Abstract
Resveratrol is a strong antioxidant that exhibits blood glucose-lowering effects, which might contribute to its usefulness in preventing complications associated with diabetes. The present study aimed to investigate resveratrol effects on catalase (CAT) and glutathione peroxidase (GPx) gene and protein expression, their phosphorylation states and activities in rat liver of STZ-induced diabetes. Diabetes increased the levels of total protein phosphorylation and p-CAT, while mRNA expression, protein levels, and activity were reduced. Although diabetes induced transcriptional repression over GPx, it did not affect the protein levels and activity. When resveratrol was administered to diabetic rats, an increase in activity was associated with an increase in p-GPx levels. Decrease in Sirtuin1 (SIRT1) and nuclear factor erythroid 2-related factor (Nrf2) and increase in nuclear factor kappa B (NFκB) gene expression in diabetes were associated with a decrease in CAT and GPx mRNA expression. A possible compensatory mechanism for reduced gene expression of antioxidant enzymes is proved to be nuclear translocation of redox-sensitive Nrf2 and NFκB in diabetes which is confirmed by the increase in nuclear and decrease in cytoplasmic protein levels of Nrf2 and NFκB. Taken together, these findings revealed that an increase in the oxidized state in diabetes intricately modified the cellular phosphorylation status and regulation of antioxidant enzymes. Gene regulation of antioxidant enzymes was accompanied by nuclear translocation of Nrf2 and NFκB. Resveratrol administration also activated a coordinated cytoprotective response against diabetes-induced changes in liver tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All types of diabetes mellitus are characterized by hyperglycemia due to an insufficient or complete lack of insulin activity. Both a loss of insulin secretion and insulin resistance result in diabetes, which cause alterations in carbohydrate, protein, and lipid metabolism [1]. There is considerable evidence that hyperglycemia results in the production of reactive oxygen species (ROS), which mediate oxidative stress in various tissues and, importantly, exerts damaging effects on all cellular biomolecules. Cells regulate the levels of ROS using enzymatic and non-enzymatic cellular defense mechanisms. Catalase (CAT), a strong antioxidant enzyme, is a primary defense component that catalyzes the decomposition of H2O2 to water, a function that is shared with glutathione peroxidase (GPx).
Trans-resveratrol (3,5,4′-trihydroxystilbene), a polyphenol, is present as a biologically active substance in plants. It exhibits a wide range of biological properties, and its antioxidant properties have been previously demonstrated in the prevention and treatment of diabetic complications [2, 3]. Several lines of evidence have demonstrated that resveratrol also exhibits anti-inflammatory, anti-aggregation, and neuroprotective properties [2, 4]. Recently, resveratrol has become increasingly attractive as a therapeutic agent in the treatment of a variety of pathologies, including diabetes mellitus [5]. However, the mechanisms underlying the beneficial effects of resveratrol have not been completely elucidated, although its antioxidant activity has been demonstrated to protect tissues, such as the liver, kidney, and brain against a variety of damage caused by oxidative stress [4, 6–8].
Recent studies have indicated a strong relationship between redox signaling and steady-state levels of protein phosphorylation due to reactive oxygen species (particularly H2O2), which might alter the cellular phosphorylation status, thereby affecting the activities and expression of antioxidant enzymes [9–11]. Furthermore, the activities or presence of antioxidant enzymes in cells are strongly regulated by transcriptional, translational, and post-translational mechanisms as a consequence of changes in the cellular redox potential [12].
Nuclear factor erythroid 2-related factor (Nrf2) functions as a key mediator of the redox homeostatic gene regulatory network, where under conditions of oxidative and electrophilic stress, the Nrf2 signaling pathway is activated to enhance the expression of multiple antioxidant and phase II enzymes, which can restore redox homeostasis. The function of Nrf2 and its downstream target genes have been shown to be important for protection against oxidative stress [13]. Depletion of cellular antioxidant defense mechanisms and the generation of oxygen-free radicals result in the activation of nuclear factor kappa B (NFκB) [14], an inducible transcription factor, and thus promote the expression of NFκB-regulated genes, which thereby contributes to the further enhancement of proinflammatory cytokines and defense against oxidative stress [15]. This orchestrated regulation of gene expression by Nrf2 and NFκB is very efficient in increasing cellular detoxification and antioxidant capacity, indicating an important role for NFκB-Nrf2-ARE (Antioxidant Response Elements) pathways as a cellular antioxidant defense system. In addition, sirtuins (SIRT1) are a class of NAD+-dependent deacetylases, which regulate systems that control the redox environment, thereby counteracting oxidative damage, which is associated with common diseases of aging and also contributes to aging itself. It has been previously proposed that the chemical activation of the SIRT1 gene and/or proteins with several agents, including resveratrol, may have antidiabetic and other beneficial health effects [16].
Recent studies have demonstrated that transcription of CAT and GPx may be mediated by redox-sensitive transcription factors, such as Nrf2 and NfκB, which can affect the fate of redox status in pathophysiological changes [17, 18]. Furthermore, tyrosine phosphorylation at specific tyrosine residues of CAT and GPx may regulate their activities and proteolytic degradation [19, 20]. Thus, we hypothesized that oxidative stress in diabetes is correlated, at least in part, with the gene expression and protein phosphorylation of antioxidant enzymes. We examined the expression and activity levels of hepatic CAT and GPx in combination with their phosphorylation states in resveratrol treated diabetic rats. Moreover, we investigated (1) the oxidized state of liver tissues associated with diabetes, (2) total protein phosphorylation levels in conjunction with the CAT and GPx phosphorylation status, and (3) hepatic expression of CAT and GPx and their concomitant transcription factors; Nrf2, NFκB and the cellular regulator SIRT1 in diabetic rats with or without resveratrol.
Materials and methods
Materials
Streptozotocin (STZ), nicotinamide adenine dinucleotide reduced phosphate (NADPH), and glutathione reductase (GR) were purchased from Sigma (St. Louis, MO, USA). Trans-resveratrol was obtained from Molekula (Gillingham, Dorset, UK), Total RNA isolation kits were obtained from Qaigen (Venlo, Netherlands) and reagents for cDNA synthesis were obtained from Fermentas (Burlington, Canada). Real-time PCR SYBR Green I Master Mix was purchased from Roche (Foster City, CA, USA). Phosphoprotein purification kits were obtained from Santa Cruz (Santa Cruz, CA, USA). Antibodies were obtained from Abcam (Cambridge, MA, USA) and Santa Cruz (Santa Cruz, CA, USA). PVDF membranes were purchased from Bio-Rad (Hercules, California, USA). All other chemicals used in this study were of the highest analytical grade available, and the buffers were prepared using sterile distilled water.
Animal procedure and tissue preparation
Experiments were performed on eight-week-old adult male Wistar rats weighing between 200 and 250 g. All animal procedures were approved by the Committee for the Ethical Animal Care (Kobay DHL, 2012/45) according to rules of the Guide for the Care and Use of Laboratory Animals as published by the US National Institute of Health (NIH Publication No: 85/23, revised in 1986). The rats were fed with a standard diet and were randomly divided into four groups: (1) non-diabetic control group (n = 12) given only vehicle (10 % DMSO), (2) non-diabetic control group (n = 12) given a daily i.p. dose of 20 mg/kg/day resveratrol in 10 % DMSO throughout the 4-week period, (3) diabetic group received STZ (55 mg/kg) dissolved in 0.05 M citrate buffer (pH: 4.5) (n = 12) and vehicle for each day, and (4) diabetic group treated with resveratrol, which received a daily i.p. dose of 20-mg/kg/day resveratrol (n = 9) throughout the 4-week period, starting from 2 days after STZ administration. After 4 weeks of diabetes, the rats were fasted overnight and decapitated for the removal of liver tissues, which were quickly frozen in liquid nitrogen, and kept at −85 °C for subsequent biochemical analyses.
Tissue homogenization and protein extraction
Homogenates of liver tissues were obtained with the aid of the Tissue RuptureTM (Qiagen, Venlo, Netherland) homogenizer using a homogenization solution consisting of 1.15 % (w/v) KCl, 5 mM EDTA, 0.2 mM PMSF, and 0.2 mM DTT in 25 mM phosphate buffer at pH 7.4. Next, the homogenates were centrifuged at 1.500×g for 10 min at 4 °C and the supernatants were aliquoted to perform enzyme assays and other biochemical analysis. Protein concentrations of the homogenates were determined according to the Lowry method [21].
Purification of total phosphoproteins
Total phosphoproteins obtained from liver tissues were separated using phosphoprotein purification affinity columns with commercially available kits (Santa Cruz, CA, USA) according to the manufacturer’s protocol with some modifications. Accordingly, 50 mg liver tissues were homogenized in 300-µl RIPA buffer (15 mM NaCl, 1 % NP-40, 0.5 % sodium orthovanadate, 0.1 % SDS, 50 mM Tris pH 8.0), including proteinase and phosphatase inhibitor cocktail tablets (1 tablet/10 ml) (Pierce; Brebières, France). Next, the volume was raised to 1 ml with 2 × binding and washing buffer, which were supplied in the kit. The protein concentration was determined using the Lowry method [21], where 5 mg of total protein was applied to the equilibrated phosphoprotein purification columns and incubated at 4 °C for 30 min. Unbound flow-through containing non-phosphorylated proteins was eluted and washed from the column. Next, the phosphoprotein fractions were eluted using successive 1-ml elution with elution buffer and their protein contents were determined using the Lowry method [21], which was adapted to microplate measurements in a total volume of 200 µl. After isolation, total phosphoproteins were concentrated using ultrafiltration columns, which were provided in the phosphoprotein purification kit.
Nuclear and cytoplasmic protein extraction
Nuclear and cytoplasmic proteins were extracted and isolated from liver tissues using Thermo Scientific NE-PER® Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Rockford, IL, USA) according to manufacturer’s protocol. Briefly, liver tissues underwent lysis in cytoplasm extraction reagent and were centrifuged at 16,000×g for extraction of the nuclear material. Proteins from the nuclear material were extracted by the addition of nuclear extraction reagent to the nuclei and centrifugation at 16,000×g. Nuclear and cytoplasmic extracts were stored at −85 °C until use.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and immunoblot analysis
For the determination of cytosolic CAT, GPx, SIRT1, NFκB-p65, and Nrf2 protein contents, whole homogenates containing 50 µg (10 µg for CAT) of proteins were loaded onto 4 % stacking and 10–12 % separating slab gels and separated under 200 V constant voltage. Nuclear NFκB and Nrf2 protein levels were studied from isolated nuclear fraction and p-CAT and p-GPx levels were studied using isolated total phosphoprotein fractions instead of whole homogenates. After SDS-PAGE, proteins were electroblotted onto PVDF membranes [22]. Then, blotted membranes were blocked with 5 % (w/v) bovine serum albumin (BSA) and incubated with CAT (Anti-CAT Rabbit IgG, Abcam: Cambridge, USA, 1:6.000), GPx (Anti-GPx Rabbit IgG, Abcam: Cambridge, USA, 1:1.000), SIRT1 (Anti-SIRT1 Rabbit IgG, Santa Cruz, USA, 1/100), NFκB-p65 (Anti- NFκB-p65 Rabbit IgG, Santa Cruz, USA, 1/100), Nrf2 (Anti-Nrf2 Rabbit IgG, Santa Cruz, USA, 1/100) primary antibodies for 2 h. As a cytoplasmic internal control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) proteins were also labeled with anti-GAPDH Rabbit IgG (Santa Cruz, USA, 1:2.000) and nuclear internal standard which is Lamin B (Anti-Lamin B Rabbit IgG, Santa cruz, USA, 1/250) was used for the normalization of nuclear proteins. Horseradish peroxidase (HRP)-conjugated secondary antibody (Goat anti-rabbit IgG-HRP conjugate Abcam: Cambridge, USA, 1:10.000) was incubated for 1 h and the blots were then incubated in ClarityTM Western ECL (Bio-Rad Laboratories, Hercules CA, USA) substrate solution. Images of the blots were obtained using the ChemiDocTM MP chemiluminescence detection system (Bio-Rad Laboratories, Hercules CA, USA) equipped with a CCD camera. The relative expression of proteins with respect to the reference proteins was calculated using the ImageLab4.1 software.
Determination of protein thiols, reduced glutathione (GSH), lipid peroxidation, and protein carbonyl contents
Protein thiol groups were estimated using Ellman’s procedure [23], and reduced GSH determination was performed on the basis of the oxidation of GSH by 5,5′-dithiobis-(2-nitrobenzoic acid) [24]. Lipid peroxidation was examined using TBARS measurement [25], and the protein carbonyl contents were determined as described elsewhere [26].
Determination of enzymatic activities
The CAT activities were determined using a method in which the rate of H2O2 decomposition was measured at 240 nm [27], and the GPx activities were measured by following the oxidation of NADPH at 340 nm [28]. The activities were calculated as U/mg, which reflects the amount of substrate consumed in 1 min by one mg protein containing cytosolic fraction.
Determination of CAT, GPx, SIRT1, Nrf2, and NFκB Gene Expression using Real-Time Quantitative PCR (qRT-PCR)
Total RNAs were isolated from 50 mg of liver tissue using the RNeasy total RNA isolation kit (Qiagen, Venlo, Netherlands) as described according to the manufacturer protocol. After isolation, the amount and quality of total RNA were determined using spectrophotometry at 260/280 nm and the Agilent 2100 bioanalyzer (Santa Clara, USA). cDNA synthesis was performed using 1 µg of total RNA and oligo(dT)18 primers using the commercial first strand cDNA synthesis kit (MBI Fermentas, USA). The mRNA expressions of CAT, GPx, SIRT1, Nrf2, and NFκB were determined using qRT-PCR by mixing 1 μl cDNA, 5 μl 2X SYBR Green Mastermix (Roche FastStart Universal SYBR Green Master Mix), and primer pairs (Table 1) at 0.5 mM concentrations in a final volume of 10 µl.
Next, qRT-PCR (LightCycler480 II, Roche, Basel, Switzerland) was performed as follows: initial denaturation at 95 °C for 10 min, denaturation at 95 °C for 10 s, annealing at 58 °C for 15 s, and extension at 72 °C for 15 s with 45 repeated thermal cycles measuring the green fluorescence at the end of each extension step. The PCR reactions were performed in triplicates and the specificity of PCR products was confirmed using melt analysis. In addition, negative controls lacking template were used in all reactions. The relative expression of genes with respect to the internal control GAPDH was calculated with the efficiency corrected advance relative quantification tool provided by the LightCycler® 480 SW 1.5.1 software.
Statistical analysis
The gene and protein expression data were normalized to the mean of the control groups, which was arbitrarily set to onefold, and the relative changes were given as fold changes over control. Other data were presented as the mean ± standard error of the mean. SPSS 15.0 statistical software (IBM Corporation, Armonk, NY, USA) was used to calculate the statistical significance between groups, which was determined using one-way ANOVA with the appropriate post-hoc test (Tukey’s Honestly Significant Difference). A probability of 0.05 was established as the level of significance in the data analysis and comparisons between groups.
Results
Resveratrol effect on STZ-induced hyperglycemia and body weight loss
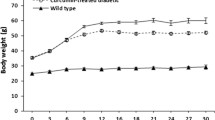
STZ-treated rats displayed a significant induction in fasting blood glucose concentration compared to age-matched controls (Fig. 1a). Resveratrol, which is known to demonstrate insulin-like effects, did not significantly change the diabetic blood glucose levels. Moreover, STZ-treatment decreased the body weight of the rats (p < 0.05) (Fig. 1b). Similarly, resveratrol resulted in a reduction in body weight since the newly recognized function of resveratrol has been found to activate a family of key enzymes in calorie restriction such as Sir2-family histone deacetylases. So resveratrol effectively mimics calorie restriction in animals [29, 30] and modulates the body weight as in this study.
Effects of diabetes and resveratrol administration on fasting blood glucose levels (a) and changes in body weight (b) of the experimental groups. Values were given as the mean ± SEM for all groups. Asterisk (*) indicates that the means were significantly different (p < 0.05) compared to control groups
Resveratrol effect on oxidative stress markers in diabetic liver
We found a statistically significant elevation in lipid peroxidation and protein carbonyl and reduction in protein thiols and glutathione levels in diabetic liver (Fig. 2a–d), suggesting diabetes-promoted oxidative stress. An increase in the levels of lipid peroxides and protein carbonyls in the liver tissues of diabetic group of rats suggested increased oxidative stress. Resveratrol did not significantly alter these oxidative markers as given to the control rats. Although there was a slight modification in previously described biomarkers with resveratrol, except protein carbonyl contents, these levels did not return to normal levels in diabetic rats.
Effect of diabetes and resveratrol on liver lipid peroxidation (a) protein thiols (b) protein carbonyl (c) and reduced glutathione contents. Asterisk (*) indicates that the means were significantly different (p < 0.05) compared to control groups. Hash (#) indicates that the means were significantly different (p < 0.05) compared to diabetic groups
Total phosphoprotein content
After phosphoprotein purification, the total protein amount was quantified in the fractions (Fig. 3). Both STZ-treatment and resveratrol application increased the total phosphorylation levels above the control group. Resveratrol administration further enhanced protein phosphorylation in diabetic rats.
Total phosphoprotein concentrations of fractions eluted from purification columns after 5-mg protein load. Values were given as the mean ± SEM for all groups. Asterisk (*) indicates that the means were significantly different (p < 0.05) compared to control groups. Hash (#) indicates that the means were significantly different (p < 0.05) compared to diabetic groups. Each bar represents at least four rats
Resveratrol effect on CAT expression, activity, and phosphorylation levels in diabetic liver
The gene and protein expressions of CAT were measured in liver tissues, which indicated that a decrease in activity was associated with a decrease in mRNA and protein expression with STZ-treatment (Fig. 4a, b). In contrast with this decrease, p-CAT levels were enhanced (Fig. 4d) indicating that CAT is over-phosphorylated in diabetes, which regulates enzymatic activities (Fig. 4c). Even though application of resveratrol further reduced mRNA expression in both control and diabetic rats, CAT protein levels and enzymatic activity were partially recovered as compared to untreated diabetic group. Resveratrol enhanced enzymatic activities of CAT while reduced the phosphorylation over CAT.
Effect of diabetes and resveratrol on CAT mRNA (a) total CAT protein (b) CAT enzymatic activity (c) and CAT phosphorylation levels (d). For the p-CAT measurement, each bar represents at least four rats. Asterisk (*) indicates that the means were significantly different (p < 0.05) compared to control groups. Hash (#) indicates that the means were significantly different (p < 0.05) compared to diabetic groups
Resveratrol effect on GPx expression, activity, and phosphorylation levels in diabetic liver
Representative western blot images for CAT, p-CAT, GPx, p-GPx, and internal standard GAPDH bands which were measured in the groups are provided in Fig. 5a. Except mRNA expression levels, STZ-treatment did not significantly change the GPx and p-GPx levels and its enzymatic activities. The decrease in gene expression levels of GPx with diabetes was further enhanced with resveratrol application (Fig. 5b). Similarly, resveratrol also suppressed the protein expression of GPx when administered to both control and diabetic animals (Fig. 5c). The enzymatic activity and p-GPx levels were elevated when resveratrol was administered to diabetic rats (Fig. 5d, e).This result indicated that the post-translational phosphorylation of GPx by resveratrol caused the enzymatic activities to be elevated in the STZ-treated group.
Representative images for CAT, p-CAT, GPx, and p-GPx protein expression as measured using Western blotting analyses (a). The intensity of the bands was quantified using densitometric analysis and normalized with corresponding GAPDH. Effect of diabetes and resveratrol on GPx mRNA (b) total GPx protein (c) GPx enzymatic activity (d) and GPx phosphorylation levels (e). For the p-GPx measurement, each bar represents at least four rats. Asterisk (*) indicates that the means were significantly different (p < 0.05) compared to control groups. Hash (#) indicates that the means were significantly different (p < 005) compared to diabetic groups
Diabetes and resveratrol effect on the mRNA expression levels of SIRT1, Nrf2, and NFκB
qRT-PCR results demonstrated that the gene expression levels of SIRT1 and Nrf2 were suppressed with diabetes (Fig. 6a,b). This was in contrast with NFκB expression, which was significantly enhanced (p < 0.05) in STZ-treated rats (Fig. 6c). Application of resveratrol to diabetic group increased Nrf2 and SIRT1 mRNA levels while, when applied to the control group, resveratrol had no effect.
Relative gene expression levels of SIRT1 (a) Nrf2 (b) and NFκB (c) with respect to internal standard GAPDH. The gene expression level of these proteins was given as fold changes over the control groups whose expression values were arbitrarily set to one. Asterisk (*) indicates that the means were significantly different (p < 0.05) compared to control groups. Hash (#) indicates that the means were significantly different (p < 0.05) compared to diabetic groups
Immunoblot analysis of SIRT1, Nrf2, and NFκB
In this study, we also analyzed the protein levels of SIRT1, Nrf2, and NFκB and their concerted modulation in diabetic liver. Immunoblot analyses were carried out in both cytoplasmic and nuclear fractions of rat liver and representative blot images are demonstrated in Fig. 7a. Densitometric analysis revealed that similar with the mRNA expression, cytoplasmic protein levels of SIRT1 were reduced in diabetic liver which was normalized to control values with the resveratrol administration (Fig. 7b). This result was in agreement with mRNA expression of the same protein. Furthermore, cytoplasmic Nrf2 and NFκB protein level was reduced and nuclear form of these two proteins was significantly higher in diabetic rats (Fig. 7c–f). Any significant effect of resveratrol was observed on protein levels of Nrf2 and NFκB.
Immunoblot analysis results of SIRT1, Nrf2, and NFκB-p65 from both nuclear and cytoplasmic fractions. Representative blot images (a), SIRT1 protein levels in cytoplasm (b), cytoplasmic and nuclear protein levels of Nrf2 (c, d), and cytoplasmic and nuclear protein levels of NFκB-p65 (e, f) were given. Asterisk (*) indicates that the means were significantly different (p < 0.05) compared to control groups. Hash (#) indicates that the means were significantly different (p < 0.05) compared to diabetic groups
Discussion
Oxidative stress can either be a cause or consequence of damage to cellular macromolecules, such as lipids, proteins, and nucleic acids in diabetes mellitus [1]. Consistent with this depiction, we determined the degree of lipid peroxidation and protein carbonylation levels, which was enhanced by diabetes. These results indicated the oxidized state of liver tissues in diabetic rats. Diabetic depletion of GSH and protein thiols also supports the oxidized state. Deterioration in glucose homeostasis is thought to be source of oxidative stress, which results in changes in the expression of oxidative markers. Although resveratrol is known to have antioxidative function [31], it partially normalizes the oxidative state by only alleviating protein carbonyls to control levels. Furthermore, diabetes-mediated oxidation of proteins and its recovery following resveratrol treatment suggests that the antioxidant and free radical scavenging activity of resveratrol may contribute in reducing the toxic effects of diabetes [32].
In this study, diabetes amended the intracellular protein phosphorylation, which may be correlated, at least partially, with oxidative stress. Recent studies have indicated a strong association between redox signaling and protein phosphorylation states and demonstrated that sources of oxidative stress, particularly hydrogen peroxide (H2O2), may modify cellular phosphorylation, which can manipulate the activities and expression levels of antioxidant enzymes [33]. An increase in intracellular protein phosphorylation, as observed in diabetes, might be due to the reversible oxidation of several protein tyrosine phosphatases (PTPs) with oxidative conditions, causing a decrease in its activities, thereby resulting in an overall amplification of phosphorylation in these tissues [9, 33]. Furthermore, an increase in protein phosphorylation levels by resveratrol may be attributed to an increase in kinase activities by allosteric modulation [34–36] or to a lesser extent, by its prooxidant effect, which has been proposed in some studies [31, 37]. Despite any statistical significance found, the trend in tissue thiobarbituric acid reactive substances (TBARS) levels, GSH and protein thiols with resveratrol may be an indicator of the probable prooxidant activity of resveratrol in the long term.
Normally, two main antioxidant enzymes, CAT and GPx, protect cells and tissues from oxidative injury and regulate redox signaling within cells [38]. The activities or presence of CAT and GPx in the cells are strongly regulated by transcriptional, translational and post-translational mechanisms as a consequence of changes in the cellular redox potential [39, 40]. Previous studies have demonstrated that CAT and GPx activities are regulated by post-translational events, such as phosphorylation at specific tyrosine residues. Moreover, GPx activity is regulated by phosphorylation at Tyr96 and the post-translational level modification of CAT occurs at Tyr231 and Tyr385 phosphorylation [19, 20]. Phosphorylation of these two enzymes was shown to be mediated by the redox-sensitive protein tyrosine kinases, c-Abl and Arg, which form a heterodimer and become activated via oxidative stress promoters [41]. Modulation of the activities with phosphorylation provides protection against oxidative conditions or induces protein turnover because over-phosphorylation might mark the proteins for ubiquitination [19]. Herein we presented evidence that diabetes induced an increase in CAT phosphorylation and decreased CAT mRNA and protein expression and catalytic activity. Because phosphorylation has the ability to change enzymatic activity, enzymatic silencing of CAT in diabetic liver tissues may have resulted in decreased mRNA and protein expression as well as its phosphorylation. However, over-phosphorylation of CAT with diabetes induces its ubiquitination, resulting in its degradation [19]. Another potential mechanism for the reduction of CAT activities may be due to the inactivation caused by the excess of free radicals and/or by non-enzymatic glycation due to persistent hyperglycemia, which has been extensively reported to occur in diabetes [42]. Resveratrol application to diabetic rats increases CAT protein expression and activity and decreases p-CAT when compared to diabetic liver. It is possible that this reduced CAT phosphorylation is involved in increased CAT protein stability and therefore CAT protein level.
Changes in the activities and protein expressions of GPx in STZ-treated rats were different compared to those observed for CAT. Although the activity and protein amounts did not significantly change with diabetes, the mRNA expressions were reduced with STZ administration. This study also reported that resveratrol demonstrated a post-translational effect over the GPx enzyme, which increased the phosphorylation level, thereby enhancing enzymatic activities in diabetes. On the basis of these findings, resveratrol may potentially modulate CAT and GPx activities, thereby enlarging the antioxidative capacity toward ROS and providing higher protection against oxidative damage induced by diabetes [42].
In tissues, some redox-sensitive proteins, which can undergo reversible oxidative and reductive reactions, can activate or delay downstream signaling pathways, including antioxidant enzyme systems [33, 43]. Some of these redox-sensitive proteins are metabolic enzymes or proteins, such as Nrf2, NFκB and phosphatases, which are capable of sensing oxidant signals [9]. The metabolic and gene regulatory responses of the liver to oxidative stress and diabetic state occur in conjunction with changes in Nrf2 and NFκB and their subsequent effect on the expression of their target genes, particularly genes encoding CAT and GPx [44, 45]. During hyperglycemia-mediated oxidative stress, the expression of Nrf2 and its down-regulated proteins, including GPx and CAT, were significantly decreased in the liver tissues of diabetic rats. However, resveratrol treatment significantly modulated the gene expression of Nrf2 in such a manner that the CAT and GPx activities were up-regulated in diabetes. Repression of Nrf2 gene expression with diabetes [44, 46] and its normalization with resveratrol treatment demonstrated that Nrf2 signaling was down-regulated with oxidative changes, which in turn affected downstream regulatory proteins, such as GPx and CAT, in the diabetic rat group [47].
Under normal oxidative conditions, Nrf2 is present in the cytoplasm and unable to operate if there is any signal but as the redox potential of the cell changes, they are activated to localize themselves into nucleus. When the redox status altered as in the case of diabetes, these transcription factors can move into the cell nucleus and impinges on the ARE found in the promoter regions of genes encoding phase II detoxification enzymes and antioxidant proteins [48]. Thus, any changes occurring in these transcription factors with oxidative redox differences may be a potential candidate mechanism for the regulation of antioxidant enzymes in these tissues. Herein we presented a transcriptional repression of GPx and CAT which could be counterbalanced with nuclear translocation of Nrf2 confirmed by immunoblot analysis from cytoplasmic and nuclear fractions. Similarly, transcription factor NFκB is also located in the cytoplasm and can move into nucleus for the transcriptional regulation of several genes, including antioxidant enzymes and proteins that are responsible for immune and inflammatory responses [17]. We also demonstrated that the gene expression levels of NFκB were elevated in STZ-treated rats, which was consistent with previous studies [14, 49], and immunoblot analysis of NFκB from both cytoplasmic and nuclear fractions indicated that diabetes induced nuclear translocation which is revealed from significant increase in the nuclear to cytoplasmic protein ratios in the liver tissues. These results indicated that the regulatory responses of the liver to inflammation were induced with STZ-treatment.
Sirtuins are a class of NAD+-dependent deacetylases, which function as metabolic regulators contributing to cellular regulation in stress response and energy metabolism. Sirtuins can regulate systems that control the redox environment to help counteract oxidative damage. One of the subtypes of sirtuins; SIRT1 modulates carbohydrate metabolism via the deacetylation of other transcription factors and can repress NFκB activity [50]. Chemical activation of SIRT1 gene and/or proteins with agents, such as resveratrol, has been shown to exhibit antidiabetic and other beneficial health effects [16]. Consistent with recently published data [51], our data also indicated that repressed SIRT1 gene and protein expression with STZ diabetes was enhanced to normal levels with resveratrol administration. Activation of SIRT1 by resveratrol in diabetes may also increase the expression of several transcription factors, which increases antioxidant target genes, such as CAT and GPx, to reduce ROS levels in diabetic rats.
In the present study, it has been presented that diabetes-induced oxidative stress may be causally related to the down-regulation of CAT and GPx combined with an attenuation of enzymatic activities. A possible compensatory mechanism for reduced gene expression of antioxidant enzymes is proved to be nuclear translocation of redox-sensitive Nrf2 and NFκB in diabetes. STZ diabetes also modulates overall protein phosphorylation as well as CAT and GPx phosphorylation, which affects cellular defense mechanisms. By inducing CAT activity and reducing p-CAT in diabetic liver, resveratrol could influence CAT protein stability and activity which is in correlation with detected increase in CAT protein expression; GPx activity and phosphorylation levels are increased by resveratrol treatment in diabetes, suggesting that resveratrol promotes GPx activation by influencing its phosphorylation. Furthermore, resveratrol exerts beneficial effects on diabetes-induced oxidative stress and hepatic repression of Nrf2 and SIRT1 by revitalizing these affected pathways. Thus, future studies should investigate whether resveratrol supplementation is useful in the alleviation of diabetes-induced alterations.
Abbreviations
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- STZ:
-
Streptozotocin
- p-CAT:
-
Phosphorylated catalase
- p-GPx:
-
Phosphorylated glutathione peroxidase
- qRT-PCR:
-
Quantitative real-time PCR
- SIRT1:
-
Sirtuin1
- Nrf2:
-
Nuclear factor erythroid 2-related factor
- NFκB:
-
Nuclear factor kappa B
- ROS:
-
Reactive oxygen species
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- PAGE:
-
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis
- HRP:
-
Horseradish peroxidase
- PTPs:
-
Protein tyrosine phosphatases
- TBARS:
-
Thiobarbituric acid reactive substances
References
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070. doi:10.1161/CIRCRESAHA.110.223545
Pandey KB (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2:270–278
Szkudelski T, Szkudelska K (2011) Anti-diabetic effects of resveratrol. Ann N Y Acad Sci 1215:34–39. doi:10.1111/j.1749-6632.2010.05844.x
Marques FZ, Markus MA, Morris BJ (2009) Resveratrol : cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol 41:2125–2128. doi:10.1016/j.biocel.2009.06.003
Szkudelska K, Szkudelski T (2010) Resveratrol, obesity and diabetes. Eur J Pharmacol 635:1–8. doi:10.1016/j.ejphar.2010.02.054
Baur J, Sinclair D (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506. doi:10.1038/nrd2060
Savouret JF, Quesne M (2002) Resveratrol and cancer: a review. Biomed Pharmacother 56:84–87
Thirunavukkarasu M, Penumathsa SV, Koneru S et al (2007) Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med 43:720–729. doi:10.1016/j.freeradbiomed.2007.05.004
Groen A, Lemeer S, van der Wijk T et al (2005) Differential oxidation of protein-tyrosine phosphatases. J Biol Chem 280:10298–10304. doi:10.1074/jbc.M412424200
Rhee S, Kang SW, Jeong W et al (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17:183–189. doi:10.1016/j.ceb.2005.02.004
Rhee SG (2003) Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 14:211S–215S. doi:10.1097/01.ASN.0000077404.45564.7E
Evans JL (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of Type 2 diabetes. Endocr Rev 23:599–622. doi:10.1210/er.2001-0039
Lee J-M, Johnson J a (2004) An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol 37:139–143
Locke M, Anderson J (2011) NF-κB activation in organs from STZ-treated rats. Appl Physiol Nutr Metab 36:121–127. doi:10.1139/H10-094
Soufi FG, Mohammad-Nejad D, Ahmadieh H (2012) Resveratrol improves diabetic retinopathy possibly through oxidative stress—nuclear factor κB—apoptosis pathway. Pharmacol Rep 64:1505–1514
Morris BJ (2013) Seven sirtuins for seven deadly diseases of aging. Free Radic Biol Med 56:133–171. doi:10.1016/j.freeradbiomed.2012.10.525
Pillai SS, Sugathan JK, Indira M (2012) Selenium downregulates RAGE and NFκB expression in diabetic rats. Biol Trace Elem Res 149:71–77. doi:10.1007/s12011-012-9401-1
Taguchi K, Motohashi H, Yamamoto M (2011) Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 16:123–140. doi:10.1111/j.1365-2443.2010.01473.x
Cao C, Leng Y, Kufe D (2003) Catalase activity is regulated by c-Abl and Arg in the oxidative stress response. J Biol Chem 278:29667–29675. doi:10.1074/jbc.M301292200
Cao C, Leng Y, Huang W et al (2003) Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem 278:39609–39614. doi:10.1074/jbc.M305770200
Lowry O, Roserbrough N, Farr A, Randall R (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Towbin H, Staehelin T, Gordon J (1992) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology 24:145–149
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Jain SK, Levine SN (1995) Elevated lipid peroxidation and vitamin E-quinone levels in heart ventricles of streptozotocin-treated diabetic rats. Free Radic Biol Med 18:337–341
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Aebi H (1984) In methods in enzymology. Catalase Vitr. Academy Press, New York, pp 121–126
Paglia D, Valentine W (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Baur JA (2010) Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev 131:261–269. doi:10.1016/j.mad.2010.02.007
Zhang J (2006) Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J 397:519–527. doi:10.1042/BJ20050977
Gadacha W, Ben-Attia M, Bonnefont-Rousselot D et al (2009) Resveratrol opposite effects on rat tissue lipoperoxidation: pro-oxidant during day-time and antioxidant at night. Redox Rep 14:154–158. doi:10.1179/135100009X466131
Upadhyay G, Singh AK, Kumar A et al (2008) Resveratrol modulates pyrogallol-induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. Eur J Pharmacol 596:146–152. doi:10.1016/j.ejphar.2008.08.019
Den Hertog J, Groen A, Van Der Wijk T (2005) Redox regulation of protein-tyrosine phosphatases. Arch Biochem Biophys 434:11–15. doi:10.1016/j.abb.2004.05.024
Conte A, Pellegrini S, Tagliazucchi D (2003) Effect of resveratrol and catechin on PC12 tyrosine kinase activities and their synergistic protection from beta-amyloid toxicity. Drugs Exp Clin Res 29:243–255
Palmieri L, Mameli M, Ronca G (1999) Effect of resveratrol and some other natural compounds on tyrosine kinase activity and on cytolysis. Drugs Exp Clin Res 25:79–85
Dasgupta B, Milbrandt J (2007) Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA 104:7217–7222. doi:10.1073/pnas.0610068104
Hassan-Khabbar S, Cottart C-H, Wendum D et al (2008) Postischemic treatment by trans-resveratrol in rat liver ischemia-reperfusion: a possible strategy in liver surgery. Liver Transpl 14:451–459. doi:10.1002/lt.21405
Meyer AJ, Hell R (2005) Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res 86:435–457. doi:10.1007/s11120-005-8425-1
Stevens MJ (2005) Redox-based mechanisms in diabetes. Antioxid Redox Signal 7:1483–1485. doi:10.1089/ars.2005.7.1483
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875. doi:10.1105/tpc.105.033589
Cao C, Leng Y, Li C, Kufe D (2003) Functional interaction between the c-Abl and Arg protein-tyrosine kinases in the oxidative stress response. J Biol Chem 278:12961–12967. doi:10.1074/jbc.M300058200
Schmatz R, Perreira LB, Stefanello N et al (2012) Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie 94:374–383. doi:10.1016/j.biochi.2011.08.005
Hongjun L, Colavitti R, Rovira II, Finkel T (2005) Redox-dependent transcriptional regulation. Circ Res 97:967–974. doi:10.1161/01.RES.0000188210.72062.10
Arambašić J, Mihailović M, Uskoković A et al (2013) Alpha-lipoic acid upregulates antioxidant enzyme gene expression and enzymatic activity in diabetic rat kidneys through an O-GlcNAc-dependent mechanism. Eur J Nutr 52:1461–1473. doi:10.1007/s00394-012-0452-z
Dinić S, Arambašić J, Mihailović M et al (2013) Decreased O-GlcNAcylation of the key proteins in kinase and redox signalling pathways is a novel mechanism of the beneficial effect of α-lipoic acid in diabetic liver. Br J Nutr 110(3):1–12. doi:10.1017/S0007114512005429
Li B, Liu S, Miao L, Cai L (2012) Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res 2012:1–7. doi:10.1155/2012/216512
Palsamy P, Subramanian S (2011) Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta 1812:719–731. doi:10.1016/j.bbadis.2011.03.008
Selcuk MY, Aygen B, Dogukan A et al (2012) Chromium picolinate and chromium histidinate protects against renal dysfunction by modulation of NF-κB pathway in high-fat diet fed and Streptozotocin-induced diabetic rats. Nutr Metab (Lond) 9:30. doi:10.1186/1743-7075-9-30
Kelleher AR, Fairchild TJ, Keslacy S (2010) STZ-induced skeletal muscle atrophy is associated with increased p65 content and downregulation of insulin pathway without NF-κB canonical cascade activation. Acta Diabetol 47:315–323. doi:10.1007/s00592-010-0209-1
Kawahara TLA, Michishita E, Adler AS et al (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136:62–74. doi:10.1016/j.cell.2008.10.052
Wu L, Zhang Y, Ma X et al (2012) The effect of resveratrol on FoxO1 expression in kidneys of diabetic nephropathy rats. Mol Biol Rep 39:9085–9093. doi:10.1007/s11033-012-1780-z
Acknowledgments
Financial support provided by grants obtained from Karamanoglu Mehmetbey University (BAP-27-M-12) and TUBITAK (112T159) is gratefully acknowledged.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadi, G., Bozan, D. & Yildiz, H.B. Redox regulation of antioxidant enzymes: post-translational modulation of catalase and glutathione peroxidase activity by resveratrol in diabetic rat liver. Mol Cell Biochem 393, 111–122 (2014). https://doi.org/10.1007/s11010-014-2051-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2051-1