetics for juvenile A. nyassae. The recommended concentrations of benzocaine are between 75 and 125 mg L−1 for Juvenile I and between 50 and 125 mg L−1 for Juvenile II size classes. For menthol, concentrations between 50 and 125 mg L−1 can be used for both juvenile size classes.
References
Alvarenga CMD, Volpato GL (1995) Agonistic profile and metabolism in alevins of the Nile tilapia. Physiol Behav 57(1):75–80. https://doi.org/10.1016/0031-9384(94)00206-K
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42(3):517–525. https://doi.org/10.1093/icb/42.3.517
Becker AG, Parodi TV, Heldwein CG, Zeppenfeld CC, Heinzmann BM, Baldisserotto B (2012) Transportation of silver catfish, Rhamdia quelen, in water with eugenol and the essential oil of Lippia alba. Fish Physiol Biochem 38(3):789–796. https://doi.org/10.1007/s10695-011-9562-4
Becker AJ, Fogliarini CDO, Souza CDF, Becker AG, Mourão RHV, Silva LVFD, Baldisserotto B (2018) Ventilatory frequency and anesthetic efficacy in silver catfish, Rhamdia quelen: a comparative approach between different essential oils. R Bras Zootec 47:e20170185. https://doi.org/10.1590/rbz4720170185
Bittencourt F, Souza BED, Neu DH, Rarato RR, Boscolo WR, Feiden A (2013) Eugenol e benzocaına como anestesicos para juvenis de Cyprinus carpio Linnaeus, 1758 (Carpa Comum). R Bras Cienc Agrar 8(1):163–167. https://doi.org/10.5039/agraria.v8i1a2225
Facanha MF, Gomes LC (2005) A eficácia do mentol como anestésico para Tambaqui (Colossoma macropomum, Characiformes: Characidae). Acta Amaz 35(1):71–75. https://doi.org/10.1590/s0044-59672005000100011
Fernandes IM, Bastos YF, Barreto DS, Lourenço LS, Penha JM (2016) The efficacy of clove oil as an anaesthetic and in euthanasia procedure for small-sized tropical fishes. Braz J Biol 77(3):444–450. https://doi.org/10.1590/1519-6984.15015
Gontijo AMDMC, Barreto RE, Speit G, Reyes VAV, Volpato GL, Salvadori DMF (2003) Anesthesia of fish with benzocaine does not interfere with comet assays results. Mutat Res 534(1–2):165–172. https://doi.org/10.1016/S1383-5718(02)00276-0
Hoshiba MA, Dias RMS, Moreira KMF, Cunha L, Geraldo AMR, Tamajusuku ASK (2015) Clove oil and menthol as anesthetic for platy. Bol Inst Pesca 41:737–742
Junior EFDM, Uehara SA, Rodrigues EC, Palheta GDA, Melo NFACD, Freire LDS, Takata R (2018) Menthol and eugenol as natural anesthetics for early juveniles of curimba. R Bras Zootec 47:e20170266. https://doi.org/10.1590/rbz4720170266
Junior EFDM, Uehara SA, Freitas TM, Melo NFAC, Palheta GDA, Takata R (2019) Effectiveness of benzocaine as anesthetic at different water temperatures for early juvenile curimba (Prochilodus lineatus Valenciennes, 1836), a neotropical fish species. Bol Inst Pesca 45:e474. https://doi.org/10.20950/1678-2305.2019.45.3.474
Keene JI, Noakes DIG, Moccia RD, Soto GC (1998) The efficacy of clove oil as an anesthetic for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 29(2):89–101. https://doi.org/10.1046/j.1365-2109.1998.00927.x
King HR (2009) Fish transport in the aquaculture sector: an overview of the road transport of Atlantic salmon in Tasmania. J Vet Behav Clinical Applications and Research 4(4):163–168. https://doi.org/10.1016/j.jveb.2008.09.034
Morgan JD, Iwama GK (1997) Measurements of stressed states in the field. In: Iwama GK, Pickering AD, Sumpter JP, Shreck CB (eds) Fish stress and health in aquaculture. Cambridge, Cambridge University Press, pp 247–268
Okamoto MH, Tesser MB, Louzada LR, Santos RA, Sampaio LA (2009) Benzocaína e eugenol como anestésicos para juvenis do pampo (Trachinotus marginatus). Cienc Rural 39(3):866–870. https://doi.org/10.1590/S0103-84782008005000100
Okamura D, Araújo FGD, Rosa PV, Freitas RTFD, Murgas LDS, Cesar MP (2010) Effect of benzocaine concentration and fish size on anesthesia and recovery in Nile tilapia. R Bras Zootec 39(5):971–976. https://doi.org/10.1590/S1516-35982010000500005
Oliveira CPB, Paixao LCH, Silva AF, Souza SA, Albinati ACL, Lima AO, Copatti CE (2019) Use of eugenol for the anaesthesia and transportation of freshwater angelfish (Pterophyllum scalare). Aquaculture 513:e734409. https://doi.org/10.1016/j.aquaculture.2019.734409
Padua SB, Ventura AS, Satake F, Ishikawa MM, Hisano H, Rotta MA, Arantes FC (2012) Respostas hematológicas em tuvira após anestesia com diferentes concentrações de óleo de cravo. Bol Inst Pesca 38(3):181–188
Patel T, Ishiuji Y, Yosipovitch G (2007) Menthol: a refreshing look at this ancient compound. J Am Acad Dermatol 57(5):873–878. https://doi.org/10.1016/j.jaad.2007.04.008
Pickering AD (ed) (1981) Stress and fish. Academic Press, New York
Ribeiro PAP, Miranda FKC, Melillo FR, Santos AEH, Silva WDS, Rodrigues LA, Luz RK (2013) Efeito anestésico do eugenol em juvenis de pacamã. Pesqui Agropecu Bras 48(8):1136–1139. https://doi.org/10.1590/S0100-204X2013000800048
Ribeiro PAP, Miranda FKC, Melo DCD, Luz RK (2015) Efficiency of eugenol as anesthetic for the early life stages of Nile tilapia (Oreochromis niloticus). An Acad Bras Cienc 87(1):529–535. https://doi.org/10.1590/00013765201520140024
Ribeiro PAP, Melo HDC, Oliveira CG, Della FMAL, Luz RK (2019) Eugenol and benzocaine as anesthetics for Lophiosilurus alexandri juvenile, a freshwater carnivorous catfish. Aquac Int 27(1):313–321. https://doi.org/10.1007/s10499-018-0326-3
Romaneli RDS, Boaratti AZ, Rodrigues AT, Queiroz DMDA, Khan KU, Nascimento TMT, Mansano CFM (2018) Efficacy of benzocaine, eugenol and menthol as anesthetics for freshwater angelfish. J Aquat Anim Health 30(3):210–216. https://doi.org/10.1002/aah.10030
Ross LG, Ross B (2008) Anaesthetic and sedative techniques for aquatic animals. Blackwell Science, Oxford
Rothwell SE, Black SE, Jerrett AR, Forster ME (2005) Cardiovascular changes and catecholamine release following anesthesia in Chinook salmon (Oncorhynchus tshawy tscha) and snapper (Pagrus auratus). Comp Biochem Physiol A Mol Integr Physiol 140(3):289–298. https://doi.org/10.1016/j.cbpb.2005.01.007
Schwalbe MAB, Bassett DK, Webb JF (2012) Feeding in the dark: lateral-line-mediated prey detection in the peacock cichlid Aulonocara stuartgranti. J Exp Biol 215(12):2060–2071. https://doi.org/10.1242/jeb.065920
Silva HNPD, Carvalho BCFD, Maia JLDS, Becker AG, Baldisserotto B, Heinzmann BM, Silva LVFD (2019) Anesthetic potential of the essential oils of Lippia alba and Lippia origanoides in tambaqui juveniles. Cienc Rural 49:e20181059. https://doi.org/10.1590/0103-8478cr20181059
Summerfelt RC, Smith LS (1990) Anesthesia, surgery, and related techniques. In: Schreck CB, Moyle PB (eds) Methods for fish biology, Bethesda, pp 213–272
Tarkhani R, Imani A, Jamali H, Farsani HG (2017) Anaesthetic efficacy of eugenol on various size classes of angelfish (Pterophyllum scalare Schultze, 1823). Aquac Res 48(10):5263–5270. https://doi.org/10.1111/are.13339
Teta C, Kaiser H (2019) Menthol as an alternative anaesthetic and sedative for rainbow trout, Oncorhynchus mykiss. Afr J Aquat Sci 44(1):71–76. https://doi.org/10.2989/16085914.2018.1548342
Toni C, Becker AG, Simões LN, Pinheiro CG, Lima-Silva L, Heinzmann BM, Baldisserotto B (2014) Fish anesthesia: effects of the essential oils of Hesperozygis ringens and Lippia alba on the biochemistry and physiology of silver catfish (Rhamdia quelen). Fish Physiol Biochem 40(3):701–714. https://doi.org/10.1007/s10695-013-9877-4
Uehara SA, Andrade DR, Takata R, Junior AG, Vidal MV (2019) The effectiveness of tricaine, benzocaine, clove oil, and menthol as anesthetics for lambari-bocarra Oligosarcus argenteus. Aquaculture 502:326–331. https://doi.org/10.1016/j.aquaculture.2018.12.054
Weber ES (2011) Fish analgesia: pain, stress, fear aversion, or nociception? Vet Clin N Am 14(1):21–32. https://doi.org/10.1016/j.cvex.2010.09.002
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77(3):591–625. https://doi.org/10.1152/physrev.1997.77.3.591
Woody CA, Nelson J, Ramstad K (2002) Clove oil as anaesthetic for adult sockeye salmon: field trials. J Fish Biol 60(2):340–347. https://doi.org/10.1111/j.1095-8649.2002.tb00284.x
Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Astaneh SA, Rasooli I (2006) Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 67(12):1249–1255. https://doi.org/10.1016/j.phytochem.2006.04.025
Funding
The present research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-Brazil). LUZ, R.K. received a research grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq No. 308547/2018-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anesthetics for Aulonocara nyassae
Rights and permissions
About this article
Cite this article
Ferreira, A.L., de Souza e Silva, W., Neves, L.d.C. et al. Benzocaine and menthol as anesthetics for the African cichlid Aulonocara nyassae. Aquacult Int 28, 1837–1846 (2020). https://doi.org/10.1007/s10499-020-00561-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00561-w