Abstract
This study is aimed to evaluate the toxic effects of triclosan (TCS) in an Indian major carp Labeo rohita. The 96-h LC50 value of triclosan to L. rohita was found to be 0.39 mg L−1. Fish were exposed to two sublethal concentrations (0.039 mg L−1, treatment I and 0.078 mg L−1, treatment II) of TCS for 35 days, and certain hematobiochemical, antioxidant, histopathological responses were measured. Compared to the control group, there was a significant (p < 0.05) decrease in the values and genotoxicity of hematological parameters such as hemoglobin (Hb), hematocrit (Hct), and erythrocyte (RBC) in TCS-exposed fish, but the values of leucocyte count (WBC), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) were found to be increased. A biphasic response in mean corpuscular hemoglobin concentration (MCHC) value was observed during the study period (35 days). Significant (p < 0.05) alterations in plasma biochemical parameters (glucose and protein), electrolytes (Na+, K+, and Cl−), and transaminases (GOT and GPT) were observed in fish treated with TCS in both treatments. Gill Na+/K+-ATPase activity was found to be decreased in fish treated with TCS in both treatments. Enzymatic and nonenzymatic antioxidant index levels have also fluctuated in all the tissues (gill, liver, and kidney). The histological lesions were comparatively more severe in the gill than the liver and kidney. Comet assay showed DNA damage on exposure at two sublethal concentrations. The present results suggest that TCS is highly toxic to fish even at sublethal concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The accumulation and persistence of chemicals in the aquatic environment lead to the deterioration of water quality and can cause long-term adverse effects in aquatic environments (Toni et al. 2010). Pharmaceuticals and personal care products (PPCPs) have gained a great deal of attention of both public as well as the scientific community as being one of the most significant emerging environmental contaminants of this century (Matozzo et al. 2012b; Geiger et al. 2016). PCPs are used to improve the quality of daily life and include products such as lotions, toothpaste, fragrances, shampoos, antibacterial soaps, detergents, insect repellents, and other cosmetics (Boxall et al. 2012; Hopkins and Blaney 2016). Unlike pharmaceuticals, PCPs are intended for external use on the human body and some ingredients are not subjected to metabolic alterations; therefore, large quantities of PCPs enter the environment through regular usage (Ternes et al. 2004; Zhang et al. 2015). Even though the concentration of these compounds in the environment is tiny, there is a concern about their potential long-term impacts to ecological and public health (Stephanie and Fraker 2004: Serra-Roig et al. 2016).
Triclosan (TCS), 5-chloro-2-(2,4-dichlorophenoxy)-phenol, is an antimicrobial agent added to a variety of consumer products such as cosmetics (i.e., deodorants, soaps, hand washes, toothpastes, domestics, and medical disinfectants), household detergents, plastics, skin care creams, textiles, and surgical scrub solutions (Ahn et al. 2008; Liang et al. 2013; Hu et al. 2016). The content of TCS in these products is typically in the range of 0.1 to 0.3% by weight (Sabaliunas et al. 2003; Miller et al. 2008). On the global front, the production of TCS has exceeded 1500 tons per year (Singer et al. 2002). Because of their widespread use, TCS has been detected in a wide variety of environmental samples including raw and treated wastewater, natural streams, sewage sludge, fish, and also human samples of urine, plasma, and breast milk (Adolfsson-Erici et al. 2002; Han et al. 2016). For example, TCS has been detected in the range between 18 and 98 ng L−1 in Swiss freshwaters (Singer et al. 2002) and 30 to 90 ng L−1 in Germany (Wind et al. 2004). Furthermore, a high level of TCS has been reported in wastewater treatment plants (WWTPs) in the USA (608–86,200 ng L−1), Europe (52–21,900 idem), and Asia (140–2301 ng L−1) (Bedoux et al. 2012).
Even though trace amounts of TCS enter the aquatic environment, they may still have some significant biological effects due to its high lipophilicity (log Kow 4.8). TCS is persistent in the environment with a half-life of at least 11 days in river water (Bester 2005) and 18 to 108 days in soil (Luzano et al. 2012). A major environmental concern is the tendency of TCS to be transformed into a series of chlorinated triclosan derivatives (CTDs) (Latch et al. 2005). In addition, TCS may be biotransformed into methyl-triclosan, a more persistent compound than the parent compound, by biological methylation (Lindstrom et al. 2002). The aquatic organisms that appeared most sensitive to the toxic effects of TCS were algae, molluscs, crustaceans, and fish (Chalew and Halden 2009). Accumulation of TCS has been reported in the bile of fish (Adolfsson-Erici et al. 2002; Houtman et al. 2004). Likewise, TCS has also been detected in the range of 750 to > 10,000 pg g−1 of wet weight in the plasma of fish collected from Detroit River (Valters et al. 2005). Low concentrations of TCS (313 μg L−1, 1.47 mg L−1, and 250 μg L−1) delayed the hatching time of fertilized eggs in medaka and decreased the hatchability of each egg (Ishibashi et al. 2004), altered androgenic or estrogenic pathways in fish indicating its endocrine disruption potentials (Foran et al. 2000; Ishibashi et al. 2004), altered the enzymatic activities (Liang et al. 2013), and impaired lipid metabolism in zebra fish (Ho et al. 2016).
Biochemical responses in organisms against environmental stress are widely used as early warning signs and are known as biomarkers of exposure. Inhibition and induction of biomarkers is a good approach to measure the potential impact of pollutants on environmental organisms (Rendon-von Osten et al. 2005). The most frequently used biomarkers of exposure to xenobiotics in fish are blood index, biochemical status, electrolytes, marker enzymes, detoxification of reactive oxygen species, DNA damage, and histopathological examinations of vital organs (Mayer et al. 1992; Wendelaar Bonga 1997; Mela et al. 2007). Changes in the hematological, biochemical (glucose and protein), electrolytes (Na+, K+, and Cl−), and enzymological (GOT, GPT, and Na+/K+-ATPase) parameters of fish exposed to toxicants indicate the physiological and immune status and health status of the fish (Ramesh et al. 2018). In general, the alterations in hematological, biochemical, ionoregulation, and enzymological parameters of fish provide warning signals in the field of environmental risk assessment.
The generation of the reactive oxygen species (ROS) in organisms exposed to pollutants can produce oxidative damage to macromolecules. To counteract the oxidative damage, enzymatic and nonenzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), glutathione peroxidase (GPx), and reduced glutathione (GSH) are produced in living organisms, and the alteration of their activities is widely used as potential biomarkers to assess the toxicity of aquatic pollutants (Wu et al. 2011; Maharajana et al. 2018). Likewise, generation of ROS also affects the membrane lipids that undergo peroxidation, and lipid peroxidation (LPO) level has been widely used in toxicological assays (Hemalatha et al. 2016). The single-cell gel electrophoresis assay also known as comet assay is widely used in aquatic toxicology to detect DNA strand breaks in fish exposed to toxicants (Lin et al. 2010). The health condition of the fish can also be evaluated by histopathological studies which provide the direct effects of the toxicants on organs/tissues (Figueiredo-Fernandes et al. 2007).
TCS level has been reported in the Indian rivers such as Kaveri, Vellar, and Tamiraparani (Ramaswamy et al. 2011). These freshwater bodies harbor many fish species including the Indian major carp Labeo rohita and fishing is a regular practice. Moreover, the information on the toxicity of TCS on aquatic organisms is limited. Furthermore, to our best knowledge, there is no report on the impact of TCS on Indian major carps at sublethal concentrations. Hence, the objectives of the present study are to determine the 96-h LC50 value of triclosan and to investigate the sublethal toxicity of triclosan on hematological, biochemical, enzymatic, and nonenzymatic antioxidants and histopathological and genotoxic effects in the Indian major carp L. rohita.
Materials and methods
Chemicals
Analytical grade PPCP, 5-chloro-2-(2,4-dichlorophenoxy) phenol (TCS, CAS # 3380345, 97% purity), and dimethyl sulfoxide (DMSO, CAS # 67-68-5, 99.9% purity) were purchased from Sigma-Aldrich, USA. TCS was dissolved in DMSO to make a stock solution at a concentration of 1000 mg L−1. DMSO in the test solution was kept below 0.1%.
Test fish and acclimatization
The Department of Zoology, School of Life Sciences, Bharathiar University, Coimbatore 46, Tamil Nadu, India, has been registered with the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA), Government of India. The experiments and the handling of the organisms were carried out as per the guidelines of the CPCSEA.
Fingerlings of Indian major carp Labeo rohita (14 ± 0.20 g in body weight and 7.3 ± 0.04 cm in body length) were collected from a government fish farm, Aliyar, Tamil Nadu, India. The fish were held in a large cement tank (1000 L capacity) containing 1000 L of freshwater with continuous aeration, and they were acclimated to the experimental condition (temperature (28.0 ± 1.0 °C), pH (7.4 ± 0.2), and total hardness (16.2 ± 0.4 mg L−1)) for a month. Fish were fed ad libitum once in a day with rice bran and groundnut oil cake, and 75% of the water volume was renewed to assure water quality. The excess food material and fecal matter were also removed. Feeding was stopped 24 h prior to the experiment.
Determination of the median lethal concentration and toxicity symptoms
Prior to performing 96 h static renewal acute toxicity tests (LC50), preliminary range-finding tests (data not shown) were conducted to determine the appropriate dose ranges for conducting the definitive test (APHA 1998). Briefly, a set of 10 fish were randomly selected and carefully distributed to each of the eight TCS concentrations. The experiment was repeated in triplicate to obtain the LC50 96-h value of the test chemical for the target species. A control without TCS and a solvent control (DMSO) for each concentration were also run simultaneously with three replicates. The fish were not fed during the experiment in order to maintain water quality. The survival at the end of every 24, 48, 72, and 96 h were recorded. Dead fish were removed immediately.
Treatment with sublethal concentrations
To evaluate the TCS toxicity at sublethal concentrations, fish were randomly collected from the stock and were housed in four glass aquarium (100 L capacity) with 50 fish in each; 0.039 mg L−1 (treatment I) and 0.078 mg L−1 (treatment II) of TCS were introduced into two glass aquarium tanks. Fish were exposed to the above concentrations of TCS for 35 days. The remaining two glass aquaria were maintained as control (no toxicant) and solvent control (DMSO). Three replicates were also maintained simultaneously along with treatments and control groups, with a similar setup. The glass aquariums were aerated continuously, and at the end of every 24 h, the test water was renewed, and the freshly prepared TCS solution was added to maintain the TCS at a constant level.
Fish sampling and homogenization
Blood samples were collected from the control and TCS-exposed groups prior to anesthetizing of fish to prevent hemolysis (McKnight 1966). The blood samples were transferred into clean vials and hematological parameters were immediately determined. A portion of the diluted blood sample was used for the genotoxicity assay. The remainder of the blood samples was centrifuged for 15 min at 10,000 rpm; the plasma was withdrawn and transferred into clean vials and kept at low temperature (4 °C) until use (for glucose, protein electrolytes, glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT)). After drawing the blood, fish were washed with double-distilled water and blotted dry with absorbent paper, and the tissues such as the gill, liver, and kidney were quickly removed and stored at − 80 °C. Tissues were weighed and homogenized with potassium phosphate buffer (pH 7.0, containing 0.5 mM EDTA) in ice-cold condition. Before storing, a portion of the tissue homogenate was used for the estimation of LPO activity, and the remaining portion was centrifuged at 12,000g for 20 min at 4 °C and the supernatant was used for the other antioxidant assays. For histopathological studies, gill, liver, and kidney tissues were carefully dissected and fixed in 35% formaldehyde solution.
Hematological analysis
The whole blood was used for the hematological assays such as erythrocyte (RBC) and leukocyte (WBC) counts (Rusia and Sood Routine 1992), hemoglobin (Hb) (Drabkin 1946), and hematocrit (HCT) (Nelson and Morris 1989). The other hematological indices like mean corpuscular volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC) were calculated using standard formulas.
Plasma biochemical analysis
Biochemical parameters such as glucose and protein in plasma of fish were estimated following standard procedures. Plasma glucose was estimated by the method of Cooper and Mc Daniel (1970) and protein by the method of Lowry et al. (1951).
Analysis of plasma electrolytes
Plasma electrolytes such as Na+, K+, and Cl− were estimated using commercially available kits. Na+ and K+ were analyzed by the method of Maruna (1958) and Cl− was estimated by the method of Tietz (1990) and Young et al. (1975) using Diagnostic Reagent kit supplied by Monozyme, In vitro Diagnostics, Secunderabad, Hyderabad, India. All the colorimetric assays were carried out using UV–VIS spectrophotometer.
Estimation of GOT and GPT activity
Plasma GOT and GPT activities were estimated following the method of Reitman and Franckel (1957) using commercially available kits.
Estimation of gill Na+/K+-ATPase activity
100 mg of gill tissue was separated from the control and TCS-treated fish, homogenized with 1.0 mL of 0.1 M Tris-HCl buffer (pH 7.4), and centrifuged at 1000 rpm at 4 °C for 15 min, and the supernatant was used as an enzyme source for the analysis of Na+/K+-ATPase activity (Shiosaka et al. 1971).
Antioxidant indices
Indices of oxidative stress
LPO was determined by the thiobarbituric acid (TBA) reaction with malondialdehyde (MDA), as the product formed due to peroxidation of lipids by the method of Devasagayam and Tarachand (1987). The MDA of the samples was expressed as nanomoles of MDA formed/mg protein.
Antioxidant enzyme assays
Superoxide dismutase (SOD; EC.1.15.1.1) activity was estimated following the method of Marklund and Marklund (1974) based on the autoxidation of pyrogallol, and the activity was expressed as SOD per mg of protein. The catalase (CAT; EC.1.11.1.6) activity was determined according to Aebi (1974) by the decrease in absorbance at 240 nm due to H2O2 consumption, and the enzyme activity was expressed in micromoles H2O2 consumed/min/mg protein. Glutathione-S-transferase (GST; EC.2.5.1.18) activity was estimated by measuring the conjugation of 1-chloro-2,4-dinitrobenzene with reduced glutathione at 340 nm (Habig et al. 1974) and the enzyme activity was expressed as micromoles of CDNB conjugate formed/min/mg protein. Glutathione peroxidase (GPx; EC.1.11.1.9) activity was assayed by the method of Rotruck et al. (1973), and the enzyme activity was expressed as units/mg protein (one unit is the amount of enzyme that converts 1 μmol GSH to GSSG in the presence of H2O2/min).
Reduced glutathione assay
Reduced glutathione level was measured at 412 nm using the method described by Ellman (1959), and the level of glutathione was expressed as micrograms of GSH formed/mg protein.
Histopathological analysis
Liver tissues were fixed in 10% neutral buffered formalin for 24 h, passed through graded series of alcohol, cleared with xylene, embedded in paraffin wax, sectioned (7 μm), and stained with hematoxylin and eosin (HE). Histopathological assessment was done and photographed using Leica photomicroscope.
Single-cell gel electrophoresis
Single-cell gel electrophoresis/comet assay was performed according to Singh et al. (1988) with some modifications. A 30-μL subaliquot of diluted blood sample was taken, mixed with 100 μL of 0.65% low melting agarose (LMA) in PBS, and pipetted onto fully frosted slides with precoated layer of 100 μL of 0.65% normal agarose followed by incubation in a refrigerator for solidification (10 min). Subsequently, the slides were immersed in freshly prepared cold lysing solution (2.5 M NaCl, 100 M EDTA-2Na, 10 Mm Tris, 10% DMSO, 1% Na sarcosinate, 1%Triton-X; pH 10.0) for 4 h in a refrigerator. Further, the slides were transferred to an electrophoresis unit (15 × 10 cm) filled with denaturation buffer (300 mM NaOH, 1 mM EDTA, pH 13.0) for 20 min and followed by electrophoresis in the same buffer for 20 min at 0.6 V/cm and 300 mA. The buffer volume was adjusted to cover the slides. Then slides were neutralized with Tris buffer (pH 7.5) for 2 min and stained with ethidium bromide (20 μg mL−1) for microscopic examination. Approximately, 100 cells per slide were scored randomly and examined using a florescent microscope, and the comet images were assayed using Comet Assay Software Project (CASP). The DNA damage was quantified as % DNA tail which indicates damage in the cell (Imanikia et al. 2016).
Statistical analysis
Data are expressed as mean ± SE. The data were normalized by Levene’s test. The statistical significance was evaluated by using one-way ANOVA following Duncan’s multiple range test (DMRT). Differences were considered significant at p < 0.05 using the statistical software SPSS version 20.
Results
Determination of LC50 and behavioral observation
The calculated 96-h LC50 value (95% confidence limits) of TCS to L. rohita was 0.39 mg L−1. During the exposure period, behavioral changes such as erratic movement, rapid operculum movements, swimming at the water surface, and gulping for air were noticed in fish exposed to TCS.
During the sublethal exposure period, fish exposed to TCS concentrations exhibited behavior similar to the control group in the earlier stages, but at later stages, fish exhibited abnormal behavioral changes in the form of sluggish movement, swimming imbalance, and rapid opercular movement. Clinically, TCS-exposed fish showed increased mucous secretion and hemorrhages on the ventral skin surface. There were no mortalities throughout the exposure period. Since no significant differences were observed between the control and DMSO group, the toxicant-free group (control) was maintained as the reference group for comparison.
Hematological parameters
Hematological responses of L. rohita to sublethal concentrations of TCS (treatments I and II) showed a significant decrease in Hb, Hct, and RBC values compared to the control group. However, the values of WBCs, MCH, and MCV were increased with increasing concentrations of TCS. A biphasic response was observed for MCHC. Statistical analysis showed that the changes in hematological parameters were found to be significant (p < 0.05) and concentration-dependent (Table 1).
Biochemical and electrolytes analysis
Mean values of plasma glucose and protein levels were significantly decreased (p < 0.05) in the TCS-exposed group (treatments I and II) when compared with the control group (Table 2). Sodium level was increased significantly (p < 0.05) in the TCS-exposed group in both the treatments compared to the control. In contrast to sodium level, potassium level was found to be decreased in both treatments. A significant increase (p < 0.05) in plasma chloride (except at the end of the 21st day in treatment I) was noticed when compared with their respective control group (Table 2).
Enzymological parameters
Na+/K+-ATPase activity in the gill was significantly (p < 0.05) decreased in both TCS-exposed groups when compared to the control group (Table 2). Moreover, the decreases in Na+/K+-ATPase activity in the gills were found to be concentration and exposure period dependent. The activities of GOT and GPT enzymes were significantly (p < 0.05) increased in both TCS-exposed groups compared to the control (Table 2).
Antioxidant responses
During the first week of exposure, the TCS-exposed fish showed concentration-dependent changes in SOD activity (Fig. 1a–c). Noticeably, there was a significant increase (p < 0.05) in SOD activity on day 14 in all the tissues/organs in both the treatment groups (except treatment I group in the liver). At 21 days, no statistically significant differences in SOD activities between any exposure concentration and the control were observed. A significant decrease (p < 0.05) in SOD activity in treatment groups at days 28 and 35 in all the tissues. In all the tissues, significant differences (p < 0.05) in CAT activity between TCS-exposed fish and the controls were observed in the first week of exposure (Fig. 2a–c). After this time point, no differences were observed among the treatment groups and the control group on day 14. But in the case of the liver, the condition persists till 21 days. After 21 days, a significant decrease (p < 0.05) in CAT activity was observed for the rest of the study period compared to the control.
Bar diagram showing changes in the gill (a), liver (b), and kidney (c) SOD activity of a freshwater fish L. rohita exposed to sublethal concentrations of TCS (0.039 and 0.078 mg L−1) for 35 days. All values are expressed as means ± SEs. * Indicate significantly different from the respective reference group (p < 0.05)
Bar diagram showing changes in the gill (a), liver (b), and kidney (c) CAT activity of a freshwater fish L. rohita exposed to sublethal concentrations of TCS (0.039 and 0.078 mg L−1) for 35 days. All values are expressed as means ± SEs. * Indicate significantly different from the respective reference group (p < 0.05)
Compared with the control, a significant difference in GST activities was observed in the tissues (gill, liver, and kidney) of fish exposed to TCS throughout the exposure period (7, 14, 21, and 28 days) (Fig. 3a–c). The GST activities in fish were significantly higher than those of the control on days 7, 14, and 21. After this time point, the enzyme activity was inhibited (except 14th day of treatment I in kidney) throughout the experimental period. The activity of GPx in the gill, liver, and kidney (except in gill) of TCS-exposed groups was significantly increased (p < 0.05) at the time intervals of 7 and 14 days (Fig. 4a–c). After 14 days, GPx activity in the TCS-exposed groups was decreased to control levels. Further, the enzyme activity was significantly inhibited on day 21. (p < 0.05) at days 28 and 35 compared with the control.
Bar diagram showing changes in the gill (a), liver (b), and kidney (c) GST activity of a freshwater fish L. rohita exposed to sublethal concentrations of TCS (0.039 and 0.078 mg L−1) for 35 days. All values are expressed as means ± SEs. * Indicate significantly different from the respective reference group (p < 0.05)
Bar diagram showing changes in the gill (a), liver (b), and kidney (c) GPx activity of a freshwater fish L. rohita exposed to sublethal concentrations of TCS (0.039 and 0.078 mg L−1) for 35 days. All values are expressed as means ± SEs. * Indicate significantly different from the respective reference group (p < 0.05)
A significant increase in GSH level relative to the control group was observed on days 7 and 14 (Fig. 5a–c). However, there were no significant differences among treatments compared with the control on day 21. After the 21st day, the GSH level was inhibited in the treated groups throughout the exposure period compared to the control. In all the tissues, there was a significant increase in MDA levels for both test concentrations (0.039 and 0.078 mg L−1) when compared with the control group (Fig. 6a–c).
Bar diagram showing changes in the gill (a), liver (b), and kidney (c) GSH level of a freshwater fish L. rohita exposed to sublethal concentrations of TCS (0.039 and 0.078 mg L−1) for 35 days. All values are expressed as means ± SEs. * Indicate significantly different from the respective reference group (p < 0.05)
Bar diagram showing changes in the gill (a), liver (b), and kidney (c) MDA level of a freshwater fish L. rohita exposed to sublethal concentrations of TCS (0.039 and 0.078 mg L−1) for 35 days. All values are expressed as means ± SEs. * Indicate significantly different from the respective reference group (p < 0.05)
Histopathological analysis
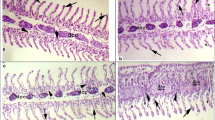
The degree of damages of the gill, liver, and kidney tissues within the TCS-treated groups is determined as none (−), mild (+), moderate (++), and severe (+++) according to Thophon et al. (2003) (Table 3). Histopathologically, no changes were observed in the gills (Fig. 7a), liver (Fig. 7d), and kidney (Fig. 7g) of the control fish. TCS-exposed fish gill showed hypertrophy in the primary and secondary lamella, lamellar fusion, and rupture and necrosis in the tips of the lamellae (Fig. 7b, c). The liver of TCS-exposed fish showed swelling of hepatocytes, pyknotic nuclei, cellular edema, vacuolation of hepatic cells, and nuclear degeneration (Fig. 7e, f). Likewise, in the kidney of TCS-exposed fish, shrinking of the glomeruli, edema in Bowman’s capsule, pyknotic nuclei, and renal tubular degeneration were observed (Fig. 7h, i).
a Control gill. L—lamellae. b, c HP—hyperplasia; LF—lamellar fusion; HT—hypertrophy; EN—epithelial necrosis; EL—epithelial lifting. d Control liver. H—hepatocytes. e, f V—vacuolation; PN—pyknotic nucleus; ND—nuclear degeneration; NH—necrotic hepatocytes; CE—cellular edema. g Control kidney. h, i GN—glomerular necrosis; PN—pyknotic nucleus; HDD—hyaline droplets degeneration; SG—shrinking of the glomeruli; RTD—renal tubular degeneration; RTS—renal tubular separation; TN—tubular necrosis. (H & E, × 100). Scale bar = 50 μm
Single-cell gel electrophoresis
SCGE/comet assay was employed to evaluate the genotoxic effects of TCS on the fish L. rohita. The comet assay results showed significantly higher DNA damage in fish exposed to TCS than in the control group (Figs. 8 and 9). Further, the damage was found to be concentration dependent, peaking on the 21st day of the exposure period. Subsequently, there was a decreasing gradient of the percentage of DNA in the tail region in both the exposed groups with the increase in exposure time in the peripheral blood of the exposed fish.
Discussion
In the present study, the sublethal toxicity of TCS to L. rohita was assessed using hematological, biochemical, histological, and oxidative stress parameters. The 96-h LC50 value calculated in this study (0.37 mg L−1) is very similar to the values found by Oliveira et al. (2009) for Danio rerio (0.34 mg L−1) and Orvos et al. (2002) for Lepomis macrochirus (0.37 mg L−1) and Pimephales promelas (0.26 mg L−1). Likewise, the 96-h LC50 values of TCS to medaka larvae were found to be 0.6 mg L−1 (Falisse et al. 2017) and 1.47 mg L−1 to Xiphophorus helleri (Liang et al. 2013). The differences within or among fish species regarding their sensitivity to TCS may depend on size, age, health status, and test conditions (Eaton and Gilbert 2008). Fish are vulnerable to TCS during their early development (Dann and Hontela 2011). The present results show that TCS is highly toxic to L. rohita, and the toxicity of TCS on L. rohita increased with increasing concentration and exposure time. Recent studies also indicate that the effects of TCS on the early life stage of zebra fish are specific to exposure concentration (Falisse et al. 2017). TCS may exert its toxic effect through thyroid axis disruption and oxidative stress induction (Oliveira et al. 2009; Schnitzler et al. 2016). In the present study, oxidative stress induction has been noticed in fish upon exposure to different concentrations of TCS which may be one of the toxic mechanisms of TCS as suggested by previous authors (Binelli et al. 2009a, 2009b; Riva et al. 2012). Recent studies indicate that TCS may interfere with iodide uptake (Wu et al. 2016).
The study on the behavior of animals under toxicant stress may be useful for studying environmental pollutants and their effects. Fish are widely used as an excellent model in this regard to understand the ecotoxicity of many toxicants in a controlled environment (Scott and Sloman 2004). In the present study, abnormal behavioral changes induced in the TCS-exposed fish appear to be a manifestation of TCS toxicity. The increase in surfacing and gulping of surface water upon exposure to TCS appears to be an attempt by the fish to avoid breathing in poisoned water. Erratic swimming may be due to the effects of TCS on the central nervous system. The clinical signs of excessive mucus secretion may have been caused by melanosis, due to exposure of the fish to stress, which irritates the gills (Ferguson 1989). The observed behavioral and clinical signs in L. rohita are consistent with those reported by Oliveira et al. (2009). Similar observations were also reported by Orvos et al. (2002) with Oncorhynchus mykiss, exposed to TCS. On the basis of the study by Fritsch et al. (2013), we inferred that altered swimming pattern occurred in L. rohita after TCS exposure. The observed swimming behavior of fish may also be due to neurological dysfunction due to TCS toxicity (Falisse et al. 2017).
Hematological parameters are widely used for the measurement of physiological disturbances in toxicant-exposed fish (Schlenk et al. 2008). To the best of our knowledge, there is no available information on the effects of TCS on hematological parameters of L. rohita. Hb, Hct, and RBC play a vital role in the transportation of nutrition, oxygen, and metabolic wastes in many organisms (Min and Kang 2008). In the present study, the decrease of hematological parameters such as Hb, Hct, and RBC content indicates shrinkage of red blood cells due to the toxic action of TCS. A similar observation was also reported in C. carpio after exposure to pharmaceutical drugs such as clofibric acid and diclofenac (Saravanan et al. 2011). The higher values of erythrocyte indices may be due to hypoxia, impaired water balance, or macrocytic anemia. Increased WBC count in the present study indicates that TCS may activate the immune system in fish thus signaling an adaptive immune response (Modesto and Martinez 2010). Moreover, the increase in WBC content might have resulted from hypoxia and gill damage due to the accumulation of toxicants in the gill region (El-Sayed et al. 2015).
Changes in the biochemical parameters are widely used to assess the health condition of the fish exposed to contaminants both in laboratory and field studies (Shailaja and D’Silva 2003) because they are sensitive to environmental changes and provide important information about the internal environment of the organism (Qiu et al. 2009). The decrease in the plasma glucose level of fish exposed to toxicants may be due to enhanced energy demand that stimulates utilization and exhaustion of glucose (El-Sayed et al. 2007) or may be due to the rapid utilization of blood glucose during stress condition (Agrahari et al. 2007). In the present study, the decrease in plasma glucose level indicates high utilization of glucose to tolerate the stress condition of TCS. The decrease in plasma protein level noted in this study may be due to the necrosis of cells which may lead to impairment in protein synthesis machinery (Fontana et al. 1998). Alterations in heat shock proteins were also reported in zebra fish embryos upon exposure to TCS (Falisse et al. 2017). The significant decrease in plasma protein in toxicant-exposed fish may also be caused due to impaired protein synthesis due to liver disorder (Saravanan et al. 2011). In the present investigation, also the observed decrease in plasma protein in TCS-exposed fish indicates liver disorder due to the accumulation of TCS in the liver.
The alterations in the GOT and GPT activities may offer an early warning of potentially harmful alterations in aquatic organisms under stress conditions (Abdel-Khalek et al. 2015). Moreover, the alterations in these enzyme activities can be used as sensitive biomarkers in the field of ecotoxicology. In general, alterations in the activities of these two enzymes in blood plasma indicate tissue impairment caused by stress (Svoboda 2001). In the present study, there was a significant increase in GOT and GPT activities in the plasma of L. rohita exposed to TCS for 35 days which may result from the accumulation of TCS in target organs resulting in damage or injury in the target organs. Chen et al. (2004) reported that the elevated levels of these two enzymes in tilapia are linked with hepatic injury. Alterations in GOT and GPT activities were also noticed in goldfish and rainbow trout upon treated with sencor and carbamazepine, respectively (Li et al. 2010; Maksymiv et al. 2015). Furthermore, any changes in the protein and carbohydrate metabolism due to toxicant stress may also affect transaminase activity (Ramesh et al. 2018).
Sodium, potassium, and chloride concentrations in fish blood play a vital role for active regulation of water influx, and ion efflux and their alterations upon exposure to toxicants can be used as sensitive biomarkers of chemical exposure and effects (Mayer et al. 1992). In general, plasma Na+ and Cl− levels in aquatic organisms tend to be affected by waterborne toxicants (Pelgrom et al. 1995). In the present study, plasma Na+ and Cl− concentrations were more strongly affected in TCS-exposed fish. These ion increases could be the result of plasma water moving out of the circulation and into the tissues (Wendelaar Bonga 1997). Unlike the other plasma ions, plasma K+ concentrations decreased in fish exposed to TCS. This decrease in plasma K+ concentration may reflect an increase in potassium influx to erythrocytes and/or other cell types in response to TCS present in the water. Furthermore, the action of aquatic pollutants on organs involved in osmoregulation, endocrine system, and metabolism and on active transport processes may cause a shift in the hydromineral balance of the organisms (Martinez and Colus 2002). Osmoregulatory failure due to toxicant stress may also cause a decrease in plasma electrolyte levels (Poopal et al. 2017). In general, organic pollutants in the environment affect the Na+/K+-ATPase by decreasing its activity. In the present investigation, inhibition in the activity of Na+/K+-ATPase in gill tissues after TCS exposure suggests reduced NAK transport across the membrane. Furthermore, the alterations in the plasma Na+, Cl−, and K+ in the present study might be due to inhibition of Na+/K+-ATPase by TCS toxicity.
There is ample evidence indicating that TCS may alter the pro-oxidant/antioxidant balance and induce oxidative stress in diverse aquatic species. The knowledge of oxidative stress in fish has great importance for environmental and aquatic toxicology. The enzyme SOD plays a vital role in the body’s antioxidant system under stress conditions (Kappus 1985). SOD, which catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide, plays an important role in defending against the accumulation of toxic activated oxygen species (AOS) (Salin 1987; Lin et al. 2010). In the present study, SOD activity increased at sublethal concentrations of TCS during the early exposure period (7 and 14 days). A significant increase in SOD activity has been reported in the gills of clam Ruditapes philippinarum exposed to 600 and 900 ng of TCS/L (Matozzo et al. 2012a). Liu et al. (2010) reported that an increase in oxygen free radical production occurred in fish under a low level of stress condition. Consequently, excessive ROS may have induced the synthesis of more SOD or increased its activity to protect against oxidative stress. As the exposure time increased (28 and 35 days), SOD activity decreased below the levels detected in the control groups. This decrease in SOD activity may be because of the increased oxygen free radicals in fish that rendered SOD inactive by oxidation. Previous research demonstrated that SOD activity was activated under mild adverse stress and declined under more intense stress (Shao et al. 2012). Inhibition of SOD activity has also been reported in the digestive gland of the clam Ruditapes philippinarum exposed to 600 and 900 ng TCS/L (Matozzo et al. 2012a). The inhibition of SOD activity can be explained by the metabolism of TCS over time, which may have reduced the toxicant concentrations during prolonged exposure, and the adverse effects exerted on SOD synthesis, and its activity was induced by excess ROS. These results show that the toxic effect of TCS on SOD activity is much more evident at higher concentrations.
CAT located in the peroxisomes plays a vital role in the reduction in hydrogen peroxide produced from the metabolism of long chain fatty acids to water and oxygen (Stanic et al. 2006). In this assay, CAT activities in fish exposed to different TCS concentrations increased during the first week of exposure and then decreased to control levels and even inhibited at later time points. Elevation of CAT activity has also been reported in Ruditapes philippinarum exposed to different concentrations of TCS (Binelli et al. 2011; Matozzo et al. 2012a). Lin et al. (2010) reported a biphasic response in CAT activity in the earthworm Eisenia fetida following TCS treatment which is in agreement with our results. They also suggested that TCS can induce CAT synthesis in fish in response to scavenging H2O2 into H2O and O2 to maintain free radical balance. According to Sanchez et al. (2015), an increase in the CAT activity may be caused by the increase of pro-oxidants. In the present study, the decrease in the CAT activity indicates damage to the antioxidant defense system due to TCS toxicity (Lin et al. 2010).
Glutathione-S-transferases catalyze the conjugation of glutathione to a variety of compounds and also involved in the cellular detoxification of xenobiotic compounds (Livingstone 2003). In our study, GST activities were activated after 7, 14, and 21 days of exposure in all tissues and then suppressed at the end of the 28th and 35th day. The fluctuations in GST activity have been demonstrated (except gill) in several fishes in response to organic contaminants (Kubrak et al. 2010; Ge et al. 2015). An elevation in GST activity has been reported in zebra fish (Danio rerio) exposed to TCS at 0.25 and 0.35 mg L−1 concentrations (Oliveira et al. 2009). Likewise, GST activity was increased in the digestive gland of Mytilus galloprovincialis when exposed to TCS (Canesi et al. 2007). However, no significant effect of TCS toxicity on GST activity was noticed in zebra fish upon exposure to 50 and 100 μg TCS/L (Falisse et al. 2017).
In the present study, the observed increase in GST activity in all tissues suggests an increase in detoxification processes in L. rohita. Furthermore, the decline in GST activity reflects impairment in the detoxifying capacity of the fish and the occurrence of oxidative stress. The GST was more active in liver tissue than in the gill and kidney, which indicates the effective role of the liver in xenobiotic detoxification (Basha and Rani 2003).
Glutathione peroxidase catalyzes GSH-dependent reduction of many peroxides, including H2O2 and LOOH, thereby protecting cells from ROS-induced damage (Lushchak 2012). In this assay, GPx activities in L. rohita exposed to different TCS concentrations increased after 7, 14, and 21 days of exposure in the gill, liver, and kidney and then decreased to control levels and even inhibited at later time points. This biphasic response is considered an adaptation to a detoxification activity increase. The initial elevated level of GPx activity in fish exposed to TCS indicates stimulation of the antioxidant pathway due to increasing peroxide concentrations. On the contrary, a reduced GPx activity in a given tissue could indicate that its antioxidant capacity was exceeded by the amount of hydroperoxide products. Thus, inhibition of GPx activity might reflect a possible failure of the antioxidant system in tissues of exposed fish (Ballesteros et al. 2009). Ahmad et al. (2006) observed an altered GPx activity in the tissues of European eel after polluted water exposure. Likewise, GPx activity was significantly increased in zebrafish embryos when exposed to 50 and 100 μg TCS/L (Falisse et al. 2017).
Reduced glutathione is the main nonprotein thiol and one of the main reductants found in cells (Siegers 1989). It possesses antioxidant properties and its protective role against oxidative-stress-induced toxicity in aquatic animals is well established (Hasspielar et al. 1994). We found an initial increase in L. rohita gill, liver, and kidney GSH levels exposed to TCS. However, the tissue GSH levels decreased to the control level after 21 days of exposure, whereas it significantly decreased in later exposure periods. This altered response is considered as an adaptation to a detoxification activity increase (Buchner et al. 1993). Moreover, the protective effects of GSH were attributed to its ability to stabilize the pollutants by scavenging the free radicals and thereby blocking LPO development (Meister and Anderson 1983). The significant decrease in GSH in all treated groups indicates the imbalance between the oxidative and antioxidant systems with longer exposure time. Peroxidation of lipids is essential for aquatic animals since they usually contain greater amounts of highly unsaturated fatty acids (Huang et al. 2003). In the present study, fish exposed to TCS for 35 days showed enhanced LPO levels in the gill, liver, and kidney which indicate an overproduction of free radicals and/or an insufficient antioxidant response to scavenger ROS and the prevention of oxidative stress. Earlier studies show that LPO may be induced in various tissues by a variety of environmental pollutants (Ahmad et al. 2003; Isik and Celik 2008). Recently, Pan et al. (2018) reported that the increase of LPO level in Chlamydomonas reinhardtii cell membranes indicates that TCS induced oxidative stress or the increase of ROS production. The increase of MDA content in E. fetida exposed to TCS may be a response to oxidative stress caused by the toxicant (Lin et al. 2010).
Histopathological investigations of organ sections of TCS-exposed fish revealed adverse effects in the gill, liver, and kidney. In fish, gills play an important role in respiratory, osmoregulatory, and excretory functions and also a target organ for pollutants (Evans 1993). Toxicants may affect the structural morphology of the gill or damage the organ which results in respiratory distress in fish (Magare and Patil 2000). In the present investigation, exposure of L. rohita to TCS resulted in structural alterations of the gill lamellae including hypertrophy and necrosis of the epithelium, epithelial lifting, lamellar fusion, and hyperplasia of the lamella. Similar results were also observed by Barja-Fernández et al. (2013), Barišić et al. (2015), and Samanta et al. (2015). Histopathological lesions such as lamellar fusion, hyperplasia, and epithelial necrosis were noticed in fish gills of rainbow trout (Oncorhynchus mykiss) upon exposure to TCS (Capkin et al. 2017). The observed epithelial necrosis of the gill epithelium is a direct response to TCS toxicity, whereas lifting of the epithelium, hyperplasia, and lamellar fusion indicate the defense responses of the fish. The lifting up of the epithelium increases the distance through which the toxicant has to travel or reach the bloodstream. Lifting is reported to be induced by the incidence of severe edema (Schwaiger et al. 2004) as it was found in the present experiment. Changes in the structural morphology of the gill may allow the toxicants to enter which results in disruption mechanism of the fish (Ramesh et al. 2018).
Fish liver is one of the most receptive organs which show alterations in histoarchitecture following exposure to various types of environmental pollutants (Ahmed et al. 2013). The histopathological lesions observed in the liver were vacuolation, cellular edema, low number of necrotic hepatocytes, nuclear degeneration, and pyknotic nuclei. Pyknotic nucleus and necrotic hepatocytes were observed in the liver of the TCS-exposed fish Oncorhynchus mykiss (Capkin et al. 2017). Vacuolation of hepatocytes is associated with the excessive accumulation of fat in the cytoplasm (Bogiswariy et al. 2008). Degeneration of liver tissue and necrosis of central vein could be due to the accumulation of neutrophils and lymphocytes. Mohamed (2001) reported that the cellular degeneration in the liver might be due to oxygen deficiency as a result of gill degeneration. Myers et al. (1987) reported that pyknosis is the state of condensed nuclei present in the hepatocytes; it might be due to the deposition of lipids and glycogen.
The teleostean kidney is one of the first organs to be affected by contaminants in the water (Thophon et al. 2003). Most common alterations found in the kidney of fishes exposed to TCS are hyaline droplet degeneration, renal tubular separation, renal tubular degeneration, shrinking of the glomeruli and pyknotic nuclei. Likewise, pyknotic nuclei and glomerulus degeneration were observed in the kidney of the TCS-exposed fish Oncorhynchus mykiss (Capkin et al. 2017). The earlier results correlate with the findings of Bucher and Hofer (1993). Samanta et al. (2015) observed shrinkage of the glomerulus, damaged and hypertrophied proximal convoluted tubule, and loss of hematopoietic tissue in the fish Anabas testudineus (Bloch) treated with almix. In the present study, among the three organs studied, the structural alterations were more in the gill which may be due to its direct contact with the environment. In general, due to its lipophilic nature, TCS may accumulate in the target organs of fish, which may lead to morphological changes (Dann and Hontela 2011).
The analysis of DNA integrity in aquatic organisms has been shown to be a highly suitable method for evaluating the impact of environmental genotoxicants, allowing the detection of exposure to low concentrations of contaminants (Scalon et al. 2010). Furthermore, DNA damage along with nuclear abnormalities can be used as biomarkers to monitor the pollution of freshwater bodies (Hussain et al. 2018). In the present study, TCS exposure to fish caused a pronounced increase in the percentage of DNA in the tail indicating DNA damage in both treatment groups. Such results are concordant with the previous reports of the effects of TCS on other species such as zebra mussel (Binelli et al. 2009b), aquatic insect (Martínez-Paz et al. 2013), protozoan (Gao et al. 2015), and in Oncorhynchus mykiss (Capkin et al. 2017). Recently, Wang et al. (2018) also reported the genotoxic effect of TCS through micronucleus formation and nuclear abnormalities in the peripheral blood and liver of goldfish. Moreover, reports on the genotoxicity of TCS, particularly on fish, are rather scarce. Our results provide the first evidence for genotoxicity effects of TCS on the freshwater fish L. rohita. The precise mechanism of TCS-induced DNA damage is unclear, but it is suggested that TCS affects the stability of DNA both by generating ROS and by preventing the base excision repair system (Binelli et al. 2009a, 2009b). Further, this may be associated with necrotic or apoptotic DNA fragmentation (Gichner et al. 2006). The present study shows that DNA damage was significantly higher as the concentration of TCS was increased which indicates that TCS is genotoxic to the fish Labeo rohita. Furthermore, the damage can be used to monitor the potential risk of TCS to aquatic organisms.
Conclusion
The results of our study suggest that the exposure of L. rohita to TCS alters hematological, biochemical, and physiological parameters together with histopathological changes in certain target tissues. This study also confirms that TCS is genotoxic to fish since it induces DNA strand breaks. Furthermore, these parameters can be used as potential biomarkers for risk assessment in the aquatic ecosystem. Studies on TCS toxicity to fish are rare, and the present findings may contribute to the scarce literature regarding fish sublethal exposure to TCS. In addition to this, our results implied that constant TCS release to the environment is able to cause toxic effects to aquatic organisms.
References
Abdel-Khalek AA, Kadry MAM, Badran SR, Marie MAS (2015) Comparative toxicity of copper oxide bulk and nano particles in Nile tilapia; Oreochromis niloticus: biochemical and oxidative stress. J Basic Appl Zool 72:43–57
Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J (2002) Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 46(9–10):1485–1489
Aebi H (1974) Catalase. In: Bergmeyer HV (ed) Methods in enzymatic analysis. Academic, New York, pp 674–684
Agrahari S, Kashev C, Pandey KC, Krishna Gopal K (2007) Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch). Pestic Biochem Physiol 88:268–272
Ahmad I, Pacheco M, Santos MA (2003) Naphthalene-induced differential tissue damage association with circulating fish phagocyte induction. Ecotoxicol Environ Saf 54(1):7–15
Ahmad I, Pacheco M, Santos MA (2006) Anguilla anguilla L. oxidative stress biomarkers: an in situ study of freshwater wetland ecosystem (Pateira de Fermentelos, Portugal). Chemosphere 65(6):952–962
Ahmed MK, Habibullah-Al-Mamuna M, Parvina E, Akter MS (2013) Arsenic induced toxicity and histopathological changes in gill and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus). Exp Toxicol Pathol 65(6):903–909
Ahn KC, Zhao F, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DPY, Gee S, Hammock BD (2008) In vitro biologic activities of the antimicrobials triclocarban, it analogs, and triclosan in bioassay screen: receptor-based bioassay screens. Environ Health Perspect 116(9):1203–1210
APHA (1998) American Public Health Association: Standard Method for examination of water and waste 20th ed., Washington DC: America Public Health Association.
Ballesteros ML, Wunderlin DA, Bistoni MA (2009) Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan. Ecotoxicol Environ Saf 72:199–205
Barišić J, Dragun Z, Ramani S, Filipović Marijić V, Krasnići N, Čož-Rakovac R, Kostov V, Rebok K, Jordanova M (2015) Evaluation of histopathological alterations in the gills of Vardar chub (Squalius vardarensis Karaman) as an indicator of river pollution. Ecotoxicol Environ Saf 118:158–166
Barja-Fernández S, Míguez JM, Alvarez-Otero R (2013) Histopathological effects of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in the gills, intestine and liver of turbot (Psetta maxima). Ecotoxicol Environ Saf 95:60–68
Basha PS, Rani AU (2003) Cadmium induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (tilapia). Ecotoxicol Environ Saf 56:218–221
Bedoux G, Roig B, Thomas O, Dupont V, Le Bot B (2012) Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res 19:1044–1065
Bester K (2005) Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch Environ Contam Toxicol 49:9–17
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009a) Cytotoxic and genotoxic effects of in vitro exposure to triclosan and trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp Biochem Physiol C Toxicol Pharmacol 150:50–56
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009b) In vivo experiments for the evaluation of genotoxic and cytotoxic effects of triclosan in zebra mussel hemocytes. Aquat Toxicol 91:238–244
Binelli A, Parolini M, Pedriali A, Provini A (2011) Antioxidant activity in the zebra mussel (Dreissena polymorpha) in response to triclosan exposure. Water Air Soil Pollut 217:421–430
Bogiswariy S, Jegathambigai R, Marimuthu K (2008) Effect of acute exposure of cadmium chloride in the morphology of the liver and kidney of mice. In: Proceedings of the international conference on environmental research and technology (ICERT), May 28–30. Parkroyal, Penang, Malaysia, pp 1036–1042
Boxall AB, Rudd MA, Brooks BW (2012) Pharmaceuticals and personal care products in the environment: what are the big questions? Environ Health Perspect 120:1221–1229
Bucher F, Hofer R (1993) The effects of treated domestic sewage on three organs (gills, kidney, liver) of brown trout (Salmo trutta). Water Res 27:255–261
Buchner F, Hofer R, Krumschnabel G, Doblander C (1993) Disturbances in the pro-oxidant-antioxidant balance in the liver of bullhead (Cottus gobio L.) exposed to treated paper mill effluents. Chemosphere 27:1329–1338
Canesi L, Ciacci C, Lorusso LC, Betti M, Gallo G, Pojana G, Marcomini A (2007) Effects of triclosan on Mytilus galloprovincialis hemocyte function and digestive gland enzyme activities: possible modes of action on non target organisms. Comp Biochem Physiol C 145:464–472
Capkin E, Ozcelep T, Kayis S, Altinok I (2017) Antimicrobial agents, triclosan, chloroxylenol, methylisothiazolinone and borax, used in cleaning had genotoxic and histopathologic effects on rainbow trout. Chemosphere 182:720–729
Chalew TEA, Halden RU (2009) Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J Am Water Resour Assoc 45:4–13
Chen C, Wooster GA, Bowser PR (2004) Comparative blood chemistry and histopathology of tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin or copper sulfate. Aquaculture 239:421–443
Cooper GR, Mc Daniel V (1970) The determination of glucose by the O-toluidine method. Stand Methods Clin Chem 6:159–170
Dann AB, Hontela A (2011) Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol 31:285–311
Devasagayam TPA, Tarachand U (1987) Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun 145:134–138
Drabkin DL (1946) Spectrometric studies, XIV—the crystallographic and optimal properties of the hemoglobin of man in comparison with those of other species. J Biol Chem 164:703–723
Eaton DL, Gilbert SG (2008) Principles of toxicology. In: Klaassen CD (ed) Casarett and Doull’s toxicology. The basic science of poisons, 7th edn. McGraw-Hill, New York, pp 11–44
Ellman GL (1959) Tissue sulfhydril groups. Arch Biochem Biophys 82:70–77
El-Sayed YS, Saad TT, El-Bahr SM (2007) Acute intoxification of deltamethrin in monosex Nile tilapia, Oreochromis niloticus with special reference to the clinical, biochemical and haematological effects. Environ Toxicol Pharmacol 24:212–217
El-Sayed YS, Samak DH, Abou-Ghanema IY, Soliman MK (2015) Physiological and oxidative stress biomarkers in the freshwater monosex Nile tilapia, Oreochromis niloticus L., exposed to pendimethalin-based herbicide. Environ Toxicol 30:430–438
Evans DH (1993) Osmotic and ionic regulation. In: Evans DH (ed) The physiology of fishes. CRC, Boca Raton, FL, pp 315–341
Falisse E, Voisin AS, Silvestre F (2017) Impacts of triclosan exposure on zebrafish early-life stage: toxicity and acclimation mechanisms. Aquat Toxicol 189:97–107
Ferguson HW (1989) Systemic pathology of fish. Iowa State University Press, Ames, Iowa
Figueiredo-Fernandes A, Ferreira-Cardoso JV, Garcia-Santos S, Monteiro SM, Carrola J, Matos P, Fontaínhas-Fernandes A (2007) Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesqui Vet Bras 27(3):103–109
Fontana L, Moreira E, Torres M, Fernandez I, Rios A, Sanchezde Medina F, Gi A (1998) Dietary nucleotides correct plasma and liver microsomal fatty acids alterations in rats with liver cirrhosis induced by oral intake of thioacetamide. J Hepatol 28:662–669
Foran CM, Bennett ER, Benson WH (2000) Developmental evaluation of a potential non-steroidal estrogen: triclosan. Mar Environ Res 50(1–5):153–156
Fritsch EB, Connon RE, Werner I, Davies RE, Beggel S, Feng W, Pessah IN (2013) Triclosan impairs swimming behavior and alters expression of excitation-contraction coupling proteins in fathead minnow (Pimephales promelas). Environ Sci Technol 47:2008–2017
Gao L, Yuan T, Cheng P, Bai Q, Zhou C, Ao J, Wang W, Zhang H (2015) Effects of triclosan and triclocarban on the growth inhibition, cell viability, genotoxicity and multixenobiotic resistance responses of Tetrahymena thermophila. Chemosphere 139:434–440
Ge W, Yan S, Wang J, Zhu L, Chen A, Wang J (2015) Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J Agric Food Chem 63:1856–1862
Geiger E, Hornek-Gausterer R, Saçan MT (2016) Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotoxicol Environ Saf 129:189–198
Gichner T, Patkova Z, Szakova J, Demnerova K (2006) Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotoxicol Environ Saf 54:420–426
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7713
Han J, Won EJ, Hwang UK, Kim LC, Yim JH, Lee JS (2016) Triclosan (TCS) and triclocarban (TCC) cause lifespan reduction and reproductive impairment through oxidative stress-mediated expression of the defensome in the monogonont rotifer (Brachionus koreanus). Comp Biochem Physiol C Toxicol Pharmacol 185-186:131–137
Hasspielar BM, Behar JV, Di Giulio RT (1994) Glutathione-dependent defense in channel catfish (Ictalurus punctatus) and brown bullhead (Ameriurus nebulosus). Ecotoxicol Environ Saf 28:82–90
Hemalatha D, Amala A, Rangasamy B, Nataraj B, Ramesh M (2016) Sublethal toxicity of quinalphos on oxidative stress and antioxidant responses in a freshwater fish Cyprinus carpio. Environ Toxicol 31:1399–1406
Ho JC, Hsiao CD, Kawakami K, Tse WK (2016) Triclosan (TCS) exposure impairs lipid metabolism in zebrafish embryos. Aquat Toxicol 173:29–35
Hopkins ZR, Blaney L (2016) An aggregate analysis of personal care products in the environment: identifying the distribution of environmentally-relevant concentrations. Environ Int 92-93:301–316
Houtman CJ, Van Oostveen AM, Brouwer A, Lamoree MH, Legler J (2004) Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environ Sci Technol 38:6415–6423
Hu Y, Ren G, Deng L, Zhang J, Liu H, Mu SW, Wu T (2016) Degradable UV-cross linked hydrogel for the controlled release of triclosan with reduced cytotoxicity. Mater Sci Eng C Mater Biol Appl 67:151–158
Huang CH, Chang RJ, Huang SL, Chen W (2003) Dietary vitamin E supplementation affects tissue lipid peroxidation of hybrid tilapia, Oreochromis niloticus x O. aureus. Comp Biochem Physiol B Biochem Mol Biol 134:265–270
Hussain B, Sultana T, Sultana S, Masoud MS, Ahmed Z, Mahboobb S (2018) Fish eco-genotoxicology: comet and micronucleus assay in fish erythrocytes as in situ biomarker of freshwater pollution. Saudi J Biol Sci 25(2):393–398
Imanikia S, Galea F, Nagy E, Phillips DH, Stürzenbaum SR, Arlt VM (2016) The application of the comet assay to assess the genotoxicity of environmental pollutants in the nematode Caenorhabditis elegans. Environ Toxicol Pharmacol 45:356–361
Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, Arizono K (2004) Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol 67:167–179
Isik I, Celik I (2008) Acute effects of methyl parathion and diazinon as inducers for oxidative stress on certain biomarkers in various tissues of rainbow trout (Oncorhynchus mykiss). Pestic Biochem Physiol 92:38–42
Kappus H (1985) Lipid peroxidation; mechanisms, analysis, enzymology and biological relevance. In: Sies H (ed) Oxidative stress. Academic, London, pp 273–310
Kubrak OI, Lushchak OV, Lushchak JV, Torous IM, Storey JM, Storey KB, Lushchak VI (2010) Chromium effects on free radical processes in goldfish tissues: comparison of Cr (III) and Cr (VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp Biochem Physiol C Toxicol Pharmacol 152:360–370
Latch DE, Packer JL, Stender BL, Van Overbek J, Arnold WA, McNeill K (2005) Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ Toxicol Chem 24:517–525
Li ZH, Velisek J, Zlabek V, Grabic R, Machova J, Kolarova J, Randak T (2010) Hepatic antioxidant status and hematological parameters in rainbow trout, Oncorhynchus mykiss, after chronic exposure to carbamazepine. Chem Biol Interact 183:98–104
Liang X, Nie X, Ying G, An T, Li K (2013) Assessment of toxic effects of triclosan on the swordtail fish (Xiphophorus helleri) by a multi-biomarker approach. Chemosphere 90:1281–1288
Lin DS, Zhou QX, Xie XJ, Liu Y (2010) Potential biochemical and genetic toxicity of triclosan as an emerging pollutant on earthworms (Eisenia fetida). Chemosphere 81:1328–1333
Lindstrom A, Buerge IJ, Poiger T, Bergqvist PA, Muller MD, Buser HR (2002) Occurrence and environmental behaviour of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ Sci Technol 36:2322–2329
Liu Y, Zhou QX, Xie XJ, Lin DS, Dong LX (2010) Oxidative stress and DNA damage in the earthworm Eisenia fetida induced by toluene, ethylbenzene and xylene. Ecotoxicology 19:1551–1559
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev Med Ve 154:427–430
Lowry OH, Rosebrough NJ, Farr AL, Randall L (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Biol Chem 26:736837
Luzano N, Rice CP, Ramirez M, Torrents A (2012) Fate of triclosan and methyltriclosan in soil from biosolids application. Environ Pollut 160:103–108
Magare SR, Patil HT (2000) Effect of pesticides on oxygen consumption, red blood cell count and metabolites of a fish, Puntius ticto. Environ Ecol 18:891–894
Maharajana K, Muthulakshmia S, Nataraj B, Ramesh M, Kadirvelu K (2018) Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos (Danio rerio): a multi biomarker study. Aquat Toxicol 196:132–145
Maksymiv IV, Husak VV, Mosiichuk NM, Matviishyn TM, Sluchyk IY, Storey JM, Storey KB, Lushchak VI (2015) Hepatotoxicity of herbicide Sencor in goldfish may result from induction of mild oxidative stress. Pestic Biochem Physiol 122:67–75
Marklund S, Marklund G (1974) Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Martinez CBR, Colus IMS (2002) Biomarcadores em peixes neotropicais para o monitoramento da poluicao aquatic na bacia do rio Tibagi. In: Medri ME, Bianchini E, Shibatta AO, Pimenta JA (eds) A bacia do rio Tibagi. Parana, Londrina, pp 551–577
Martínez-Paz P, Morales M, Martínez-Guitarte JL, Morcillo G (2013) Genotoxic effects of environmental endocrine disruptors on the aquatic insect Chironomus riparius evaluated using the comet assay. Mutat Res 758:41–47
Maruna RFL (1958) Quantitative estimation of sodium (Na+) and potassium (K+) in human serum by colorimetric method. Clin Chim Acta 2:581–585
Matozzo V, Formenti A, Donadello G, Marin MG (2012a) A multi-biomarker approach to assess effects of triclosan in the clam Ruditapes philippinarum. Mar Environ Res 74:40–46
Matozzo V, Rova S, Marin MG (2012b) The nonsteroidal anti-inflammatory drug, ibuprofen, affects the immune parameters in the clam Ruditaps philippinarum. Mar Environ Res 79:116–121
Mayer FL, Versteeg DJ, McKee MJ, Folmar LC, Graney RL, McCume DC, Rattner BA (1992) Physiological and non-specific biomarkers. In: Hugget RJ, Kimerle RA Jr, Mehrle PM, Bergman HL (eds) Biomarkers: biochemical, physiological, and histological markers of anthropogenic stress. Lewis, Chelsea, pp 5–85
McKnight IMA (1966) Hematological study on the mountain white fish, Popium willasemi. J Fish Res Board Can 23:45–64
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Mela M, Randi MAF, Ventura DF, Carvalho CEV, Pelletier E, Oliveira Ribeiro CA (2007) Effects of dietary methyl mercury on liver and kidney histology in the neotrophical fish Hoplias malabaricus. Ecotoxicol Environ Saf 68:426–435
Miller TR, Heidler J, Chillrud SN, Delaquil A, Ritchie JC, Mihalic JN, Bopp R, Halden RU (2008) Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ Sci Technol 42:4570–4576
Min EY, Kang JC (2008) Effect of waterborne benomyl on the hematological and antioxidant parameters of the Nile tilapia, Oreochromis niloticus. Pestic Biochem Physiol 92:138–143
Modesto KA, Martinez CBR (2010) Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299
Mohamed FA (2001) Impacts of environmental pollution in the southern region of Lake Manzalah, Egypt, on the histological structures of the liver and intestine of Oreochromis niloticus and Tilapia zilli. J Egypt Acad Soc Environ Dev 2:25–42
Myers MS, Rhodes LD, McCain BB (1987) Pathologic anatomy and patterns of occurrence of hepatic neoplasms, putative preneoplastic lesions and other idiopathic hepatic conditions in English sole (Parophrys vetulus) from Puget Sound, Washington, USA. J Natl Cancer Inst 78:333–363
Nelson DA, Morris MW (1989) Basic methodology. Hematology and coagulation, part IV. In: Nelson DA, Henry JB (eds) Clinical diagnosis and management by laboratory methods, 17th edn. Saunders Company, Philadelphia, pp 578–625 (Chapter 27)
Oliveira R, Domingues I, Koppe Grisolia C, Soares AM (2009) Effects of triclosan on zebrafish early-life stages and adult. Environ Sci Pollut Res 16:679–688
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rodethenstein A, Cunningham V (2002) Aquatic toxicity of triclosan. Environ Toxicol Chem 21:1338–1349
Pan CG, Peng FJ, Shi WJ, Hu LX, Wei XD, Ying GG (2018) Triclosan-induced transcriptional and biochemical alterations in the freshwater green algae Chlamydomonas reinhardtii. Ecotoxicol Environ Saf 148:393–401
Pelgrom SMGJ, Lock RAC, Balm PHM, Wendelaar Bonga SE (1995) Integrated physiological response of tilapia, Oreochromis mossambicus, to sublethal copper exposure. Aquat Toxicol 32:303–320
Poopal RK, Ramesh M, Maruthappan V, Babu Rajendran R (2017) Potential effects of low molecular weight phthalate esters (C16H22O4 and C12H14O4) on the freshwater fish Cyprinus carpio. Toxicol Res 6:505–520
Qiu T, Xie P, Guo L, Zhang D (2009) Plasma biochemical responses of the planktivorous filter-feeding silver carp (Hypophthalmichthys molitrix) and bighead carp (Aristichthys nobilis) to prolonged toxic cyanobacterial blooms in natural waters. Environ Toxicol Pharmacol 27:350–356
Ramaswamy BR, Shanmugam G, Velu G, Rengarajan B, Larsson DG (2011) GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J Hazard Mater 186:1586–1593
Ramesh M, Thilagavathi T, Rathika R, Poopal RK (2018) Antioxidant status, biochemical, and hematological responses in a cultivable fish Cirrhinus mrigala exposed to an aquaculture antibiotic sulfamethazine. Aquaculture 491:10–19
Reitman S, Franckel S (1957) A colorimetric method for the determination of serum glutamic oxalo acetic and glutamic pyruvic transaminase. Am J Clin Pathol 28:56–63
Rendon-von Osten J, Ortız-Arana A, Guilhermino L, Soares AMVM (2005) In vivo evaluation of three biomarkers in the mosquito fish (Gambusia yucatana) exposed to pesticides. Chemosphere 58:627–636
Riva C, Cristoni S, Binelli A (2012) Effects of triclosan in the freshwater mussel Dreissena polymorpha: a proteomic investigation. Aquat Toxicol 118–119:62–71
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Rusia V, Sood Routine SK (1992) Haematological tests. In: Kanai L, Mukerjee I (eds) Medical laboratory technology. McGraw Hill, New Delhi Tata, pp 252–258
Sabaliunas D, Webb SF, Hauk A, Jacob M, Eckhoff WS (2003) Environmental fate of triclosan in the River Aire Basin, UK. Water Res 37:3145–3154
Salin ML (1987) Toxic oxygen species and protective systems of the chloroplast. Physiol Plant 72:681–689
Samanta P, Bandyopadhyay N, Pal S, Mukherjee AK, Ghosh AR (2015) Histopathological and ultramicroscopical changes in gill, liver and kidney of Anabas testudineus (Bloch) after chronic intoxication of almix (metsulfuron methyl 10.1% + chlorimuron ethyl 10.1%) herbicide. Ecotoxicol Environ Saf 122:360–367
Sanchez D, Houde M, Douville M, De Silva AO, Spence C, Verreault J (2015) Transcriptional and cellular responses of the green alga Chlamydomonas reinhardtii to perfluoroalkyl phosphonic acids. Aquat Toxicol 160:31–38
Saravanan M, Karthika S, Malarvizhi A, Ramesh M (2011) Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: hematological, biochemical, ionoregulatory and enzymological responses. J Hazard Mater 195:188–194
Scalon MCS, Rechenmacher C, Siebel AM, Kayser ML, Rodrigues MTAS, Maluf SW, Silva LB (2010) Evaluation of Sinos river water genotoxicity using the comet assay in fish. Braz J Biol 70:1217–1222
Schlenk D, Celander M, Gallagher EP, George S, James M, Kullman SW, van der Hurk P, Willett K (2008) Biotransformation in fishes. In: Di Giulio RT, Hinton DE (eds) The toxicology of fishes. Taylor and Francis Group, Boca Raton, pp 153–234
Schnitzler JG, Frédérich B, Dussenne M, Klaren PH, Silvestre F, Das K (2016) Triclosan-induced alterations in thyroid hormone status result in retarded early development and metamorphosis in Cyprinodon variegatus. Aquat Toxicol 181:1–10
Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele RD (2004) Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I. Histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol 68:141–150
Scott GR, Sloman KA (2004) The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68:369–392
Serra-Roig MP, Jurado A, Díaz-Cruz MS, Vázquez-Suñé E, Pujades E, Barceló D (2016) Occurrence, fate and risk assessment of personal care products in river–groundwater interface. Sci Total Environ 568:829–837
Shailaja MS, D’Silva C (2003) Evaluation of impact of PAH on a tropical fish, Oreochromis mossambicus using multiple biomarkers. Chemosphere 53:835–841
Shao B, Zhu LS, Dong M, Wang J, Wang JH, Xie H, Zhang QM, Du ZQ, Zhu SY (2012) DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio). Ecotoxicology 21:1533–1540
Shiosaka T, Okuda H, Fujii S (1971) Mechanism of the phosphorylation of thymidine by the culture filtrates of Clostridium perfringens and rat liver extract. Biochim Biophys Acta 246:171–183
Siegers CP (1989) Glutathione and glutathione dependent enzymes. In: A. S. Koster, E. Richter, F. Lauterbach, F. Hartman (Eds.), Intestinal metabolism of xenobiotics. Prog in Pharmacol Clin Pharmacol 7:171–180
Singer HP, Muller SR, Tixier C, Pillonel L (2002) Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol 36:4998–5004
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Stanic B, Andric N, Zoric S, Grubor-Lajsic G, Kovacevic R (2006) Assessing pollution in the Danube River near Novi Sad (Serbia) using several biomarkers in starlet (Acipenser ruthenus L.). Ecotoxicol Environ Saf 65:395–402
Stephanie L, Fraker GRS (2004) Direct and interactive effects of ecologically relevant concentrations of organic wastewater contaminants on Rana pipiens tadpoles. Environ Toxicol 19:250–256
Svoboda M (2001) Stress in fish—review. Bul VURH Vodnany 37:69–191
Ternes TA, Joss A, Siegrist H (2004) Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ Sci Technol 38:392–399
Thophon S, Kruatrachue M, Upatham ES, Pokethitiyook P, Sahaphong S, Jaritkhuan S (2003) Histopathological alterations of white sea bass, Lates calcacifer, in acute and sub acute cadmium exposure. Environ Pollut 121:307–320
Tietz NW (1990) Clinical guide to laboratory test, 2nd edn. W.B. Saunders Co, Philadelphia, p 118
Toni C, Menezes CC, Loro VL, Clasen BE, Cattaneo R, Santi A, Pretto A, Zanella R, Leitemperger J (2010) Oxidative stress biomarkers in Cyprinus carpio exposed to commercial herbicide bispyribac-sodium. J Appl Toxicol 30:590–595
Valters K, Li HX, Alaee M, D’Sa I, Marsh G, Bergman A, Letcher RJ (2005) Polybrominated diphenyl ethers and hydroxylated and methoxy-lated brominated and chlorinated analogues in the plasma of fish from the Detroit River. Environ Sci Technol 39:5612–5619
Wang F, Xu R, Zheng F, Liu H (2018) Effects of triclosan on acute toxicity, genetic toxicity and oxidative stress in goldfish (Carassius auratus). Exp Anim 67(2):219–227
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Wind T, Werner U, Jacob M, Hauk A (2004) Environmental concentrations of boron, LAS, EDTA, NTA and triclosan simulated with GREAT-ER in the River Utter. Chemosphere. 54:1135–1144
Wu Y, Beland FA, Fang JL (2016) Effect of triclosan, triclocarban, 2,2′,4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol in Vitro 32:310–319
Wu M, Xu H, Shen Y, Qiu W, Yang M (2011) Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ Toxicol Chem 30:2335–2341.
Young DS, Pestaner LC, Gibberman V (1975) Effects of drugs on clinical laboratory tests. Clin Chem 21:1D–432D
Zhang NS, Liu YS, Van den Brink PJ, Price OR, Ying GG (2015) Ecological risks of home and personal care products in the riverine environment of a rural region in South China without domestic wastewater treatment facilities. Ecotoxicol Environ Saf 122:417–425
Funding
D. Hemalatha is thankful to UGC, New Delhi for the grant of Basic Science Research Fellowship No. F.7-24/2007 (BSR) and DST-FIST, New Delhi for providing basic facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments and the handling of the organisms were carried out as per the guidelines of the CPCSEA.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hemalatha, D., Nataraj, B., Rangasamy, B. et al. DNA damage and physiological responses in an Indian major carp Labeo rohita exposed to an antimicrobial agent triclosan. Fish Physiol Biochem 45, 1463–1484 (2019). https://doi.org/10.1007/s10695-019-00661-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00661-2