Abstract
Oxidative stress is a possible source of spermatozoa function deterioration. Seminal fluid (SF) protects spermatozoa against reactive oxygen species (ROS) attack during development in testes and transit through the reproductive tract. Spermatozoa curvilinear velocity and percent of motile cells as well as changes in thiobarbituric acid-reactive substance (TBARS) content, superoxide dismutase, and catalase activity, and uric acid concentration in SF were evaluated in sterlet sperm collected from testes 24 h after hormone induction of spermiation and from Wolffian ducts at 12, 24, 36, and 60 h after hormone injection (HI). While testicular spermatozoa motility was not initiated in activating medium, Wolffian duct sperm showed low motility at 12 h, significant increase at 24 and 36 h, and decrease at 60 h. Testicular SF was characterized by the highest level of TBARS and activity of studied enzymes compared with SF from Wolffian duct sperm at 24 h post-HI. In fluid from Wolffian duct sperm, a significant increase in TBARS content was shown at 36–60 h post-HI. In contrast to testicular SF, in SF from Wolffian duct sperm, this increase was not counterbalanced by changes in the studied variables of antioxidant system. This may be the source of the observed decrease in spermatozoa motility parameters 60 h post-HI. The results may confirm a dual role of ROS in fish sperm physiology. The data with respect to decrease in sturgeon spermatozoa motility parameters at 60 h post-HI should be taken into account in artificial sturgeon propagation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The process through which spermatozoa acquire the capability of motility and fertilization is called maturation (Schulz et al. 2010). This process is well investigated in many animal species, especially in humans and domestic mammals (de Lamirande et al. 1997; Marengo 2008; Sostaric et al. 2008), because of its importance for success in assisted reproductive technologies. In mammals, spermatozoa acquire the potential for motility as they leave the testis and pass along the epididymis. Fish spermatozoa maturation has not commonly been considered a strongly limiting factor for success in aquaculture and has been investigated in only a few teleost species (Morisawa and Morisawa 1986, 1988; Miura et al. 1995; Ohta et al. 1997; Miura and Miura 2001). These studies have shown that substances in the sperm duct are required for spermatozoa to acquire the potential for motility.
We have recently established that the sperm maturation process in sturgeon takes place outside the testes and suggested that high molecular weight substances present in seminal fluid (SF) and/or urine are involved (Dzyuba et al. 2014).
Sturgeon possesses an excretory system in which sperm and urinary ducts are not completely separated. We observed significant differences between sterlet testicular sperm and sperm collected from Wolffian ducts, the latter being commonly used in aquaculture. Testicular spermatozoa did not become motile in activating medium (AM). Motility took place after in vitro incubation in urine or SF from Wolffian duct sperm in a temperature and time-dependent manner (Dzyuba et al. 2014). We found a lack of motility in testicular spermatozoa after incubation in SF from Wolffian duct sperm from which high molecular weight substances had been removed, indicating that the presence of high molecular weight substances in SF is a prerequisite for sturgeon spermatozoa maturation.
The antioxidant system of SF and spermatozoa plays an important role in maintaining the semen viability under in vivo conditions and in vitro storage (Lahnsteiner and Mansour 2010). To counteract oxidative damage, SF and spermatozoa possess low molecular weight antioxidants and antioxidant enzymes. In the present study, superoxide dismutase (SOD), catalase (CAT), and uric acid (UrAc) were chosen as representatives of enzymatic and non-enzymatic antioxidant systems. SOD and CAT are the most important elements in the enzymatic protective system, since they are, respectively, the scavengers of superoxide anion radicals and hydrogen peroxide, the latter being partially produced during enzymatic dismutation of superoxide anion radicals. For this reason, it is better to analyze the combined activity of SOD and CAT. Uric acid is probably the main non-enzymatic antioxidant in fish SF, as it is present in high concentrations in SF of a range of teleost species (Ciereszko et al. 1999; Lahnsteiner et al. 2010). There are no data available with respect to the UrAc level in SF of chondrosteans. Available data concerning the intensity of peroxidation processes and antioxidant system stability under in vitro fish sperm storage are somewhat contradictory. In brown trout, thiobarbituric acid-reactive substance (TBARS) content in spermatozoa and SF was essentially increased after 48 h of in vitro storage (Lahnsteiner et al. 2010), while the concentration of all studied non-enzymatic substances and antioxidant enzymes did not change in SF or in spermatozoa during storage. In Siberian and Russian sturgeon, significant increase in TBARS content has also been shown in spermatozoa after 3 and 6 days in vitro storage, respectively (Shaliutina et al. 2013), but an increase in SOD activity preceded the increase in TBARS concentration. Data on intensity of peroxidation processes and the antioxidant system capacity of sturgeon SF are lacking.
The primary goal of the present study was to evaluate changes in TBARS content, SOD and catalase activity, and uric acid concentration of SF of sterlet Acipenser ruthenus sperm collected from testes and from the Wolffian ducts at various times after carp pituitary induction of spermiation.
Materials and methods
All experiments were conducted according to the principles of the Ethics Committee for the Protection of Animals in Research of the University of South Bohemia in Ceske Budejovice, Research Institute of Fish Culture and Hydrobiology, Vodnany (based on the EU-harmonized Animal Welfare Act of the Czech Republic).
Fish rearing conditions
During the natural spawning season, 24 sterlet males (3–4 years old, 0.6–1.0 kg weight) were transferred from aquaculture ponds (water temperature 8–10 °C) into a 0.8 m3 closed water recirculation system, located at the hatchery of South Bohemian Research Center of Aquaculture and Biodiversity of Hydrocenoses, Vodnany, Czech Republic. Water temperature was increased to 15 °C within 24 h, and fish were held 4 days without feeding before beginning the experiments.
Sperm collection and seminal fluid processing
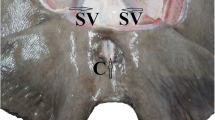
Sperm was collected from the Wolffian ducts (this sperm is used for artificial sturgeon propagation) and from testes. Wolffian duct sperm was collected at the urogenital sinus by aspiration using a 4-mm plastic catheter connected to a 10-ml syringe. Spermiation was stimulated by intramuscular injection of carp pituitary powder dissolved in 0.9 % (w/v) NaCl solution at 4 mg kg−1 of body weight. Fish were randomly separated into four groups. In these groups, Wolffian duct sperm was collected only once at different time after stimulation of spermiation: 12, 24, 36, or 60 h. Fish from which sperm was collected 24 h post-hormone injection (HI) were euthanized by a blow to the head and exsanguination to collect testicular sperm by incision of efferent ducts (Fig. 1).
Structure of sterlet male urogenital tract. UGS urogenital sinus, T testis, K kidney, WD Wolffian ducts, ED efferent ducts. a Urogenital sinus (black arrowhead) with sperm–urinary duct catheter inserted during urine or Wolffian duct sperm collection; oval area dissected to allow access to the testis–kidney junction to collect testicular sperm. b Overall view of body cavity after removal of skin and digestive tract; Wolffian ducts are shown by arrowheads; oval the area of the testis–kidney junction. c View of the testis–kidney junction. d Simplified schematic view of sturgeon urogenital system according to Hoar (1969). Testicular sperm was collected from incision of efferent ducts
The sperm samples were centrifuged at 300×g at 4 °C for 10 min, and the supernatants were collected and centrifuged a second time for 15 min at 5,000×g. Supernatants obtained after the second centrifugation were referred to as SF and were frozen at −80 °C until use.
Motility analysis
For motility activation, 10 mM Tris–HCl buffer, pH 8.0, containing 0.25 % pluronic acid was used as AM. For triggering motility, Wolffian duct sperm was diluted in AM at 1:100, and testicular sperm was added to 50 µl AM with a tip of a dissecting needle (dilution approximately 1:1,000). Sperm suspensions were carefully mixed for 2 s. Motility was recorded for 1–2 min post-activation using video microscopy combined with stroboscopic illumination (ExposureScope®, Czech Republic). Video records were analyzed to estimate spermatozoa curvilinear velocity (VCL) and percent of motile cells (motility rate) by microimage analyzer (Olympus Micro Image 4.0.1. for Windows, Japan).
Spermatozoa concentration determination
Spermatozoa concentration was determined using a Burker cell hemocytometer (Meopta, Czech Republic) and Olympus BX 50 phase contrast microscope (200× magnification; Olympus, Japan).
Spermatozoa membrane integrity assessment
Spermatozoa membrane integrity was determined using the Live/Dead Sperm Viability kit (L-7011; Molecular Probes) by the method of Flajshans et al. (2004). Briefly, the sperm samples from all groups were diluted with 150 mM NaCl into a suspension containing 5 × 106 cells/ml. Subsequently, SYBR 14 dye and propidium iodide were added to 1 ml of sperm suspension to a final concentration of 100 nM and 12 μM, respectively. After 10-min incubation in dark, results were observed by fluorescence microscopy with an Olympus BX60 equipped with Olympus MWB filter cube (wide band, blue excitation 450–480 nm, emission above 515 nm), and images were recorded using 3CCD Sony DXC-9100P color camera and analyzed by microimage analyzer (Olympus Micro Image 4.0.1. for Windows, Japan). Sixty to 100 images per sperm sample were analyzed. Percent of live (green fluorescence) spermatozoa was used as a variable of spermatozoa membrane integrity.
Evaluation of thiobarbituric acid-reactive substance content in seminal fluid
The TBARS content was measured spectrophotometrically according to Asakawa and Matsushita (1980). Briefly, to 0.08–0.25 ml SF, 0.025 ml butylated hydroxytoluene solution (22 mg in 10 ml ethanol), 0.025 ml ferric chloride solution (27 mg of FeCl3·6H2O in 10 ml water), 0.375 ml of 0.2 M glycine–hydrochloric acid buffer, pH 3.6, and 0.375 ml TBA reagent (0.5 % TBA and 0.3 % sodium dodecyl sulfate) were added. The tubes were capped and heated for 15 min in a boiling water bath. After cooling, 0.25 ml glacial acetic acid and 0.5 ml chloroform were added. The mixture was vigorously shaken and centrifuged for 10 min at 1,500×g. The absorbance of samples was determined at 535 nm against a blank with deionized water substituted for the biological sample. A molar extinction coefficient of 1.56 × 105 M−1 cm−1 was used for calculation of TBARS content. The concentration of TBARS was expressed as nmol ml−1 SF.
Evaluation of enzymatic antioxidant system variables in seminal fluid
Superoxide dismutase (EC 1.15.1.1) activity was measured spectrophotometrically at 420 nm according to the method of Marklund and Marklund (1974). The inhibition of pyrogallol autoxidation by SOD-containing sample was used for the determination of the enzyme activity. The autoxidation of 0.2 mM pyrogallol in air-equilibrated 50 mM Tris–HCl buffer, pH 8.2, containing 1 mM EDTA, was inhibited by the addition of the assayed sample. One unit of the enzyme is generally defined as the amount of enzyme that inhibits the reaction (in this case, pyrogallol autoxidation) by 50 %. The results were expressed in units per ml of SF.
Catalase (EC 1.11.1.6) activity was measured spectrophotometrically at 240 nm. Reaction medium contained 10 mM К+-phosphate buffer with 0.1 mM EDTA, pH 7.4, and 15 mM H2O2 according to the method of Marklund et al. (1981). Catalase activity was calculated from H2O2 decomposition rate, using molar extinction coefficient 39.4 M−1 cm−1, and expressed in µmol min−1 ml−1 of SF.
Evaluation of uric acid content in seminal fluid
The uric acid content was used as an index of the non-enzymatic antioxidant system of SF. UrAc content was determined by the uricase method (Duncan et al. 1982) using absorption spectrophotometry and expressed as µmol per l of SF.
Data presentation and statistical analysis
The values of spermatozoa velocity were checked for distribution characteristics and homogeneity of dispersion using Shapiro–Wilk’s and Levene’s tests, respectively. As they were normally distributed with similar dispersion values, parametric one-way ANOVAs were applied and Tukey’s honest significant difference test (HSD test) was used for contrasting the differences between groups. Because of the small number of observations (n = 6), nonparametric statistics using the Kruskal–Wallis test followed by the Mann–Whitney U test with Bonferroni correction were performed for comparison of percent of motile spermatozoa, CAT and SOD activity, uric acid and TBARS content, and membrane integrity.
Results were presented as mean ± standard deviation, and statistical significance was considered at P < 0.05. All analyses and plots were conducted using STATISTICA V 9.1 computer program (Statsoft Inc., USA).
Results
Spermatozoa motility parameters
Table 1 represents motility parameters of sterlet testicular spermatozoa and Wolffian duct spermatozoa. Significant differences were observed in testicular spermatozoa and Wolffian duct spermatozoa responses to AM treatment. AM did not initiate testicular spermatozoa motility, but did activate Wolffian duct spermatozoa. Wolffian duct sperm showed low motility at 12 h post-HI and a significant increase at 24 and 36 h post-HI. Wolffian duct spermatozoa at 60 h showed a decrease in motility rate. The same trend was shown for spermatozoa VCL (Table 1). Average VCL at 12 and 60 h post-HI was characterized by low values, while high, typical for sterlet (Dzyuba et al. 2012), levels were observed at 24 and 36 h post-HI.
TBA-reactive substance content in seminal fluid
Seminal fluid from testicular sperm was characterized by more than threefold (7.6 vs. 2.1 nmol ml−1) content of these products compared with SF from Wolffian duct sperm collected 24 h post-HI (time at which sperm is commonly collected in aquaculture) (Fig. 2). A lower level of TBARS was found in SF from Wolffian duct sperm compared with SF from testicular sperm at all sperm collections. Content of TBARS in SF from Wolffian duct sperm was the lowest at 12 h post-HI and was significantly increased at 36 h post-HI. Sperm collected at 60 h post-HI did not show a rise in TBARS content.
Enzymatic antioxidant activity and uric acid concentration in seminal fluid
The activities of SOD and CAT were 5–10 and 42–50 times, respectively, in SF from testicular sperm that of SF from Wolffian duct sperm at all collection times (Fig. 3a, b). The activity of the analyzed antioxidant enzymes in SF from Wolffian duct sperm was similar at all collection times.
In contrast to TBARS, SOD, and CAT, UrAc concentration in SF from testicular sperm was not different from the values in SF from Wolffian duct sperm (Fig. 4). There were no significant changes in this variable in SF from Wolffian duct sperm at any time post-HI.
Membrane integrity and spermatozoa concentration
Testicular spermatozoa and Wolffian duct spermatozoa membrane integrity at 12, 24, and 36 h post-HI were similar. Membrane integrity at 60 h post-HI was significantly reduced compared with values at 12–36 h post-HI (Fig. 5a).
Membrane integrity (a) and spermatozoa concentration (b) of sterlet sperm, collected at various times after hormone stimulation of spermiation. 24(T) SF from testicular sperm collected at 24 h after stimulation. Values with different letters are significantly different (P < 0.05, Mann–Whitney U test, n = 6)
The highest spermatozoa concentration was found in testicular sperm (Fig. 5b). Spermatozoa concentration was extremely low (0.016 ± 0.008 × 109 cell ml−1) in Wolffian duct sperm collected at 12 h post-HI. Sperm collected at 24 h post-HI was characterized by increased concentration (0.372 ± 0.160 × 109 cell ml−1). Wolffian duct sperm at 36 and 60 h post-HI showed an increase in spermatozoa concentration to 3.1 ± 1.5 × 109 and 3.2 ± 1.8 × 109 cell ml−1, respectively. No significant difference between spermatozoa concentration at 36 and 60 h post-HI was observed.
Discussion
Results of the present study confirmed our previous observation of differences in motility of spermatozoa collected from testes and from Wolffian ducts (Dzyuba et al. 2014) and determined that motility parameters of Wolffian duct spermatozoa are altered as a function of time after induction of spermiation. Wolffian duct spermatozoa collected 60 h post-HI were characterized by a significant decrease in velocity and motility (Table 1).
Oxidative stress is among the reasons for spermatozoa function deterioration in various animal species, including mammals and fish (Sikka et al. 1995; Agarwal and Saleh 2002; Aitken et al. 2003; Kasimanickam et al. 2007; Turner and Lysiak 2008; Shiva et al. 2011; Hagedorn et al. 2012), and a function of SF is the protection of spermatozoa from oxidative damage (Potts et al. 2000; Baumber and Ball 2005; Tavilani et al. 2008; Lahnsteiner et al. 2010), as effectiveness of spermatozoa antioxidant system is insufficient to cope with oxidative stress.
Seminal fluid from testicular sperm was characterized by a higher level of TBARS than that from Wolffian duct sperm at all collection times (Fig. 2). As we did not study the changes in TBARS content in SF from testicular sperm at different time post-HI, correct comparison can be done only for two groups of results: (1) SF from testicular sperm and Wolffian duct sperm, collected at 24 h post-HI, and (2) SF from Wolffian duct sperm, collected at different time post-HI. Content of TBARS in SF from Wolffian duct sperm changed with the time post-HI, showing a significant increase at 36 h. Intensification of lipid peroxidation at 36 h post-HI was not accompanied by deterioration of spermatozoa motility parameters. At 60 h post-HI, the level of TBARS was unchanged, with a significant decrease in spermatozoa VCL and motility rate (Table 1).
We observed that an increased amount of TBARS in SF from testicular sperm was followed by a rise in SOD and CAT activity (Fig. 3). The high level of lipid peroxidation products in SF from testicular sperm, which is immature in sturgeon, may be an indication of excessive production of reactive oxygen species (ROS). In mammals, enhancement of superoxide production by the addition of exogenous NADPH has been shown to correlate with epididymal development and peaks in immature sperm (Fisher and Aitken 1997). The involvement of ROS in mammalian sperm maturation was reviewed by Ford (2004) and Aitken et al. (2012). As fish possess cystic type of spermatogenesis, during spermiation, spermatozoa are released in the lumen of testis as a result of the breakdown of the Sertoli cell layer (Vizziano et al. 2008). It could be supposed that exactly this breakdown leads to activation of lipid peroxidation and at the same time to increase in activity of enzymatic antioxidant system. It looks like that the conditions of increased oxidative processes physiologically could be involved in sturgeon sperm maturation in a way similar to one occurring during mammalian sperm epididymal transit (Ford 2004; Aitken et al. 2012). So, ROS seem to be a factor involved in the spermatozoa maturation process. At the same time, spermatozoa are extremely sensitive to the damaging effects of these highly reactive species. In order to prevent the development of oxidative stress, spermatozoa are equipped with non-enzymatic and enzymatic antioxidants (Rhemrev et al. 2000; Chabory et al. 2010). Because of the limited cytoplasmic volume and, therefore, the low amount of endogenous antioxidant, sperm cells rely chiefly on the antioxidant capacity of SF.
Seminal fluid from testicular sperm was characterized by an extremely high level of SOD and CAT activity compared with SF from Wolffian duct sperm (Fig. 3). Uric acid of sterlet SF exhibited stable concentration in all investigated groups (Fig. 4). It is quite understandable that the testicular SF is more concentrated in the different components as it is not diluted yet by the mixing with some urine (the later explaining the homogenous uric acid concentration between samples). Uric acid concentration determined in the present study for sterlet SF was significantly lower than that has been reported for other fish species (Ciereszko et al. 1999; Lahnsteiner et al. 2010). The high activity of the enzymatic antioxidant system allowed testicular spermatozoa to cope with the deleterious effects of excessive ROS production and to retain the ability to become motile after passing through the kidneys and Wolffian ducts.
The increase observed in TBARS content with time following spermiation was not accompanied by a corresponding increase in activity of the studied antioxidant enzymes. We suggest that extended time in the Wolffian ducts resulted in spermatozoa oxidative stress and, finally, in decrease in motility parameters. The assessment of spermatozoa membrane integrity may be an additional confirmation of this. Membrane integrity was stable to 36 h (Fig. 5a), a time characterized by a significant increase in TBARS content. As this increase was not compensated for by a rise in antioxidant enzyme activity, the spermatozoa membrane lost its integrity, supposedly due to lipid peroxidation. Combined results with respect to sperm motility parameters, spermatozoa concentration, and membrane integrity (Table 1; Fig. 5a, b) lead to the speculation that low-motility parameters observed at different time post-HI may have different sources. In testis and a short time post-HI, low motility may be due to immature spermatozoa. The cell membrane is intact, and a certain amount of time in the Wolffian duct is probably required for completion of maturation. Shortly after HI, SF and/or urine is actively produced, keeping spermatozoa concentration low. With time, spermatozoa became damaged due to oxidative stress, and this process is accompanied by decrease in SF production. As a result, at completion of the spermiation period, spermatozoa at high concentrations with compromised membrane integrity are present in the Wolffian duct.
The present study shows that sturgeon spermatozoa maturation and time in Wolffian ducts are accompanied by significant alterations in motility parameters and in SF pro-oxidant–antioxidant balance. High content of TBARS and activity of antioxidant enzymes in SF from testicular sperm may indicate a regulatory role of ROS in sturgeon sperm maturation process. Extended time in the Wolffian duct was associated with oxidative damage resulting from inadequate enzymatic antioxidant system efficacy in scavenging ROS. The obtained results may confirm a dual role of ROS in fish sperm physiology. The data concerning decrease in sturgeon sperm motility parameters 60 h after induction of spermiation should be taken into account in artificial sturgeon propagation.
References
Agarwal A, Saleh RA (2002) Role of oxidants in male infertility: rationale, significance, and treatment. Urol Clin N Am 29:817–827
Aitken RJ, Baker MA, Sawyer D (2003) Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online 7:65–70
Aitken RJ, Jones KT, Robertson SA (2012) Reactive oxygen species and sperm function—in sickness and in health. J Androl 33:1096–1106
Asakawa T, Matsushita S (1980) Coloring conditions of thiobarbituric acid test for detecting lipid hydroperoxides. Lipids 15:137–140
Baumber J, Ball BA (2005) Determination of glutathione peroxidase and superoxide dismutase-like activities in equine spermatozoa, seminal plasma, and reproductive tissues. Am J Vet Res 66:1415–1419
Chabory E, Damon C, Lenoir A, Henry-Berger J, Vernet P, Cadet R, Saez F, Drevet JR (2010) Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. J Anim Sci 88:1321–1331
Ciereszko A, Dabrowski K, Kucharczyk D, Dobosz S, Goryczko K, Glogowski J (1999) The presence of uric acid, an antioxidantive substance, in fish seminal plasma. Fish Physiol Biochem 21:313–315
de Lamirande E, Leclerc P, Gagnon C (1997) Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod 3:175–194
Duncan PH, Gochman N, Cooper T, Smith E, Bayse D (1982) A candidate reference method for uric acid in serum. I. Optimization and evaluation. Clin Chem 28:284–290
Dzyuba B, Boryshpolets S, Shaliutina A, Rodina M, Yamaner G, Gela D, Dzyuba V, Linhart O (2012) Spermatozoa motility, cryoresistance, and fertilizing ability in sterlet Acipenser ruthenus during sequential stripping. Aquaculture 356–357:272–278
Dzyuba B, Cosson J, Boryshpolets S, Bondarenko O, Dzyuba V, Prokopchuk G, Gazo I, Rodina M, Linhart O (2014) In vitro sperm maturation in sterlet, Acipencer ruthenus. Reprod Biol 14:160–163
Fisher HM, Aitken RJ (1997) Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool 277:390–400
Flajshans M, Cosson J, Rodina M, Linhart O (2004) The application of image cytometry to viability assessment in dual fluorescence-stained fish spermatozoa. Cell Biol Int 28:955–959
Ford WCL (2004) Regulation of sperm function by reactive oxygen species. Hum Reprod Update 10:387–399
Hagedorn M, McCarthy M, Carter VL, Meyers SA (2012) Oxidative stress in zebrafish (Danio rerio) sperm. PLoS One 7:e39397
Hoar WS (1969) Reproduction. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 3, 1st edn. Academic Press, New York and London, pp 1–72
Kasimanickam R, Kasimanickam V, Thatcher CD, Nebel RL, Cassell BG (2007) Relationships among lipid peroxidation, glutathione peroxidase, superoxide dismutase, sperm parameters, and competitive index in dairy bulls. Theriogenology 67:1004–1012
Lahnsteiner F, Mansour N (2010) A comparative study on antioxidant systems in semen of species of the Percidae, Salmonidae, Cyprinidae, and Lotidae for improving semen storage techniques. Aquaculture 307:130–140
Lahnsteiner F, Mansour N, Plaetzer K (2010) Antioxidant systems of brown trout (Salmo trutta f. fario) semen. Anim Reprod Sci 119:314–321
Marengo SR (2008) Maturing the sperm: unique mechanisms for modifying integral proteins in the sperm plasma membrane. Anim Reprod Sci 105:52–63
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Marklund S, Nordensson I, Back O (1981) Normal CuZn superoxide dismutase, Mn superoxide dismutase, catalase and glutathione peroxidase in Werner’s syndrome. J Gerontol 36:405–409
Miura T, Miura C (2001) Japanese eel: a model for analysis of spermatogenesis. Zool Sci 18:1055–1563
Miura T, Kasugai T, Nagahama Y, Yamauchi K (1995) Acquisition of potential for sperm motility in vitro in Japanese eel Anguilla japonica. Fish Sci 61:533–534
Morisawa S, Morisawa M (1986) Acquisition of potential for sperm motility in rainbow trout and chum salmon. J Exp Biol 126:89–96
Morisawa S, Morisawa M (1988) Induction of potential for sperm motility by bicarbonate and pH in rainbow trout and chum salmon. J Exp Biol 136:13–22
Ohta H, Ikeda K, Izawa T (1997) Increases in concentrations of potassium and bicarbonate ions promote acquisition of motility in vitro by Japanese eel spermatozoa. J Exp Zool 277:171–180
Potts RJ, Notarianni LJ, Jefferies TM (2000) Seminal plasma reduces exogenous oxidative damage to human sperm, determined by the measurement of DNA strand breaks and lipid peroxidation. Mutat Res 447:249–256
Rhemrev JP, van Overveld FW, Haenen GR, Teerlink T, Bast A, Vermeiden JP (2000) Quantification of the nonenzymatic fast and slow TRAP in a postaddition assay in human seminal plasma and the antioxidant contributions of various seminal compounds. J Androl 21:913–920
Schulz RW, de Franca LR, Lareyre JJ, LeGac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Shaliutina A, Hulak M, Gazo I, Linhartova P, Linhart O (2013) Effect of short-term storage on quality parameters, DNA integrity, and oxidative stress in Russian (Acipenser gueldenstaedtii) and Siberian (Acipenser baerii) sturgeon sperm. Anim Reprod Sci 139:127–135
Shiva M, Gautam AK, Verma Y, Shivgotra V, Doshi H, Kumar S (2011) Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin Biochem 44:319–324
Sikka SC, Rajasekaran M, Hellstrom WJG (1995) Role of oxidative stress and antioxidants in male infertility. J Androl 16:464–468
Sostaric E, Aalberts M, Gadella BM, Stout TAE (2008) The roles of the epididymis and prostasomes in the attainment of fertilizing capacity by stallion sperm. Anim Reprod Sci 107:237–248
Tavilani H, Goodarzi MT, Vaisi-raygani A, Salimi S, Hassanzadeh T (2008) Activity of antioxidant enzymes in seminal plasma and their relationship with lipid peroxidation of spermatozoa. Int Braz J Urol 34:485–491
Turner TT, Lysiak JJ (2008) Oxidative stress: a common factor in testicular dysfunction. J Androl 29:488–498
Vizziano D, Fostier A, Loir M, Le Gac F (2008) Testis development, its hormonal regulation and spermiation induction in teleost fish. In: Alavi SMH, Cosson JJ, Coward K, Rafiee G (eds) Fish spermatology, 1st edn. Alpha Science International Ltd, Oxford, pp 103–139
Acknowledgments
The authors acknowledge financial support from projects: CENAKVA CZ.1.05/2.1.00/01.0024, Strengthening of excellence scientific teams in USB FFPW CZ.1.07/2.3.00/20.0024, GAJU 114/2013/Z, GACR P502/11/0090, and 502/12/1973. The results of the project LO1205 were obtained with financial support from the MEYS of the CR under the NPU I program. The Lucidus Consultancy, UK, is gratefully acknowledged for the English correction and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dzyuba, V., Dzyuba, B., Cosson, J. et al. The antioxidant system of sterlet seminal fluid in testes and Wolffian ducts. Fish Physiol Biochem 40, 1731–1739 (2014). https://doi.org/10.1007/s10695-014-9963-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9963-2