Abstract
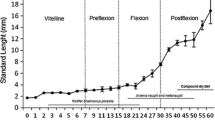
The process of differentiation of digestive tract structures in the sterlet Acipenser ruthenus (L.) larvae was studied from hatching to the beginning of exogenous feeding [9 dph (day post hatching)] using histological procedures. On the day of hatching the digestive tract was closed and completely filled with nutrients (the yolk platelets) that were successively utilized during development. A liver primordium was present in the ventral region of the yolksac. The pancreas was observed on the 2 dph. At the same time, the mouth opening took place. Glandular and nonglandular stomach and anterior and intermediate intestine developed from the yolksac walls. Gastric glands became visible on the 7 dph. The primary intestine developed into the spiral intestine. At the moment of onset of exogenous feeding the yolk material was completely exhausted and there was not mixed feeding observed in sterlet larvae. The fish started exogenous feeding on the 9 dph, which was accompanied with evacuation of melanin plug. At the end of endogenous feeding the digestive tract of sterlet larvae was developed and functional, so they could properly utilize food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sterlet (Acipenser ruthenus (L.) 1758) belong to one of the oldest phylogenetical group of Acipenseridae family (Birstein and DeSalle 1998). They inhabit regions including Caspian, Black, White, and Kara Sea catchment areas, and are rarely found in the Baltic Sea catchment. Sterlet becomes mature relatively early, at the age of 4–5 years (males), and 7–9 years (females) (Berg et al. 1949). Due to their fast maturation, and environmental requirements (freshwater), sterlet became an object of aquaculture. Despite their relatively small body size (maximum 1 m of body length, and 20 kg), for years sterlet have been of great interest of fishermen, fish farmers, and consumers due to the excellent taste of meat and caviar. Particularly high level of harvest resulted in a considerable population reduction, and nowadays sterlet is included to the list of endangered species. An overexploitation, together with the exceptionally high poaching level and environment degradation resulted in a drastic decrease in the population of all Acipenseridae, including sterlet (Birstein et al. 1997). According to the Washington Convention all Acipenseridae species are protected now (Lenhardt et al. 2006), and all of them are included into the international Red Book (IUCN 2006). Reduction of wild populations resulted in a decrease in sterlet harvest in their natural sites (CITES 2000; Hensel and Holcík 1997), which brought about an increased interest in rearing of these valuable fish under controlled conditions for both—commercial purposes and future restocking. Despite successful results of rearing of various sturgeon species, further studies are necessary to optimize rearing techniques, particularly in early developmental stages. Success of fish culture strongly depends on an application of appropriate strategy of larval rearing. Detailed knowledge of structure and function of larval and juvenile digestive system is of key importance for development of such optimum rearing strategies. From the practical and economical point of view, the appropriate moment of the beginning of feeding larvae exogenous food must be established and thus, detailed observation of the changes in digestive tract of larvae including development of digestion and nutrient absorption processes is particularly important. Over the past years, many studies were carried out concerning larval development of various species of sturgeons but the data on digestive tract histology are very scarce (Gawlicka et al. 1995; Gisbert et al. 1998; Gisbert and Doroshov 2003). Little data are available on general development of sterlet larvae (Dettlaff et al. 1993) but nothing is known on developmental changes in anatomy of these fish. Up till now no information on the development of sterlet digestive tract was available, thus the present study was undertaken, and the obtained results may be useful for aquaculture practice.
Materials and methods
The study was carried out in the Division of Ichthyobiology and Fisheries of the Warsaw University of Life Science. The sturgeon larvae used in experiment were obtained from a private fish farm “RYBA” in Olesnica, Poland.
The larvae were reared from hatching until the beginning of exogenous feeding. On the 9 day post hatching ad libitum feeding with Artemia salina nauplii was started. Experiment was terminated on the 10 dph. Rearing took place in two tanks of 1 m3 each in water recirculation system. Each tank was stocked with 50 thousand fish per m3. The temperature of water was 16 ± 0.8°C. The dissolved oxygen concentration was 10.2 ± 0.3 mg l−1.
During the experiment, fish were daily sampled (10 ind.) for histological and histochemical analyses. The sampled fish were anesthetized with MS 222 (tricaine methanesulphonate, Sigma–Aldrich Chemie GmbH, Taufkirchen, Germany). The samples were preserved in Bouin’s solution and subjected to the standard histological procedures. The paraffin blocks were cut longitudinally and transversely into the 5 μm sections using the rotational microtome Leica RM2265 (Leica Microsystems, Nussloch, Germany). Then the sections were stained with alcian blue and Periodic acid Schiff’s (AB/PAS) pH 2.5, 1.0, and 0.5 (Pearse 1985). The preparations were used to observe changes in the digestive tract structure during larval development and for identification of carbohydrate compounds. The observations were performed using a Nikon ECLIPSE 90i microscope connected with a digital camera Nikon DS5-U1 and a computer image analysis system NIS—Elements AR (Nikon Corporation, Tokyo, Japan).
Results
On the day of hatching, the larvae showed a primary digestive tract divided into two main sections. The anterior region included a large yolk sac filled with yolk and covered with unilayered squamous epithelium that later would develop into the glandular (cardiac) and nonglandular (pyloric) stomach, and anterior and intermediate intestine. The posterior region included developing primary intestine, which later would differentiate into the spiral intestine and the anal channel. In the newly hatched larvae the mouth was closed and buccopharynx was not connected with the yolk sac. The buccopharynx was covered with pseudo stratified epithelium with irregular cells filled with yolk and pigment granules. The posterior region of buccopharynx and esophagus were undeveloped. In the ventral section of the yolk sac, a liver primordium was visible. Hepatocytes showed irregular shape and were filled with large lipid vacuoles, yolk platelets and pigment granules. The primary intestine was closed, and appeared as a straight tube lined with ciliate cylindrical epithelium, which cells were filled with yolk and pigment granules.
Development of buccopharynx
On the first day post hatching the buccopharynx remained closed. In its anterior section a small lumen was observed but the posterior part was still completely filled with yolk. The buccopharynx was not connected with the yolksac since its lumen was filled with the yolk platelets. Epithelium of both, anterior and posterior part of buccopharynx showed yolk platelets and pigment granules (Fig. 1). Opening of the buccopharynx took place on the 2 dph. Mouth opening consisted of two folds lined with thin multilayered cubical epithelium. The yolk platelets disappeared completely from the anterior part of the buccopharynx but were still visible until the 3 dph in the epithelium. The posterior region of buccopharynx showed yolk presence until the 4 dph. First mucous cells that stained with alcian blue (AB/PAS, pH 2.5, 0.5 and 1.0) appeared in the buccopharynx epithelium on the 5 dph. These cells secreted acidic carboxyl, and sulfate mucins. In the larvae which were 5 days old, first teeth started to develop in folds surrounding mouth opening (Fig. 2), and at the onset of exogenous feeding they were already clearly visible in the mouth. The other teeth started to growing from the 7 dph, and appeared later in the posterior region of buccal cavity towards the pharynx. It was observed that teeth growth was synchronized and they developed in pairs: in upper and lower mouth region. Taste buds started to differentiate on the 6 dph.
Development of esophagus
Esophagus started to differentiate on the 3 dph, and development proceeded from the stomach towards the buccal cavity. Initially, esophagus was visible as a short tube of narrow lumen and its epithelial cells contained vacuoles filled with yolk platelets.
The esophageal lumen increased in developing larvae. The anterior section was filed with yolk and pigment granules until the 7 dph and at this time buccopharynx was not yet connected with the prospective stomach (Fig. 3). Between the 7 and 8 dph, irregular epithelial cells in the posterior esophagus part transformed into the cylindrical epithelium and the yolk material gradually disappeared.
Between the 8 and 9 dph, the esophagus became completely pervious, showing two distinct regions of different epithelium histological structure. Anterior section was straight, and lined with pseudostratified cylindrical epithelium with numerous mucous cells secreting mainly acidic mucins (AB/PAS, pH 2.5, 0.5 and 1.0) (Fig. 4a) and only single cells stained magenta indicating the presence of neutral carbohydrate compounds. Posterior esophagus section connected with the stomach was folded and lined with ciliated cylindrical epithelium (Fig. 4b). The mucous cells secreting neutral and acidic mucins were observed between the epithelial cells but in the posterior section they were less numerous comparing to the anterior part of esophagus.
Development of glandular and nonglandular stomach
On the 2 dph, epithelium covering the yolksac developed two furrows: ventral and dorsal (Fig. 5) which on the 4 dph divided the digestive tract into two parts: glandular stomach and intestine.
Development of glandular stomach started on the 6 dph and at that time the yolksac epithelium transformed from squamous into cylindrical (Fig. 6). The first gastric glands became visible on the 7 dph (Fig. 7) and their number increased during further larval development. The developing stomach was initially filled with yolk and pigment granules that gradually disappeared and on the 8 dph only small yolk particles remained.
On the 4 dph, multilayered squamous epithelium of the anterior ventral part of the yolksac started to develop into pyloric stomach. On the 5 dph the pyloric stomach was already clearly visible and showed mucous folds covering a thick muscle layer. In both, lumen and epithelium particles of yolk were still present but gradually disappeared. During the further larval development, the folds of pyloric stomach mucosa became higher and more numerous. At that time (5 dph), pyloric stomach started to separate from the intestine (anterior) with an epithelial fold that developed into the pyloric sphincter. On the 7 dph, the pyloric sphincter was a muscular fold covered with unilayered cylindrical epithelial cells still containing yolk platelets. The cells showed also large clear vacuoles (Fig. 8) which disappeared together with yolk reduction and were no more present on the 9 dph. Epithelial cells covering the sphincter showed regular cylindrical shape and centrally located nuclei. Yolk particles present in the lumen and epithelium of pyloric stomach disappeared between the 8 and 9 dph. At that time, nonglandular stomach was already completely developed showing a thick muscle layer, and high mucosal folds lined with ciliated cylindrical epithelium (Fig. 9). Pyloric stomach was separated from the anterior intestine with fully developed pyloric sphincter.
Development of anterior, middle, and spiral intestine
The anterior and central section of intestine in sterlet larvae which was 1 day old, was undifferentiated and filled with yolk. During the larval development the intestine became longer and on the 5 dph anterior intestine differentiated. During yolksac resorption, number and size of lipid vacuoles increased in the supranuclear regions of anterior intestine epithelial cells.
These vacuoles were gradually disappearing until the beginning of exogenous feeding. They were completely absent on the 9 dph. The first mucous cells developed in the anterior intestine on the 6 dph.
On the 3 dph, the middle intestine started to differentiate showing numerous and high epithelial folds. Enterocytes of this intestine section had basally located nuclei and supranuclear vacuoles filled with yolk platelets. During development, the height of folds decreased and yolk platelets disappeared from the epithelial cells. On the 4 dph, mucous cells became visible in the middle intestine (staining AB/PAS, pH 2.5, 0.5 and 1.0 positive), and their number increased with the age of larvae.
The middle intestine differed from the anterior intestine with the number and size of mucosal folds—in the former they were lower and less numerous. The epithelium was similar in both sections but in the anterior intestine enterocytes were less vacuolated.
The differentiation of spiral intestine started on the 2 dph from small folds in its posterior section that developed into the spiral valve and proceeded frontward. At that time, the lumen of spiral intestine was filled with yolk platelets, and the walls were lined with simply ciliated cylindrical epithelium with yolk platelets inside the supranuclear vacuoles. Large lipid vacuoles, and yolk platelets quickly disappeared and on the 3 dph they were no more visible. During the larval development, spiral intestine grew in length and its mucosal folds forming a spiral valve became higher. The spiral intestine, initially filled with yolk, on the 2 dph started to accumulate granules of embryonic pigment that formed a melanin plug (Fig. 10). On the 3 dph, posterior section of spiral intestine showed mucous cells staining with alcian blue (AB/PAS pH 2.5) indicating the presence of acidic carboxyl and sulfate mucins (AB/PAS pH 0.5 and 1.0). The number of mucous cells increased during the larval development. The endmost part of spiral intestine developed into a short anal channel lined with ciliated cylindrical epithelium without mucous cells. Opening of anus and evacuation of melanin plug took place on the 9 dph.
Development of liver and pancreas
The liver primordium located in a ventral region of the yolksac was already observed on the day of hatching. Hepatic cells of irregular shape were filled with yolk and pigment granules and contained large lipid vacuoles. On the next day the size of liver increased due to hepatocyte differentiation and lipid vacuoles became more numerous (Fig. 11). During larval development, further increase in lipid vacuole number took place, while yolk gradually disappeared. On the 2 dph, polygonal hepatocytes showed round nuclei with distinct nuclear membrane surrounded with small volume of cytoplasm. Each nucleus contained distinct nucleoli.
Pancreas developed at the dorsal side of furrow dividing the yolksac into stomach and intestine and was visible already on the 2 dph. As larval development proceeded, further pancreas differentiation took place. On the 3 dph it distinctly increased and showed first zymogen granules (Fig. 12). Exocrine pancreatic cells were arranged into lobules. On the 3 dph pancreas was visible in ventral region of the yolksac adjacent to the liver and showed yolk platelets. At the same time, gall bladder developed between the liver and pancreas (Fig. 13).
On the 6 dph, a bile duct became visible but not fully pervious (Fig. 14). Gall bladder, and bile duct were lined with unilayered cubical epithelium.
At the same time (6 dph), hepatic yolk almost completely disappeared, while the number of lipid vacuoles increased. Hepatocyte nuclei were located peripherally, and cytoplasm was almost completely filled with lipids.
During the larval development, pancreas grew, and filled a large part of larval body cavity. On the 8 dph it was situated among the glandular and nonglandular stomach and the intestine, adjacent to the liver in front, and directly under kidney at the back (Fig. 15). At this developmental stage no yolk platelets were observed. Pancreatic cells were arranged into vesicular structures. Bile duct was pervious, and connected gall bladder with anterior intestine, over the non-glandular stomach, near the pyloric sphincter.
Histological picture of liver did not change at that time and hepatocyte cytoplasm still showed numerous lipid vacuoles (Fig. 16). Liver size increased and its middle part developed between the stomach and intestine. Distinct histological changes took place after the onset of exogenous feeding of larvae (Fig. 17). At that time, hepatocytes showed no more lipid vacuoles and their shape became regular. Numerous blood vessels filled with blood cells appeared among hepatocytes.
Discussion
The results of present study showed that digestive tract ontogenesis in sterlet larvae was similar as in other sturgeon species (Buddington and Christofferson 1985). However, some differences concerning timing of differentiation of various organs, and functional ability of food uptake, digestion and absorption were observed. Majority developmental changes in the sterlet larvae took place during the first days after hatching. Similarly as in other sturgeons, the sterlet larval digestive tract consisted of large endodermal yolksac and undifferentiated intestine at hatching (Boglione et al. 1999; Gisbert et al. 1998; Gisbert and Doroshov 2003). Mouth and anus were closed. Similar observations were made on Siberian sturgeon (Acipenser baerii, Brandt 1869) (Gisbert et al. 1998), Adriatic sturgeon (Acipenser naccarii, Bonaparte 1936) (Cataldii et al. 2002) and green sturgeon (Acipenser medirostris, Ayres 1854) (Gisbert and Doroshov 2003). The newly hatched sterlet larvae showed no pancreas but had a liver primordium situated under the yolksac. No digestive glands, liver and pancreas were observed at hatching in the green and white sturgeon larvae (Acipenser transmontanus, Richardson 1836) (Buddington and Doroshov 1986b; Gisbert and Doroshov 2003). Adriatic sturgeon larvae at the same developmental stage showed both, pancreas and liver (Boglione et al. 1999).
During the first days of larval development fish digestive tract undergoes intense differentiation process (Buddington and Christofferson 1985). In sterlet larvae, digestive tract development started from the spiral intestine and proceeded frontward, similarly as in other sturgeons: white, green and Siberian (Buddington and Doroshov 1986b; Gisbert et al. 1998; Gisbert and Doroshov 2003).
During development of sterlet digestive tract, as in other sturgeons (Buddington and Christofferson 1985), epithelium covering the yolksac differentiated into the anterior and middle intestine, and the stomach (glandular and nonglandular), the latter being developed last.
The lumen of entire digestive tract of sterlet larvae was initially filled with yolk and yolk platelets were present also in the supranuclear vacuoles of digestive tract epithelial cells. Yolk in epithelium of buccopharynx, esophagus and intestine was observed also in green and Siberian sturgeon larvae (Gisbert et al. 1998; Gisbert and Doroshov 2003). According to Dettlaff et al. (1993), it results from holoblastic cleavage, and contribution of yolk-rich endodermal cells in digestive tract development. Buddington (1985), and Buddington and Doroshov (1986b) suppose that the presence of yolk material inside the epithelial cells of developing digestive tract indicate pinocytosis and intracellular digestion.
Larvae of many fish species show three phases of feeding: endogenous, mixed, and exclusively exogenous (Mani-Ponset et al. 1996; Ostaszewska et al. 2003). In the sterlet larvae, however, no mixed feeding was observed. According to Gisbert et al. (1998), the presence of yolk in the glandular stomach and supranuclear vacuoles filled with yolk in the anterior and middle intestine after the onset of exogenous feeding may indicate mixed feeding. In the sterlet larvae, the end of endogenous feeding coincided with complete exhaustion of yolk and start of exogenous feeding (9 dph). At that time glandular stomach showed no yolk traces and anterior and middle intestine showed no supranuclear vacuoles. Histological analyses revealed that the stomach became connected with the mouth already between the 8 and 9 dph, while earlier esophagus was impervious and separated both parts making food uptake impossible. Thus, the period of mixed feeding does not occur in certain sturgeon species. This observation is in accordance with the data obtained by Dettlaff et al. (1993) and Buddington and Christofferson (1985).
The beginnig of exogenous feeding accompanied with evacuation of melanin plug. Some authors suggest that the onset of exogenous feeding should coincide with melanin plug excretion (Gawlicka et al. 1995; Monaco et al. 1981). However, according to Gisbert and Williot (1997) this is not an appropriate moment for food supply; feeding should be accompanied with yolksac exhaustion and appearance of food search behavior. Feeding before the end of endogenous period is recommended by Conte et al. (1988). On the other hand, the results obtained by Gisbert and Williot (1997) for Siberian sturgeon larvae indicate that food supply before the yolksac exhaustion is completely ineffectual. The authors observed no increase in body length and mass and survival of the larvae earlier supplied with food. The results of present study showed that evacuation of melanin plug is an appropriate moment to start feeding the larvae because at that time the digestive tract becomes pervious, thus earlier feeding is unjustified.
Most teleost fishes start exogenous feeding before their digestive tracts are fully developed (Boulhtic and Gabaudan 1992; Ostaszewska et al. 2003; Sarasquete et al. 1995). Sterlet larvae showed developed and functional digestive tract before the onset of active feeding, so they could take up, digest and absorb exogenous feed.
Histological analysis revealed that the mouth of sterlet larvae was open already on the 2 dph. At the same time mouth opening was observed in the Adriatic sturgeon (Boglione et al. 1999) and green sturgeon (Gisbert and Doroshov 2003). In the Siberian sturgeon, mouth was opened already on the first day (Gisbert et al. 1998). The first single mucous cells staining with alcian blue (pH 2.5, 1.0, and 0.5) were present in buccopharynx and among the undifferentiated esophageal epithelial cells of sterlet larvae on the 5 dph. The number and size of mucous cells increased with the fish age and their histochemical properties changed. Initially, these cells stained blue showing the presence of acidic mucins, while at the end of endogenous feeding the esophageal mucous cells secreted both, neutral and acidic carbohydrate compounds. Domeneghini et al. (1998) explained such a difference in mucus secretion with maturation and age of mucous cells. Similar type of mixed secretion pattern was observed in esophagus of gilthead seabream (Sparus aurata) (Domeneghini et al. 1998), in Mylio cuvieri (Abdullah Al Abdulhadi 2005) and in Melanogrammus aeglefinus (Hamlin et al. 2000), and also in other sturgeon species (Gisbert et al. 1998; Gisbert and Doroshov 2003). According to Gona (1979), density and type of mucous glycoprotein may be different even in closely related species under the same environmental conditions.
Mucus secreted in the digestive tract is the first protective barrier against mechanical, physical and chemical factors as well as against infections (Yashpal et al. 2007). According to Scocco et al. (1998), esophageal mucus in fish may show the same protective functions as saliva in mammals. Mucous cells and layered esophageal epithelium of fish, similarly as in other vertebrates, are responsible for transport (Abdullah Al Abdulhadi 2005). In sturgeons, this function is additionally aided by cilia covering the posterior section of esophagus (Gisbert et al. 1998; Gisbert and Doroshov 2003). At the end of endogenous feeding, esophageal epithelium of sterlet larvae was distinctly divided into two regions that differed in morphology and mucous cell histochemistry. Similar differences were observed in esophagus of Siberian and green sturgeons (Gisbert et al. 1998; Gisbert and Doroshov 2003).
Gastric region in sterlet started to differentiate on the 4 dph, which was much earlier than in most fish species lacking stomach until metamorphosis (Ostaszewska 2002). Nonglandular stomach developed first, while the first gastric glands appeared on the 7 dph, 2 days before the start of exogenous feeding. The differentiation of glandular stomach started earlier than in other sturgeon species. In Siberian, Adriatic and white sturgeons, gastric glands started to develop between the 8 and 9 dph, while in green sturgeon appeared on 12 dph (Buddington and Doroshov 1986b; Cataldii et al. 2002; Gisbert et al. 1998; Gisbert and Doroshov 2003). Sterlet stomach, similarly as in other species, showed one type of secretory cells responsible for both, hydrochloric acid and pepsinogen secretion (Domeneghini et al. 2005). Some authors suggest that the larvae have to develop functional stomach to utilize nutrients (Baglole et al. 1997). According to Hamlin et al. (2000), the presence of gastric glands indicates the beginning of chemical digestion processes. On the other hand, the results obtained by Vega-Orellana et al. (2006) suggest that morphology of stomach is not necessary indicative of its functionality. The authors proved that the main structure of stomach in Salminus brasiliensis larvae was already visible on the 3 dph, while no activity of acidic protease was detected. A considerable increase in activity of this enzyme took place on the 4 dph. This was confirmed by the results obtained by Walford and Lam (1993) who observed no pepsin activity in barramundi (Lates calcarifer) larvae at the time when gastric glands were already developed. On the other hand, the results of biochemical and histological analysis of digestive tract of the lake sturgeon (Acipenser fulvescens, Rafinesque 1817) and white sturgeon larvae done by Buddington (1985) and Buddington and Doroshov (1986a, b) indicate that at the moment of first feeding, fish showed fully developed stomach, showing pepsin presence and acidic pH. This was in accordance with the results of histochemical analyses carried out by Gisbert et al. (1999) who observed that the presence of gastric glands in Siberian sturgeon larvae coincided with high concentration of pepsin and acidic pH inside the stomach. These two properties are considered an indicator of gastric functionality. Histochemical properties of gastric mucous cells may be an additional indicator of stomach secretory activity. According to Mai et al. (2005), development of gastric glands in Pseudosciaena crocea larvae, together with the presence of neutral glycoproteins in gastric mucous cells protecting stomach wall against self-digestion by hydrochloric acid and enzymes indicate gastric functionality. Similarly, Gisbert et al. (2004) present an opinion that despite the lack of data on pepsin secretion, histological differentiation of gastric glands in California flounder (Paralichthys californicus) larvae, and the presence of gastric mucous cells abundantly secreting neutral mucin may indicate that gastric glands are functional. At the moment when sterlet larvae started exogenous feeding, gastric epithelium showed the presence of neutral carbohydrate compounds. This observation is in accordance with the data obtained by Abol-Munafi et al. (2006) who reported a PAS-positive mucous in the stomach of marble goby (Oxyeleotris marmorata) larvae immediately after development of gastric glands. Similar observation was made by Verreth et al. (1992) in African catfish (Clarias gariepinus) and by Ostos Garrido et al. (1993) in rainbow trout (Oncorhynchus mykiss).
Besides the protection of mucosa against acidic secretory products (Morrison and Wright 1999), gastric mucus produced by the cells lining stomach walls and by some supranuclear areas of gastric gland cells may participate in transport involved in gastric secretion (Domeneghini et al. 2005). In the sterlet larvae stomach was lined with ciliated cylindrical epithelial cells. According to Buddington and Doroshov (1986b), the cilia may participate in distribution and maintaining the mucus layer which helps the muscles in food transport.
In the present study, activity of digestive enzymes during endogenous feeding was not analyzed. However, the data obtained by the mentioned above authors, together with the results of histological and histochemical analysis indicate that at the moment of first feeding the sterlet larvae showed a fully functional stomach.
The results of present study revealed also that until the beginnig of exogenous feeding sterlet larvae developed anterior, middle and posterior (spiral) intestine and a short anal channel with the anus.
In the period when larvae utilized yolksac nutrients, supranuclear areas of intestine epithelial cells and hepatocytes showed large lipid vacuoles. According to Dettlaff et al. (1993), it is a result of gradual accumulation of yolk lipids. Such an accumulation of lipid droplets in epithelial cell cytoplasm indicates a temporary storage (Calzada et al. 1998) and is related to the lack of lipolytic activity (Buddington 1985). Lipid vacuoles in the intestine and liver of sterlet larvae were present until the onset of exogenous feeding and then disappeared. Gisbert et al. (1998) suggest that it may indicate alteration in lipid metabolism. The results obtained by Buddington (1985), and Buddington and Doroshov (1986a) indicate an increase in lipolytic activity after first feeding. Thus, it can be supposed that the disappearance of lipid vacuoles is related to the increase of ability of lipid utilization by the larvae.
The first mucous cells developed in the spiral intestine on the 3 dph, while in the middle and anterior intestine later, according to development process. In other fish species the first intestinal mucous cells were also observed in early developmental phase (Boulhtic and Gabaudan 1992; García Hernández et al. 2001). The number of mucous cells in sterlet intestine increased during the larval development similarly as in other fish species (Elbal et al. 2004; Ribeiro et al. 1999a). Intestinal mucus of sterlet larvae showed the presence of exclusively acidic compounds. Its histochemical properties did not change until the end of endogenous feeding. Similarly, in the nase larvae (Chondrostoma nasus) mucous cells synthesized only acidic mucins (Sysa et al. 2006). Veggetti et al. (1999) observed exclusively acidic compounds in the common sole larvae (Solea solea) at the stage of morphological differentiation. In the other fish species such as gilthead seabream (Sarasquete et al. 1995; Elbal et al. 2004) or turbot (Scophthalmus maximus) (Segner et al. 1994) intestinal epithelium secreted both, neutral and acidic mucins. It was in accordance with observation made by Gisbert et al. (1999) who found mainly mixed secretion of intestinal mucous cells in Siberian sturgeon larvae and juveniles. Also, in the sole larvae mucous cell product contained neutral mucins (Ribeiro et al. 1999a). Intestinal mucus containing mainly carbohydrate compounds (Domeneghini et al. 1998) has many physiological functions such as enhancement of digestion and nutrient absorption efficiency (Grau et al. 1992), buffering of intestine content and protection against proteolytic epithelium injury (Domeneghini et al. 2005). Additionally, it plays an osmotic role which is particularly important in fish because of participation in water and ion binding, as well as transport (Smith 1989). The presence of neutral mucins in the digestive tract is usually associated with absorption of easily digestible nutrients such as disaccharides or short-chain fatty acids (Osman and Caceci 1991). But according to Segner et al. (1994), carbohydrate compounds, particularly sulfate mucins, may regulate intestinal transport of proteins or peptides.
The results of present study indicate that the intestine of sterlet larvae before the end of endogenous feeding showed cellular structure similar as in the adult individuals (Buddington and Doroshov 1986b). Cylindrical epithelial cells lining the intestinal lumen showed the presence of brush border (microvilli), and some of them were ciliated. The larvae of certain teleost fishes also show the presence of cilia that, however, disappear later on (Tanaka 1973) and their presence in adults is considered a primitive feature typical for phylogenetically older fishes (Weisel 1973). Ferraris et al. (1987) reported the presence of cilia in both, the larvae and adults of associate Chanos chanos. According to Buddington and Christofferson (1985), the potential role of cilia is distribution of mucus and other digestive tract secretory products and facilitation of food transport (Ferraris et al. 1987).
Bisbal and Bengtson (1995) believe that the functional characteristics of intestine is related to its structure. That typical epithelium in which active nutrient transport takes place consists of cylindrical cells with brush border exposed to the intestinal lumen. Such was the structure of sterlet larval intestine epithelium at the moment of first feeding. According to García Hernández et al. (2001), differentiation of intestine is complete when mucosal folds, mucous cells, pyloric caeca are present and fish begin exogenous feeding. Folded mucosa may facilitate mixing of food with enzymes secreted by pancreas and liver and with mucus produced by the mucous cells (Grau et al. 1992), while pyloric caeca increase the absorptive surface of the intestine (Bisbal and Bengtson 1995). Although in the sterlet larvae pyloric caeca were not visible at that time, the morphology of intestine was similar to that described by García Hernández et al. (2001). According to Buddington (1985), white sturgeon at the same developmental stage (beginning of exogenous feeding) showed similar digestive enzymes as adults, which was probably genetically determined. Thus, it is possible that sterlet larvae showed a functional intestine at the beginning of active feeding.
Sterlet larvae at the onset of exogenous feeding showed completely developed digestive glands: liver and pancreas. Liver differentiated from the moment of hatching and when the fish started exogenous feeding it was already a large organ occupying much space in the peritoneal cavity. Glycogen storage is one of hepatic function indicators (Sysa et al. 2006). In the sterlet larvae glycogen storage was already detected on the 9 dph. Pancreas became visible on the 2 dph, and from the 3 dph proenzyme granules were observed in the exocrine part. Zymogen granules in exocrine pancreas were observed immediately before first feeding in many fish species (Chen et al. 2006; Ribeiro et al. 1999b).
The results of present study indicate that organization and sequence of differentiation of various digestive system structures in sterlet larvae were similar as in other sturgeon species and that the larvae at the beginning of exogenous feeding were sufficiently developed to fully utilize food.
References
Abdullah Al Abdulhadi H (2005) Some comparative histological studies on alimentary tract of tilapia fish (Tilapia spilurus) and sea bream (Mylio cuvieri). Egypt J Aquat Res 31:1
Abol-Munafi AB, Liem PT, Van MV, Ambak MA, Effendy AWM, Awang Soh M (2006) Histological ontogeny of the digestive system of marble goby (Oxyeleotris marmoratus) larvae. J Sust Sci Manage 1(2):79–86
Baglole CJ, Murray HM, Goff GP, Wright GM (1997) Ontogeny of the digestive tract during larval development of yellowtail flounder: a light microscopic and mucous histochemical study. J Fish Biol 51:120–134. doi:10.1111/j.1095-8649.1997.tb02518.x
Berg LS, Bogdanov AS, Kozhin NI, Rass TS (1949) Promyslovye ryby SSSR. Atlas tsvetnykh risunkov ryb, Mockva
Birstein VJ, DeSalle R (1998) Molecular phylogeny of Acipenserinae. Mol Phylogenet Evol 9:141–155. doi:10.1006/mpev.1997.0443
Birstein VJ, Bemis WE, Waldman JR (1997) The threatened status of acipenseriform species: a summary. Environ Biol Fishes 48:427–435. doi:10.1023/A:1007382724251
Bisbal GA, Bengtson DA (1995) Development of digestive tract in larval summer flounder. J Fish Biol 47:277–291. doi:10.1111/j.1095-8649.1995.tb01895.x
Boglione C, Bronzi P, Cataldi E, Serra S, Gagliardi F, Cataudella S (1999) Aspects of early development in the Adriatic sturgeon Acipenser naccarii. J Appl Ichthyol 15:207–213. doi:10.1111/j.1439-0426.1999.tb00236.x
Boulhtic M, Gabaudan J (1992) Histological study of the organogenesis of the digestive system and swim bladder of the Dover sole, Solea solea (Linnaeus 1758). Aquaculture 102:373–396. doi:10.1016/0044-8486(92)90190-V
Buddington RK (1985) Digestive secretions of lake sturgeon, Acipenser fulvescens, during early development. J Fish Biol 26:715–723. doi:10.1111/j.1095-8649.1985.tb04311.x
Buddington RK, Christofferson JP (1985) Digestive and feeding characteristics of the chondrosteans. Environ Biol Fishes 14:31–41. doi:10.1007/BF00001574
Buddington RK, Doroshov SI (1986a) Development of digestive secretions in white sturgeon juveniles (Acipenser transmontanus). Comp Biochem Physiol 83A(2):233–238. doi:10.1016/0300-9629(86)90567-0
Buddington RK, Doroshov SI (1986b) Structural and functional relations of the white sturgeon alimentary canal (Acipenser transmontanus). J Morphol 190:201–213. doi:10.1002/jmor.1051900205
Calzada A, Medina A, González de Canales ML (1998) Fine structure of the intestine development in cultured sea bream larvae. J Fish Biol 53:340–365. doi:10.1111/j.1095-8649.1998.tb00985.x
Cataldii E, Albano C, Boglione C, Dini L, Monaco G, Bronzi P, Cataudella S (2002) Acipenser naccarii: fine structure of the alimentary canal with references to its ontogenesis. J Appl Ichthyol 18:329–337. doi:10.1046/j.1439-0426.2002.00383.x
Chen BN, Qin JG, Kumar MS, Hutchinson WG, Clarke SM (2006) Ontogenetic development of the digestive system in yellowtail kingfish Seriola lalandi larvae. Aquaculture 256:489–501. doi:10.1016/j.aquaculture.2006.01.041
CITES (2000) Document AC.16. 7. 2. 16th meeting of the CITES Animals Committee Shepherdstown, 11–15 December. www.cites.org/eng/aC/16/16-7-2a5.pdf
Conte FS, Doroshov SI, Lutes PB, Strange EM (1988) Hatchery manual for white sturgeon, Acipenser transmontanus (R.) with application to other North American Acipenseridae. Division of Agricultural and Natural Resources, University of California, Oakland
Dettlaff TA, Ginsburg AS, Schmalhausen OI (1993) Sturgeon fishes. Developmental biology and aquaculture. Spriner, Berlin
Domeneghini C, Pannelli Straini R, Veggetti A (1998) Gut glucoconjugates in Sparus aurata L. (Pisces, Teleostei). A comparative histochemical study in larval and adult ages. Histol Histopathol 13:135–145
Domeneghini C, Arrighi S, Radaelli G, Bosi G, Veggetti A (2005) Histochemical analysis of glycoconjugate secretion in the alimentary canal Anguilla anguilla L. Acta Histochem 106:477–487. doi:10.1016/j.acthis.2004.07.007
Elbal MT, Garćia-Hernández MP, Lozano MT, Agulleiro B (2004) Development of the digestive tract of gilthead sea bream (Sparus aurata L.). Light and electron microscopic studies. Aquaculture 234:215–238
Ferraris RP, Tan JD, De la Cruz MC (1987) Development of the digestive tract of milkfish, Chanos chanos (Forsskal): histology and histochemistry. Aquaculture 61:241–257. doi:10.1016/0044-8486(87)90153-0
García Hernández MP, Lozano MT, Elbal MT, Agulleiro B (2001) Development of the digestive tract of sea bass (Dicentrarchus labrax L.). Light and electron microscopic studies. Anat Embryol (Berl) 204:39–57. doi:10.1007/s004290100173
Gawlicka A, Teh SJ, Hung SSO, Hinton DE, De la Noüe J (1995) Histological and histochemical changes in the digestive tract of white sturgeon larvae during ontogeny. Fish Physiol Biochem 14:357–371. doi:10.1007/BF00003374
Gisbert E, Doroshov SI (2003) Histology of the development digestive system and the effect of food deprivation in larval green sturgeon (Acipenser medirostris). Aquat Liv Res 16:77–89. doi:10.1016/S0990-7440(03)00029-9
Gisbert E, Williot P (1997) Larval behaviour and effect of the timing of initial feeding on growth and survival of Siberian sturgeon (Acipenser baeri) larvae under small scale hatchery production. Aquaculture 156:63–76. doi:10.1016/S0044-8486(97)00086-0
Gisbert E, Rodriguez A, Castelló-Orvay F, Williot P (1998) A histological study of the development of the digestive tract of Siberian sturgeon (Acipenser baeri) during early ontogeny. Aquaculture 167:195–209. doi:10.1016/S0044-8486(98)00312-3
Gisbert E, Sarasquete MC, Williot P, Castelló-Orvay F (1999) Histochemistry of the digestive system of Siberian sturgeon during early ontogeny. J Fish Biol 55:596–616. doi:10.1111/j.1095-8649.1999.tb00702.x
Gisbert E, Piedrahita RH, Conklin DE (2004) Ontogenetic development of the digestive system in California halibut (Paralichthys californicus) with notes on feeding practices. Aquaculture 232:455–470. doi:10.1016/S0044-8486(03)00457-5
Gona O (1979) Mucous glycoproteins of teleostean fish: a comparative histochemical study. Histochem J 11:709–771. doi:10.1007/BF01004734
Grau A, Crespo S, Sarasquete MC, González de Canales ML (1992) The digestive tract of the of the amberjack Seriola dumerili, Risso: a light and scanning electron microscope study. J Fish Biol 41:287–303. doi:10.1111/j.1095-8649.1992.tb02658.x
Hamlin HJ, Hunt von Herbing I, Kling LJ (2000) Histological and morphological evaluations of the digestive tract and associated organs of haddock throughout post-hatching ontogeny. J Fish Biol 57:716–732. doi:10.1111/j.1095-8649.2000.tb00270.x
Hensel K, Holčík J (1997) Past and current status of sturgeons in the upper and middle Danube river. Environ Biol Fishes 48:185–200. doi:10.1023/A:1007315825215
IUCN (2006) Red list of threatened species–prepared by the IUCN species survival commission. IUCN, Gland
Lenhardt M, Jaric I, Kalauzi A, Cvijanovic G (2006) Assessment of extinction risk and reasons for decline in sturgeon. Biodivers Conserv 15:1967–1976. doi:10.1007/s10531-005-4317-0
Mai K, Yu H, Ma H, Duan Q, Gisbert E, Zambonino Infante JL, Cahu CL (2005) A histological study on the development of the digestive system of Pseudosciaena crocea larvae and juveniles. J Fish Biol 67:1094–1106. doi:10.1111/j.0022-1112.2005.00812.x
Mani-Ponset L, Guyot E, Diaz JP, Connes R (1996) Utilization of yolk reserves during post-embryonic development in three teleostean species: the sea bream Sparus aurata, the sea bass Dicenfrarchus labrax, and the pike-perch Stizostedion lucioperca. Mar Biol (Berl) 126:539–547. doi:10.1007/BF00354636
Monaco G, Buddington RK, Doroshov SI (1981) Growth of white sturgeon (Acipenser transmontanus) under hatchery contitions. In: Proceedings of 12th Annual Meeting World Maricult. Soc., vol 12, pp 113–121
Morrison CM, Wright JR (1999) A study of the histology of the digestive tract of the Nile tilapia. J Fish Biol 54:597–606. doi:10.1111/j.1095-8649.1999.tb00638.x
Osman AHK, Caceci T (1991) Histology of the stomach of Tilapia nilotica (Linnaeus, 1758) from the river Nile. J Fish Biol 38:221–223. doi:10.1111/j.1095-8649.1991.tb03107.x
Ostaszewska T (2002) Zmiany morfologiczne i histologiczne układu pokarmowego i pęcherza pławnego w okresie wczesnej organogenezy larw sandacza (Stizostedion lucioperca L.) w różnych warunkach odchowu. Rozprawy naukowe i monografie. Wyd. SGGW
Ostaszewska T, Wegner A, Węgiel M (2003) Development of the digestive tract of ide, Leuciscus idus (L.) during the larval stage. Arch Pol Fish 11:181–195
Ostos Garrido MV, Nunez Torres MI, Abaurrea Equisoain MA (1993) Histological, histochemical and ultrastructural analysis of the gastric mucosa in Oncorhynchus mykiss. Aquaculture 115:121–132. doi:10.1016/0044-8486(93)90363-4
Pearse AGE (1985) Histochemistry. Theoretical and applied. vol. 2 Analytic Technology. Churchill Livingstone, New York
Ribeiro L, Sarasquete C, Dinis MT (1999a) Histological and histochemical development of the digestive system of Solea senegalensis (Kaup, 1858) larvae. Aquaculture 171:293–308. doi:10.1016/S0044-8486(98)00496-7
Ribeiro L, Zambonino-Infante JL, Cahu C, Dinis MT (1999b) Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858. Aquaculture 179:465–473. doi:10.1016/S0044-8486(99)00180-5
Sarasquete MC, Polo A, Yúfera M (1995) Histology i histochemistry of the development of the digestive system of larval gilthead seabream Sparus aurata L. Aquaculture 139:79–92. doi:10.1016/0044-8486(94)00175-N
Scocco P, Accili D, Menghi G, Ceccarelli P (1998) Unusual glycoconjugates in the oesophagus of a tilapine polyhybrid. J Fish Biol 53:39–48. doi:10.1111/j.1095-8649.1998.tb00107.x
Segner H, Storch V, Reinecker M, Kloas W, Hanke W (1994) The development of functional digestive and metabolic organs in turbot, Scophthalmus maximus. Mar Biol (Berl) 119:471–486. doi:10.1007/BF00347544
Smith LS (1989) Digestive functions in teleost fishes. In: Havler JE (ed) Fish nutrition, 2nd edn. Academic Press, London, pp 331–421
Sysa P, Ostaszewska T, Olejniczak M (2006) Development of digestive system and swim bladder of larval nase (Chondrostoma nasus L.). Aquacult Nutr 12(5):331–339. doi:10.1111/j.1365-2095.2006.00368.x
Tanaka M (1973) Studies on the structure and function of the digestive system of teleost larvae. Ph.D. dissertation, Kyoto University, pp. 136
Vega-Orellana OM, Machado Fracalossi D, Kiyoko Sugai J (2006) Dourdo (Salminus brasiliensis) larviculture: weaning and ontogenetic development of digestive proteinases. Aquaculture 252:484–493. doi:10.1016/j.aquaculture.2005.07.002
Veggetti A, Rowlerson A, Radaelli G, Arrighi S, Domeneghini C (1999) Post-hatching development of the gut and lateral muscle in the sole. J Fish Biol 55(Suppl. A):44–65
Verreth J, Toreele E, Spazier E, Sluiszen AVD, Rombout J, Booms R, Segner H (1992) Development of a functional digestive system in the African catfish Claris gariepinus (Burchell). J World Aquacult Soc 23:286–298. doi:10.1111/j.1749-7345.1992.tb00792.x
Walford J, Lam TJ (1993) Development of digestive tract and proteolytic enzyme activity in seabass (Lates calcarifer) larvae and juveniles. Aquaculture 233:305–320
Weisel GF (1973) Anatomy and Histology of the Digestive System of the Paddlefish (Polyodon spathula). J Morphol 140:243–256. doi:10.1002/jmor.1051400209
Yashpal M, Kumari U, Mittal S, Mittal AK (2007) Histochemical characterization of glycoproteins in the buccal epithelium of the catfish, Rita rita. Acta Histochem 109:285–303. doi:10.1016/j.acthis.2007.03.002
Acknowledgments
The present study was financed from the funds of the Ministry of Science and Higher Education (grant No N311 030 32/2256). The authors would like to express their thanks to Dr. Eugeniusz Bogdan from the Fish Farm “RYBA” in Olesnica, Poland for supplying fish.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wegner, A., Ostaszewska, T. & Rożek, W. The ontogenetic development of the digestive tract and accessory glands of sterlet (Acipenser ruthenus L.) larvae during endogenous feeding. Rev Fish Biol Fisheries 19, 431–444 (2009). https://doi.org/10.1007/s11160-009-9111-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-009-9111-8