Abstract
The aim of the present study was to evaluate the effects of abrupt salinity stress (12, 26 (control), and 40) on lipid peroxidation, activities and mRNA levels of antioxidant enzymes (Cu/Zn-SOD, CAT, GPx, and GR), and gene expression of the Nrf2-Keap1 signaling molecules at different times (6, 12, 24, and 48 h) in the liver of large yellow croaker Pseudosciaena crocea. The results showed that lipid peroxidation was sharply reduced at 6 h and increased at 12 h before returning to control levels in the hypo-salinity group. Similarly, lipid peroxidation was significantly decreased at 6 h followed by a sharp increase towards the end of the exposure in the hyper-salinity group. Negative relationships between lipid peroxidation and antioxidant enzyme activities and positive relationships between activities and gene expression of antioxidant enzymes were observed, suggesting that the changes at molecular levels and enzyme activity levels may provide protective roles against damage from salinity stress. Obtained results also showed a coordinated transcriptional regulation of antioxidant genes, suggesting that Nrf2 is required for regulating these genes. Furthermore, there was a positive relationship between the mRNA levels of Nrf2 and Keap1, indicating that Keap1 plays an important role in switching off the Nrf2 response. In conclusion, this is the first study to elucidate effects of salinity stress on antioxidant responses in large yellow croaker through the Keap1–Nrf2 pathway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large yellow croaker (Pseudosciaena crocea) is one of the commercially important marine species, and the annual yield from large yellow croak aquaculture is larger than that of any other net cage-farmed marine fish species in China (Liu et al. 2013). At present, larval rearing of large yellow croak mainly takes place in Ningde city, Fujian province. Then, the larvae are expanding into other coastal regions of southern China. However, environmental factors of aquaculture water in different regions are changeable, particularly the salinity. Furthermore, most of large yellow croaker are farmed in embayment; the seawater salinity is easily affected by rainstorm and surface flow, causing high mortality of cultured fish (Wang et al. 2013). It is well known that salinity stress can impose oxidative stress on organisms by accelerating the generation of reactive oxygen species (ROS) (Martinez-Alvarez et al. 2002), which leads to disturb fish’s normal physiological function. However, little information is available on the effects of salinity stress on the antioxidant system in this species by now.
To prevent damage caused by ROS, organisms have evolved an antioxidant defense system, which is composed of a complex network of protective antioxidant enzymes and nonenzymatic free radical scavengers, interacting via both direct and indirect mechanisms (Regoli and Giuliani 2014). For example, antioxidant enzyme superoxide dismutase (SOD) can detoxify superoxide anions, catalase (CAT) can scavenge H2O2, glutathione peroxidase (GPx) can scavenge both H2O2 and organic peroxides dependent on glutathione reaction, and glutathione reductase (GR) can catalyze oxidized form of glutathione (GSSG) generated by GPx to regenerate glutathione (GSH) (Halliwell 2012); some nonenzymatic antioxidants, such as GSH, can also have an effect on antioxidant enzyme activities (Srikanth et al. 2013). Extensive studies have been carried out to investigate the effects of salinity stress on lipid peroxidation and the activities of antioxidant enzymes in fish (Martinez-Alvarez et al. 2002; Liu et al. 2007). Some studies have focused on the effects of salinity stress on the expression levels of antioxidant genes in fish (Choi et al. 2008). However, these studies mentioned have only been addressed to investigate a single aspect of antioxidant responses (mRNA or enzymatic levels), and the underlying mechanisms of response to salinity stress at both enzymatic and molecular levels are poorly known in fish. On the other hand, numerous mammalian studies have shown that the NF-E2-related nuclear factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) signaling molecules play an intermediary role in defending against oxidative stress, by orchestrating the gene transcriptions of antioxidant enzymes (Dodson et al. 2015). Although few studies concerning Nrf2 antioxidant defense induced by high stocking density, PFOS or copper in fish has been conducted (Shi and Zhou 2010; Sahin et al. 2014; Jiang et al. 2015); little information is available regarding the role of Nrf2–Keap1 signaling molecules in salinity stress-induced oxidative stress in fish.
In the present study, antioxidant responses were evaluated by determining lipid peroxidation and by investigating the enzyme activities and expression of genes involved in oxidative stress. To our knowledge, this is the first study on the role of Nrf2–Keap1 signaling in fish under salinity stress and also the first time to determinate antioxidant responses to salinity stress both at enzymatic and molecular levels in large yellow croaker. The objective of this study was to elucidate the effects of abrupt salinity stress on hepatic antioxidant responses in large yellow croaker, which will contribute to further understanding the defense characteristics of the large yellow croaker against environmental stress.
Materials and methods
Abrupt salinity stress
Healthy large yellow croaker with an initial weight of 74.6 ± 4.2 g (mean ± SD) were obtained from a local offshore fish cage in the Yuyang Fisheries Co. Ltd., Zhejiang Province, China. Large yellow croaker were held in 500-L circular fiberglass tanks of a static aquarium system for a 2-week acclimatization. The fish were fed twice daily with a commercial diets (lipid and protein contents of 11 and 48% on a dry matter basis, respectively) input in slight excess of satiation during the acclimatization period. Afterward, the fish were acclimated to the target ambient salinities [12 (hypo-salinity), 26 (control, natural seawater), and 40 (hyper-salinity)] with seven salinity changes per hour, each salinity group with three replicates, and 20 fish per tank. During the challenge test, the fish were not fed, and the salinities of the seawater were measured using a handheld YSI 556 Multi-Probe System (YSI, USA) two times per day. The water was prepared at least 1 day before changing and aerated continuously to maintain dissolved oxygen near saturation levels, and 100% of the water volume was renewed daily. The salinity gradients were obtained by adding either dechlorinated tap water or marine salt to the natural seawater. The experiment was exposed to a natural photoperiod and performed at ambient temperature. The ranges of the water quality parameters were as follows: temperature was 25.3 ± 2.9 °C, salinity was 26.4 ± 0.19, dissolved oxygen was 7.38 ± 0.26 mg L−1, pH was 7.54 ± 0.31, and the hardness of the water was 134 mg L−1 as CaCO3.
Sampling and analysis
After the 6, 12, 24 and 48 h of exposure, six individuals in each group were sacrificed via anesthesia overdose by immersing the animal in benzocaine (Sigma); the liver tissues were sampled following decapitation and frozen immediately in liquid nitrogen until biochemical determinations and RNA extraction. We assured that all experiments, animal care, and protocols followed the ethical guidelines of the Zhejiang Ocean University for the care and use of laboratory animals.
Lipid peroxidation and activity of antioxidant enzyme
Liver samples were homogenized in tenfold volumes of ice-cold buffer (1 mmol L−1 of EDTA, 1 mmol L−1 of DTT, 0.5 mol L−1 of saccharose, and 0.15 mol L−1 of KCl; pH = 7.6). The homogenates were centrifuged at 900g at 4 °C for 10 min to precipitate large particles and centrifuged again at 12,000g at 4 °C for 20 min (Zeng et al. 2015). The resultant supernatant was collected and stored at −80 °C until it was measured for biochemical analysis.
Lipid peroxidation was determined by the thiobarbituric reactive species (TBARS) assay, which measured the production of malondialdehyde (MDA) that reacted with thiobarbituric acid, according to the method described by Livingstone et al. (1990). GSH level was measured as previously described by Vandeputte et al. (1994). The MDA level and GSH level were expressed as nanomole of MDA and microgram of GSH per milligram of soluble protein, respectively.
Superoxide dismutase (SOD, EC 1.15.1.1) activity was measured as previously described by Beauchamp and Fridovich (1971) based on aerobic reduction of NBT at 535 nm by superoxide radicals. Catalase activity (CAT, EC 1.11.1.6) was determined as previously described by Aebi (1984). The activity of glutathione peroxidase (GPx, EC 1.11.1.9) was determined as previously described by Drotar et al. (1985). Glutathione reductase activity (GR, EC 1.6.4.2) was assayed as previously described by Tanaka et al. (1994). Protein concentration was determined by the Bradford method (Bradford 1976) using bovine serum albumin (BSA) as the standard (Bradford protein assay kit, Tiangen Biotech, Beijing, China). The assays were run in triplicate. All enzyme activities were expressed as U (units) per milligram of soluble protein.
Gene expression
Total RNA was isolated from liver tissue using TRIzol® reagent (Invitrogen) according to the manufacturer’s instruction. The RNA quality was analyzed using 1.0% agarose gel electrophoresis, and the RNA concentration and purity were determined using an ultramicro spectrophotometer (Thermo Scientific) at 260 and 280 nm. Two milligrams of total RNA was used as templates to synthesize the first-strand complementary DNA (cDNA) using a first-strand cDNA synthesis kit (Fermentas). The cDNA synthesis reactions were diluted to 200 μL in water. Gene expression levels were determined by quantitative real-time PCR that was conducted on an Applied Biosystems Prism 7500 Sequence Detection System (Applied Biosystems, USA). The primer sequences of genes used in this analysis are shown in Table 1. The design was based on the genomic sequences in the large yellow croaker genome data (Wu et al. 2014). The qPCR amplifications were performed in a 20-μL reaction volume containing 10 μL SYBR® Premix Ex Taq Master Mix (Takara), 2.0 μL of cDNA, and 0.2 μM of each primer. PCR amplification was performed in duplicate, using the following thermal profile: initial denaturation of 1 min at 95 °C, followed by 45 cycles of 5 s at 95 °C and 10 s at 57 °C, and a final extension of 30 s at 72 °C. Each reaction was verified to contain a single product of the correct size using agarose gel electrophoresis, and dissociation curve analysis was performed after each assay to determine target specificity. Standard curves were constructed for each target gene to explain the differences in amplification efficiency between different RNA samples. A reaction was carried out without the cDNA template to ensure the absence of contamination in the reagents and primers used. A set of four housekeeping genes (EF 1α, GAPDH, β-actin, and HPRT) were selected from the literature to test their transcription stability. According to the geNorm software (Vandesompele et al. 2002), GAPDH and β-actin were used as a housekeeping gene, both of which did not change over the course of the experimental treatments. The relative expression of each gene was determined using the “delta–delta Ct” method (Pfaffl 2001), using a geNorm method to normalize the geometric mean of the best combination of two genes (GAPDH and β-actin).
Statistical analysis
Prior to statistical analysis, all data were tested for normality of distribution using the Kolmogornov–Smirnov test. The homogeneity of variances among the different treatments was tested using Barlett’s test. The results were subjected to one-way ANOVA and Tukey’s multiple range test. Nonparametric Spearman’s correlation analysis was used to examine the relationship between different parameters. Analysis was performed using the SPSS 16.0 for Windows (SPSS, Michigan Avenue, Chicago, IL, USA). For all analyses, significant levels were set at P < 0.05. All data were presented as the means ± SEM.
Results
Survival rate and MDA and GSH content
The survival rates of fish were 100% except those in the hyper-salinity group with a significantly lower survival rate (83.33 ± 5.77%, P < 0.05). The lowest and highest peaks of MDA content in the hypo-salinity group were recorded at 6 and 12 h, respectively (Fig. 1a). Compared with the control group, fish in the hyper-salinity group remarkably decreased MDA content at 6 h followed by a remarkable increase towards the end of the exposure. In comparison with the control group, fish in the hypo-salinity group significantly elevated GSH content at 6 and 24 h and sharply reduced GSH content at 12 h (Fig. 1b). Compared with the control group, fish in the hyper-salinity group remarkably increased GSH content at 6 h followed by a sharp reduction towards the end of the exposure.
Antioxidant enzyme activity
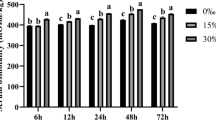
In comparison with the control group, fish in the hypo-salinity group remarkably increased SOD activity at 6 and 24 h (Fig. 2a), GPx activity at 6 and 24 h (Fig. 2c), and GR activity at 6 and 24 h (Fig. 2d) and had no effect on CAT activity (Fig. 2b). Compared with the control group, fish in the hyper-salinity group significantly enhanced SOD activity at 6 h, GPx activity at 6 and 12 h, and GR activity at 6 h, sharply inhibited SOD activity at 48 h, GPx activity at 48 h, and GR activity at 48 h, and had no effect on CAT activity.
Expression of antioxidant genes
In comparison with the control group, fish in the hypo-salinity group remarkably increased Cu/Zn-SOD expression at 6 and 24 h (Fig. 3a), Mn-SOD expression at 6 and 24 h (Fig. 3b), CAT expression at 6 and 24 h (Fig. 3c), GPx1a expression at 6 and 24 h (Fig. 3d), GPx1b expression at 6 h (Fig. 3e), GR expression at 6 and 24 h (Fig. 3f), Nrf2 expression at 6 and 24 h (Fig. 3g), and Keap1 expression during 12–48 h (Fig. 3h) and sharply reduced Cu/Zn-SOD expression at 12 h and Mn-SOD expression at 12 h. Compared with the control group, fish in the hyper-salinity group significantly stimulated Cu/Zn-SOD expression at 6 h, CAT expression at 6 h, GPx1a expression during 6–12 h, GPx1b expression during 6–12 h, GR expression at 6 h, Nrf2 expression during 6–12 h, and Keap1 expression during 24–48 h and remarkably inhibited Cu/Zn-SOD expression at 48 h, Mn-SOD expression at 48 h, CAT expression at 48 h, GPx1a expression during 48 h, GR expression during 24–48 h, Nrf2 expression at 48 h, and Keap1 expression at 6 h.
Correlation analysis
MDA content was negatively related to activities of GSH, SOD, GPx, and GR (Table 2). Pearson’s correlation coefficients between enzymatic activities and mRNA levels of according genes and between the transcription factor Nrf2 and mRNA levels of genes involved in oxidative stress are presented in Table 3. Positive correlations were observed between activity and expression of GR. There also were positive relationships between SOD activity and Cu/Zn-SOD expression, between GPx activity and GPx1a expression, and between GPx activity and GPx1b expression. No correlation was observed between SOD activity and Mn-SOD expression and between activity and expression of CAT. Nrf2 expression was positively correlated with the mRNA levels of Cu/Zn-SOD, Mn-SOD, CAT, GPx 1a, GPx 1b, and GR and was negatively related to the mRNA level of Keap1.
Discussion
Studies involved in the potential signaling molecules of antioxidant system in fish under salinity stress have not received much attention. The present study provides new experimental evidence for antioxidant defense in large yellow croaker under salinity stress and also elucidates a central role of the transcription factor Nrf2 in the salinity stress-induced oxidative stress for the first time.
The exact mechanism involved in salinity stress-induced oxidative stress is poorly understood, but it may be closely involved in changes in the activities of antioxidant enzymes (Martinez-Alvarez et al. 2002; Liu et al. 2007; Choi et al. 2008). Among the antioxidant enzymes, SOD–CAT and SOD–GPx are considered to be the vital first line of defense against oxidative stress (Winston and Di Giulio 1991), since SOD catalyzes superoxide anion radical (O2 −) and H+ into O2 and H2O2, which is subsequently transformed into nontoxic H2O by CAT and GPx. Thus, cytosolic CAT and/or GPx activities are often induced concomitantly with the activation of SOD to protect cells from oxidative stress (Di Giulio et al. 1989). In the present study, a coordinated increase was observed in activities of SOD and GPx in fish under salinity stress at 6 h, suggesting a protective role against damage by salinity stress during the early stage of exposure. However, salinity stress had no effect on CAT activity. It is known that superoxide radicals can directly inhibit CAT activity. Moreover, the antioxidant capacity of CAT could be compensated by an increase in the activity of GPx (Kang et al. 2005). The increase in GR activity suggested that a pool of reduced GSH is maintained by enhanced rates of turnover (re-conversion of oxidized GSSG to GSH) and is available as substrate for GPx. Thus, the enhancement of GR activity implied a protective and adaptive response to salinity stress in the liver and may be associated with an increase in GSH (Halliwell 2012). For example, the significant increase in GR was accompanied by a remarkable increment of GSH content in the hypo-salinity group at 6 and 24 h and in the hyper-salinity group at 6 h. In the present study, lipid peroxidation (MDA) remained relatively constant or significantly reduced while activities of antioxidant enzymes significantly increased during the early stage of exposure in both groups, further supporting the protective efficiency of antioxidant enzymes against salinity stress-induced oxidative stress. Negative relationships between lipid peroxidation and activities of antioxidant enzymes further confirmed the fact mentioned above. However, there is an accumulated risk of oxidative damage because of increased lipid peroxidation when the antioxidant system could not neutralize or eliminate the excess of ROS, which in turn inhibit the activities of antioxidant enzymes or even degrade the enzymes (Lushchak 2011). This notion was supported by the increase in lipid peroxidation and the reduction in activities of SOD, GPx, and GR at 48 h in the hyper-salinity group.
Antioxidant gene expression is considered an accurate estimate of fish antioxidant capacity, where interference of biochemical origin is not involved (Malandrakis et al. 2014). In this sense, it could be of added value to determine the onset of antioxidant gene reaction under salinity stress. In the present study, mRNA levels of Cu/Zn-SOD, Mn-SOD, GRx1a, GRx1b, and GP were upregulated in a time-dependent manner by salinity stress. The enhanced mRNA levels of these enzymes indicated that salinity stress can stimulate antioxidant capacity, which in turn correlates well with increasing activities of antioxidant enzymes in large yellow croaker. However, there was no significant correlation between SOD activity and Mn-SOD expression and between activity and expression of CAT. The possible reasons for the mismatch relationships between mRNA levels and enzyme activities are as follows: first, an SOD gene in large yellow croaker has more than two isoenzymes. The mRNA transcription level is limited to one subtype of the antioxidant gene encoding a single isoenzyme, while enzyme activity detected in the liver is equal to the total enzyme activity of the different isoenzymes. Second, enzyme activity might also be modulated at post-translational level (Sadi et al. 2014). Third, mRNA level of an antioxidant enzyme represents a snapshot of activity at any given time, while there is a time-lag effect between transcription and translation (Nam et al. 2005).
The induction of antioxidant enzyme genes is regulated by several cell signaling pathways and transcription factors (Fiol and Kültz 2007). The transcription factor Nrf2 is a master regulator of the cellular antioxidant response through the Nrf2–Keap1 signaling pathway (Dodson et al. 2015). In particular, Nrf2 is important in protecting the liver, since Nrf2 absence increases hepatic lipid peroxidation (Gong and Cederbaum 2006). In the present study, an increase in Nrf2 expression was observed in fish exposed to the hypo-salinity group at 6 and 24 h and the hyper-salinity group during 6–12 h. Enhanced expression of Nrf2 in salinity stress-induced oxidative stress indicating a protective role of Nrf2. Nrf2 expression was positively related to expression of antioxidant genes, suggesting Nrf2 may play an important role in regulating antioxidant genes. Similar results have previously been observed in Oncorhynchus mykiss (Sahin et al. 2014) and Danio rerio (Wang and Gallagher 2013). However, the persistent accumulation of Nrf2 in the nucleus may have dangerous effects, like free radical damage, apoptosis, and tumorigenesis (Katoh et al. 2005; Kensler et al., 2007). The enhancement of Keap1 expression would increase Nrf2 degradation, leading to a feedback autoregulatory loop which controls Nrf2 abundance (Katoh et al. 2005). Keap1 reversely correlated with the mRNA expression of Nrf2, indicating that Keap1 is a negative regulator to switch off the Nrf2 response. Similar result was also obtained from Anguilla anguilla exposed to H2O2 (Giuliani and Regoli 2014).
In conclusion, our study clearly elucidated abrupt salinity stress-induced antioxidant defenses, depending on salinity concentrations and time course. Compared with the hypo-salinity group, fish in the hyper-salinity group remarkably increased MDA and mortality rate while significantly reducing activities and mRNA levels of antioxidant enzymes during the late stage of exposure, suggesting that fish cannot adapt to abrupt high salinity stress. Positive correlations between gene expression and antioxidant enzyme activities were observed, suggesting transcriptional regulation may play an essential role in defending against oxidative damage of salinity stress. In the process, the Nrf2–Keap1 pathway is required for the induction of antioxidant genes. However, it should be noted that the gene mRNA only provides a portion of the transcriptional information about the de novo syntheses of these factors. Detailed mechanisms should be revealed by analyses of their protein levels and post-translational modifications in the future research.
Abbreviations
- MDA:
-
Lipid peroxidation
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- Nrf2:
-
NF-E2-related nuclear factor 2
- Keap1:
-
Kelch-like ECH-associated protein 1
References
Aebi H (1984) [13] Catalase in vitro. Method Enzymol 105:121–126
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Choi CY, An KW, An MI (2008) Molecular characterization and mRNA expression of glutathione peroxidase and glutathione S-transferase during osmotic stress in olive flounder (Paralichthys olivaceus). Comp Biochem Phys A 149:330–337
Di Giulio RT, Washburn PC, Wenning RJ, Winston GW, Jewell CS (1989) Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 8:1103–1123
Dodson M, Redmann M, Rajasekaran NS, Darley-Usmar V, Zhang J (2015) KEAP1–NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem J 469:347–355
Drotar A, Phelps P, Fall R (1985) Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci 42:35–40
Fiol DF, Kültz D (2007) Osmotic stress sensing and signaling in fishes. FEBS J 274:5790–5798
Giuliani ME, Regoli F (2014) Identification of the Nrf2–Keap1 pathway in the European eel Anguilla anguilla: role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat Toxicol 150:117–123
Gong P, Cederbaum AI (2006) Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology 43:144–153
Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70:257–265
Jiang W, Liu Y, Jiang J, Wu P, Feng L, Zhou XQ (2015) Copper exposure induces toxicity to the antioxidant system via the destruction of Nrf2/ARE signaling and caspase-3-regulated DNA damage in fish muscle: amelioration by myo-inositol. Aquat Toxicol 159:245–255
Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH (2005) 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med 11:571–578
Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, McMahon M, Hayes J, Itoh K, Yamamoto M (2005) Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys 433:342–350
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116
Liu Y, Wang WN, Wang AL, Wang JM, Suna RY (2007) Effects of dietary vitamin E supplementation on antioxidant enzyme activities in Litopenaeus vannamei (Boone, 1931) exposed to acute salinity changes. Aquaculture 265:351–358
Liu XD, Zhao GT, Cai MY, Wang ZY (2013) Estimated genetic parameters for growth-related traits in large yellow croaker Larimichthys crocea using microsatellites to assign parentage. J Fish Biol 82:34–41
Livingstone DR, Martinez PG, Michel X, Narbonne JF, O’hara S, Ribera D, Winston GW (1990) Oxyradical production as a pollution-mediated mechanism of toxicity in the common mussel, Mytilus edulis L., and other molluscs. Funct Ecol 4:415–424
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Malandrakis EE, Exadactylos A, Dadali O, Golomazou E, Klaoudatos S, Panagiotaki P (2014) Molecular cloning of four glutathione peroxidase (GPX) homologs and expression analysis during stress exposure of the marine teleost Sparus aurata. Comp Biochem Phys B 168:53–61
Martinez-Alvarez RM, Hidalgo MC, Domezain A, Morales AE (2002) Physiological changes of sturgeon Acipenser naccarii caused by increasing environmental salinity. J Exp Biol 205:3699–3706
Nam YK, Cho YS, Choi BN, Kim KH, Kim SK, Kim DS (2005) Alteration of antioxidant enzymes at the mRNA level during short-term starvation of rockbream Oplegnathus fasciatus. Fisheries Sci 71:1385–1387
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45–e45
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117
Sadi G, Bozan D, Yildiz HB (2014) Redox regulation of antioxidant enzymes: post-translational modulation of catalase and glutathione peroxidase activity by resveratrol in diabetic rat liver. Mol Cell Biochem 393:111–122
Sahin K, Yazlak H, Orhan C, Tuzcu M, Akdemir F, Sahin N (2014) The effect of lycopene on antioxidant status in rainbow trout (Oncorhynchus mykiss) reared under high stocking density. Aquaculture 418:132–138
Shi X, Zhou B (2010) The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci 115:391–400
Srikanth K, Pereira E, Duarte AC, Ahmad I (2013) Glutathione and its dependent enzymes’ modulatory responses to toxic metals and metalloids in fish—a review. Environ Sci Pollut R 20:2133–2149
Tanaka K, Sano T, Ishizuka K, Kitta K, Kawamura Y (1994) Comparison of properties of leaf and root glutathione reductases from spinach. Physiol Plantarum 91:353–358
Vandeputte C, Guizon I, Genestie-Denis I, Vannier B, Lorenzon G (1994) A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturized protocol. Cell Biol Toxicol 10:415–421
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research0034
Wang L, Gallagher EP (2013) Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol Appl Pharm 266:177–186
Wang T, Miao L, Li MY, Hu M, Chen J, Shi YH, Lu XJ (2013) Effects of abrupt salinity decrease on serum physiology, biochemistry and gill Na+/K+-atpase activity of the large yellow croaker Pseudosciaena crocea. Oceanologia Et Limnologia Sinica 44:421–426
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161
Wu C, Zhang D, Kan M, Lv Z, Zhu A, Su Y, Zhou D, Zhang J, Zhang Z, Xu M (2014) The draft genome of the large yellow croaker reveals well-developed innate immunity. Nat Commun 5:5227
Zeng L, Lei JL, Ai CX, Hong WS, Liu B (2015) Protein-sparing effect of carbohydrate in diets for juvenile turbot Scophthalmus maximus reared at different salinities. Chin J Oceanol Limnol 33:57–69
Acknowledgements
This work was supported by the Scientific Research Foundation of Zhejiang Ocean University (22115010415) and National Project of Scientific and Technical Supproting Programs funded by the Ministry of Science & Technology of China (No.2011BAD13B08). The authors thank the Yuyang Fisheries Co. Ltd. for supporting large yellow croaker.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, L., Ai, CX., Wang, YH. et al. Abrupt salinity stress induces oxidative stress via the Nrf2-Keap1 signaling pathway in large yellow croaker Pseudosciaena crocea . Fish Physiol Biochem 43, 955–964 (2017). https://doi.org/10.1007/s10695-016-0334-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0334-z