Abstract
The role of the seminal fluid antioxidant system in protection against damage to spermatozoa during in vitro sperm storage is unclear. This study investigated the effect of in vitro storage of sterlet Acipenser ruthenus spermatozoa together with seminal fluid for 36 h at 4 °C on spermatozoon motility rate and curvilinear velocity, thiobarbituric acid reactive substance level, and components of enzyme and non-enzyme antioxidant system (superoxide dismutase and catalase activity and uric acid concentration) in seminal fluid. Spermatozoon motility parameters after sperm storage were significantly decreased, while the level of thiobarbituric acid reactive substances, activity of superoxide dismutase and catalase, and uric acid concentration did not change. Our findings suggest that the antioxidant system of sterlet seminal fluid is effective in preventing oxidative stress during short-term sperm storage and prompt future investigations of changes in spermatozoon homeostasis and in spermatozoon plasma membrane structure which are other possible reasons of spermatozoon motility deterioration upon sperm storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vitro storage of sperm is an essential tool in gamete manipulation in artificial fish reproduction. The role of the sperm antioxidant system (AOS), as a network of enzyme and non-enzyme processes of cell protection against damage during in vitro storage is not fully understood. Alterations in sperm physiology, DNA integrity, and oxidative stress indices of Russian and Siberian sturgeon sperm during short-term in vitro storage were studied by Shaliutina et al. (2013). They reported that the percentage of motile spermatozoa declined only after 3 days of storage in both species, while a significant decrease in spermatozoon velocity was observed after 2 days of storage. Shaliutina et al. (2013) found the level of thiobarbituric acid (TBA) reactive substances (TBARS) to increase significantly after 6-day storage of Russian sturgeon Acipenser gueldenstaedtii sperm and after storage for 3 days in sperm of Siberian sturgeon Acipenser baerii, with the increase in products of protein oxidative damage preceding TBARS increase. According to these authors, the increase in superoxide dismutase (SOD) activity observed after 2 days of in vitro storage was insufficient to prevent cellular damage associated with oxidative stress. In contrast, no alterations in the level of non-enzyme antioxidants and activity of antioxidant enzymes were found in spermatozoa and seminal fluid of brown trout after 48-h storage (Lahnsteiner et al. 2010b). These data on sperm AOS response to storage are not sufficient to draw general conclusions and encourage further studies. As spermatozoa are characterized by low cytoplasmic volume and, therefore, low levels of endogenous antioxidants, they rely chiefly on the antioxidant capacity of seminal fluid for protection from oxidative damage (Baumber and Ball 2005; Ciereszko 2008; Lahnsteiner et al. 2010b; Potts et al. 2000; Tavilani et al. 2008). In contrast to amount of data on other fish species sperm (spermatozoa and seminal fluid) AOS and AOS of sturgeon spermatozoa, the data on sturgeon seminal fluid AOS are scarce. The goal of this study was to collect more data on the AOS of seminal fluid in sturgeons. Spermatozoa of sterlet Acipenser ruthenus were stored together with seminal fluid for 36 h at 4 °C, and the changes in spermatozoa curvilinear velocity and motility rate and seminal fluid antioxidant (superoxide dismutase, catalase, uric acid) and thiobarbituric acid reactive substance levels in comparison with freshly collected semen were investigated.
Materials and methods
Ethics
All experiments were conducted according to the principles of the Ethics Committee for the Protection of Animals in Research of the University of South Bohemia in Ceske Budejovice, Research Institute of Fish Culture and Hydrobiology, Vodnany, based on the EU-harmonized Animal Welfare Act of the Czech Republic.
Fish rearing conditions and sperm sampling
Five mature sterlet Acipenser ruthenus males (3–5 years old, weight 0.7–1.2 kg) were obtained during the natural spawning season. Fish were kept outdoors at water temperature 8–10 °C in plastic tanks located at the hatchery of the Research Institute of Fish Culture and Hydrobiology. Before hormone injection, fish were transferred to a closed recirculation system, and water temperature was increased to 15 °C within 24 h. Fish did not receive food for 4 days before beginning experimentation.
For initiation of spermiation, fish were injected intramuscularly with carp pituitary extract (4 mg kg−1). Thirty-six hours after hormone injection, sperm was collected from urogenital ducts by aspiration using a 4-mm plastic catheter connected to a 20-mL syringe. Each sperm sample was divided into two aliquots, one of which was analyzed immediately and the other was stored under aerobic conditions at 4 °C for 36 h until analysis. For this, 5 mL of sperm in 50 mL hermetically sealed plastic tubes was put in fridge. Sperm concentration of studied samples was in range 0.12–0.35 × 109 cells mL−1. A portion of the samples before and after storage was centrifuged at 10,000×g for 10 min at 4 °C to obtain seminal fluid (SF).
Spermatozoon motility analysis
Sperm was diluted 1:50 with water from tanks (15 °C, pH 7.8, dissolved oxygen 8 mg L−1) in which fish were kept, and spermatozoon motility was immediately recorded until cessation, using video microscopy combined with stroboscopic illumination (ExposureScope®, Czech Republic). Video records were analyzed to estimate spermatozoon curvilinear velocity (VCL) and percent of motile cells (motility rate) by microimage analyzer (Olympus Micro Image 4.0.1. for Windows, Japan).
Evaluation of thiobarbituric acid reactive substance content in seminal fluid
The content of TBARS in seminal fluid was measured spectrophotometrically according to Asakawa and Matsushita (1980). This test involves the reaction of secondary products of lipid peroxidation with TBA at 100 °C under acidic conditions to produce a pink-colored chromogen. The absorbance of colored samples was measured at 535 nm against a blank with deionized water replacing the SF. A molar extinction coefficient of 1.56 × 105 M−1 cm−1 was used for calculation of TBARS content, expressed as nmol mL−1 SF.
Evaluation of superoxide dismutase activity in seminal fluid
Superoxide dismutase (EC 1.15.1.1) activity was measured spectrophotometrically at 420 nm according to Marklund and Marklund (1974). The test involves inhibition of pyrogallol (0.2 mM) autoxidation in air-equilibrated 50 mM Tris–HCl buffer, pH 8.2, containing 1 mM EDTA, by SOD present in the assayed seminal fluid. One unit (U) of SOD was defined as the amount of the enzyme that inhibited the reaction by 50 %. Results were expressed as U mL−1 of SF.
Evaluation of catalase activity in seminal fluid
Catalase (EC 1.11.1.6) activity was measured spectrophotometrically at 240 nm according to Marklund et al. (1981). Reaction medium contained 10 мM potassium phosphate buffer, pH 7.4, 0.1 mM EDTA, and 15 мM H2O2. Catalase activity was calculated from H2O2 decomposition rate with the use of molar extinction coefficient 39.4 M−1 cm−1 and expressed as µmol min−1 mL−1 of SF.
Evaluation of uric acid content of seminal fluid
The UrAc content was evaluated as an index of the non-enzyme antioxidant system of seminal fluid. The UrAc content was determined by the uricase method (Duncan et al. 1982) using absorption spectrophotometry and expressed as µmol L−1 of SF.
Statistical analysis
A nonparametric Mann–Whitney U test was used for comparison of studied parameters, because of the low number of observations (n = 5). Results were presented as mean ± SE. Statistical significance was accepted at p < 0.05. All analyses were conducted using Statistica v. 9.1 (StatSoft Inc, Tulsa, OK, USA).
Results and discussion
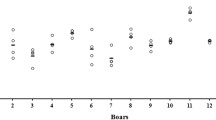
Immediately after collection, spermatozoa were characterized by the high motility and VCL values typical of sterlet (Dzyuba et al. 2012, 2014). After in vitro storage for 36 h, both parameters were significantly decreased (Fig. 1). The decrease in spermatozoon motility parameters during in vitro storage has been detected for diverse animal species including mammals, birds, and fishes (Aramli et al. 2013, 2014; DeGraaf and Berlinsky 2004; Douard et al. 2005; Guthrie et al. 2011; Kadirvel et al. 2009; Shaliutina et al. 2013; Zaniboni and Cerolini 2009). It should be stressed that in other sturgeon species the decline in motility parameters was shown to happen later compared to our study. For example, VCL of Russian and Siberian sturgeon spermatozoa was significantly decreased after 2 days of storage, while percent of motile spermatozoa essentially dropped after 3 days of storage for Persian sturgeon and after 6 days of storage for Russian and Siberian sturgeon (Aramli et al. 2013; Shaliutina et al. 2013).
Oxidative stress development during in vitro storage is considered to be a major source of spermatozoon motility deterioration (Aramli et al. 2013, 2014; Shaliutina et al. 2013; Zaniboni and Cerolini 2009). Accumulation of lipid, protein, and DNA oxidative damage products has been reported to occur in sturgeon concurrently with reduction in motility parameters (Aramli et al. 2013, 2014; Shaliutina et al. 2013). Notwithstanding the observed increase in SOD activity in these studies, antioxidant enzyme activity in spermatozoa has been suggested to be insufficient to prevent cellular damage (Shaliutina et al. 2013). Considering these data and the fact that antioxidant capacity of seminal fluid is essential for the protection of spermatozoa from oxidative stress (Ciereszko 2008; Lahnsteiner et al. 2010b), we assessed the possibility of oxidative stress development in SF of stored sterlet sperm and its impact on spermatozoon motility parameters.
In the present study, TBARS content in seminal fluid of sterlet sperm before and after 36 h of in vitro storage did not show significant changes (Fig. 2). Short-term storage at 4 °C was also not associated with changes in CAT and SOD activity (Fig. 3) or UrAc concentration (Fig. 4) in seminal fluid of A. ruthenus.
The concentration of UrAc in sterlet seminal fluid was found to be significantly lower compared to the values which were reported previously for seminal fluid of Salmonidae, Cyprinidae, Ecosidae, Percidae and Lotidae (Ciereszko et al. 1999; Lahnsteiner and Mansour 2010). At the same time, enzymatic antioxidant system of sturgeon seminal fluid seems to be more forceful, as the levels of SOD and CAT activity determined in our study were significantly higher than the levels shown for other fish species (Lahnsteiner and Mansour 2010).
No change in SOD and CAT activity or UrAc concentration was reported in brown trout seminal fluid after 48 h of sperm storage at 4 °C (Lahnsteiner et al. 2010b). Lahnsteiner et al. (2010b) demonstrated also that intensity of lipid peroxidation stimulated by ferrous sulfate and ascorbic acid was lower in brown trout spermatozoa incubated for 72 h in seminal fluid at 4 °C than in spermatozoa incubated in sperm motility-inhibiting saline solution. It confirms antioxidant functions of seminal fluid.
As we did not find significant alterations in levels of lipid peroxidation products and the studied enzyme and non-enzyme components of AOS as a result of the short-term in vitro storage of sterlet sperm at 4 °C, we hypothesize that AOS of sterlet sperm is effective in preventing the development of oxidative stress over the short-term storage. Thus, the observed significant decrease in spermatozoon motility parameters after 36 h of in vitro storage could be attributed to sources other than oxidative stress.
There are several possible reasons of spermatozoa quality deterioration after sperm storage. Spermatozoon motility could decline as a result of storage-induced disturbance of spermatozoon calcium homeostasis, which may be detrimental to sperm activation (Guthrie et al. 2011). Reduced sperm quality during liquid storage can also be associated with changes in spermatozoon plasma membrane lipid organization and functions (Douard et al. 2005; Lahnsteiner et al. 2010a; Zaniboni and Cerolini 2009). The question arises whether above-mentioned processes are involved in sterlet spermatozoon motility decrease during short-term sperm storage, and it prompts future investigations of changes in calcium homeostasis and in spermatozoon plasma membrane structure upon sperm storage in presence of seminal fluid.
References
Aramli MS (2014) ATP content, oxidative stress and motility of beluga (Huso huso) semen: effect of short-term storage. Reprod Domest Anim 49:636–640
Aramli MS, Kalbassi MR, Nazari RM, Aramli S (2013) Effects of short-term storage on the motility, oxidative stress, and ATP content of Persian sturgeon (Acipenser persicus) sperm. Anim Reprod Sci 143:112–117
Asakawa T, Matsushita S (1980) Coloring conditions of thiobarbituric acid test for detecting lipid hydroperoxides. Lipids 15:137–140
Baumber J, Ball BA (2005) Determination of glutathione peroxidase and superoxide dismutase-like activities in equine spermatozoa, seminal plasma, and reproductive tissues. Am J Vet Res 66:1415–1419
Ciereszko A (2008) Chemical composition of seminal plasma and its physiological relationship with sperm motility, fertilizing capacity and cryopreservation success in fish. In: Alavi SMH, Cosson JJ, Coward K, Rafiee G (eds) Fish spermatology. Alpha Science International Ltd., Oxford, pp 215–240
Ciereszko A, Dabrowski K, Kucharczyk D, Dobosz S, Goryczko K, Glogowski J (1999) The presence of uric acid, an antioxidantive substance, in fish seminal plasma. Fish Physiol Biochem 21:313–315
DeGraaf JD, Berlinsky DL (2004) Cryogenic and refrigerated storage of Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) spermatozoa. Aquaculture 234:527–540
Douard V, Hermier D, Labbe C, Magistrini M, Blesbois E (2005) Role of seminal plasma in damage to turkey spermatozoa during in vitro storage. Theriogenology 63:126–137
Duncan PH, Gochman N, Cooper T, Smith E, Bayse D (1982) A candidate reference method for uric acid in serum. I. Optimization and evaluation. Clin Chem 28:284–290
Dzyuba B, Boryshpolets S, Shaliutina A, Rodina M, Yamaner G, Gela D, Dzyuba V, Linhart O (2012) Spermatozoa motility, cryoresistance, and fertilizing ability in sterlet Acipenser ruthenus during sequential stripping. Aquaculture 356–357:272–278
Dzyuba V, Dzyuba B, Cosson J, Boryshpolets S, Yamaner G, Kholodniy V, Rodina M (2014) The antioxidant system of sterlet seminal fluid in testes and Wolffian ducts. Fish Physiol Biochem 40:1731–1739
Guthrie HD, Welch GR, Theisen DD, Woods LC III (2011) Effects of hypothermic storage on intracellular calcium, reactive oxygen species formation, mitochondrial function, motility, and plasma membrane integrity in striped bass (Morone saxatilis) sperm. Theriogenology 75:951–961
Kadirvel G, Satish Kumar, Kumaresan A (2009) Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Anim Reprod Sci 114:125–134
Lahnsteiner F, Mansour N (2010) A comparative study on antioxidant systems in semen of species of the Percidae, Salmonidae, Cyprinidae, and Lotidae for improving semen storage techniques. Aquaculture 307:130–140
Lahnsteiner F, Mansour N, Caberlotto S (2010a) Composition and metabolism of carbohydrates and lipids in Sparus aurata semen and its relation to viability expressed as sperm motility when activated. Comp Biochem Physiol B 157:39–45
Lahnsteiner F, Mansour N, Plaetzer K (2010b) Antioxidant systems of brown trout (Salmo trutta f. fario) semen. Anim Reprod Sci 119:314–321
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Marklund S, Nordensson I, Back O (1981) Normal CuZn superoxide dismutase, Mn superoxide dismutase, catalase and glutathione peroxidase in Werner’s syndrome. J Gerontol 36:405–409
Potts RJ, Notarianni LJ, Jefferies TM (2000) Seminal plasma reduces exogenous oxidative damage to human sperm, determined by the measurement of DNA strand breaks and lipid peroxidation. Mutat Res 447:249–256
Shaliutina A, Hulak M, Gazo I, Linhartova P, Linhart O (2013) Effect of short-term storage on quality parameters, DNA integrity, and oxidative stress in Russian (Acipenser gueldenstaedtii) and Siberian (Acipenser baerii) sturgeon sperm. Anim Reprod Sci 139:127–135
Tavilani H, Goodarzi MT, Vaisi-raygani A, Salimi S, Hassanzadeh T (2008) Activity of antioxidant enzymes in seminal plasma and their relationship with lipid peroxidation of spermatozoa. Int Braz J Urol 34:485–491
Zaniboni L, Cerolini S (2009) Liquid storage of Turkey semen: changes in quality parameters, lipid composition and susceptibility to induced in vitro peroxidation in control, n-3 fatty acids and alpha-tocopherol rich spermatozoa. Anim Reprod Sci 112:51–65
Acknowledgments
The study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic—projects CENAKVA (No. CZ.1.05/2.1.00/01.0024), CENAKVA II (No. LO1205 under the NPU I program) and COST (No. LD14119), by the Grant Agency of the University of South Bohemia in Ceske Budejovice (No. 114/2013/Z), and by the Czech Science Foundation (No. P502/15-12034S). The Lucidus Consultancy, UK, is gratefully acknowledged for the English correction and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dzyuba, V., Cosson, J., Dzyuba, B. et al. The antioxidant system of seminal fluid during in vitro storage of sterlet Acipenser ruthenus sperm. Fish Physiol Biochem 42, 563–568 (2016). https://doi.org/10.1007/s10695-015-0159-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0159-1