Abstract
This study examined the effects of acclimation temperature (10, 15, 20, or 25 °C) and an acute exposure to various temperatures on the routine metabolism of juvenile (~11 g) shortnose sturgeon (Acipenser brevirostrum). For the acclimation experiment, the minimum, mean, and maximum routine metabolic rates were established for sturgeon at each temperature. Mean routine metabolic rates for 10, 15, 20, and 25 °C were 134, 277, 313, and 309 mg O2 kg−1 h−1, respectively, with significant differences occurring between 10 and 15, 10 and 20, and 10 and 25 °C. For the acute exposure, similar patterns and significant differences were observed. Temperature quotient (Q 10) values indicate that the greatest effect of temperature occurred between 10 and 15 °C for both the acclimation and acute temperature experiments. In addition, the effect of temperature on the metabolic rate of sturgeon was nearly negligible between 15 and 25 °C. These results suggest that juvenile shortnose sturgeon are sensitive to temperature changes at the lower end of the range, and less sensitive in the mid-to-upper temperature range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature has been coined as the “abiotic master factor” (Brett 1971) by researchers (Beitinger and Lutterschmidt 2011). Since most fish are ectotherms, temperature plays a strong role in determining their distribution and abundance. In addition, temperature plays a significant part in setting both upper and lower limits on a fish’s metabolism. As a result, any changes in water temperature should influence their behaviour, metabolic processes, migration, reproduction, growth, and ultimately, survival (e.g. Fry 1971). A well-documented relationship exists for fish where metabolic rates (oxygen consumption) increase with increases in temperature, typically in a linear or exponential fashion (e.g. Fry and Hart 1948; Brett and Glass 1973; Brett and Groves 1979; Carlson and Parsons 1999; Lee et al. 2003; Kieffer and Cooke 2009). It is also known that the effects of temperature on metabolism vary depending on whether the fish has been acclimated to a temperature, or is acutely exposed to a different temperature (Kieffer et al. 1998; Kieffer and Wakefield 2009). Due to seasonal and daily temperature patterns, both temperature scenarios are likely to occur in most river systems in North America. While there have been numerous studies on the effects of acclimation and acute temperature changes on oxygen consumption in various species of fish (see above references), less is known about the effects of temperature changes on oxygen consumption rates in “primitive” fish, such as sturgeon (Mayfield and Cech 2004).
The shortnose sturgeon is a medium-sized, heavy-bodied species (Scott and Crossman 1973). Distribution is confined to the coastal rivers of eastern North America and ranges from the Saint John River (Canada) to the St. Johns River (Florida) (Scott and Crossman 1973; Wilson and McKinley 2004). Shortnose sturgeon are considered freshwater amphidromous (Kynard 1997), and like nearly all species of sturgeon are listed as vulnerable or threatened in some part of their range (Birstein and Bemis 1997; Billard and Lecointre 2001). Many populations of shortnose sturgeon are severely depleted across their range, due to overfishing (Dadswell et al. 1984) or habitat alteration (e.g. temperature change, flow change, agriculture run-off). As a result of both natural (seasonal, daily) and anthropogenic impacts, sturgeon are likely exposed to various temperatures. The effects of temperature on the general metabolism of sturgeon species, however, are not well understood.
This study focuses on establishing a baseline of routine metabolism for juvenile shortnose sturgeon that are exposed to either acclimated temperature conditions (10, 15, 20, and 25 °C) or acute temperature conditions (acutely exposed from 10 to 15, 20, and 25 °C). The temperatures used in the present study reflect those that would normally be encountered by shortnose sturgeon living along the coast of North America across seasons. How temperature affects a fish’s physiology may directly indicate its tolerance to temperature (Beitinger and Lutterschmidt 2011). Examining the effects of both acclimated and acute temperature exposure is important: (1) both acclimated and acute temperature exposure can be encountered in the natural habitat of a fish, due to both ordinary seasonal temperature fluctuations and anthropogenic influences that can alter water flows/temperatures; (2) by exposing shortnose sturgeon to acclimated and acute temperature conditions, it will be possible to determine if one type of exposure is more temperature-dependent than the other by calculating temperature quotient (Q 10) values.
Methods
Animal husbandry
Juvenile shortnose sturgeon (~11 g) were obtained from Acadian Sturgeon and Caviar (Carter’s Point, Kingston, New Brunswick). The fish were raised from eggs originating from Saint John River (New Brunswick, Canada) shortnose sturgeon. Fish were held in large (1-m diameter; approx. 160 L), flow-through tanks at the University of New Brunswick Saint John campus and were continuously supplied with a flow-through of fresh, aerated, de-chlorinated, municipal city water at approximately 15 °C. Fish were fed once in the morning and again in the afternoon until satiation with ground trout food pellets (Corey Aquafeeds: 1.5 mm optimum, 52 % protein). Fish were not fed for 24 h prior to experimental trials.
Experimental protocols
Intermittent flow respirometry: experimental set-up
Rates of oxygen consumption were assessed using 1 L intermittent flow respirometry systems (Loligo Systems, Denmark). Each respirometry system (3 in total) was connected to a computer via an integration box whereby trials were monitored by a programmed computer (Loligo Systems, Denmark). The respirometry system consisted of a flush pump, re-circulation pump, oxygen electrode, and temperature probe. A single experimental fish was placed into each respirometer at its acclimation temperature, which was immersed in an ambient tank of water at the specific temperature being tested. The re-circulating pump of the respirometer continually runs to ensure that both proper mixing within the respirometer occurs, and adequate water flows past the oxygen electrode. The second pump, i.e. the flush pump, exchanges water from inside the respirometer with water from the ambient tank between the measurement periods. During the periods of oxygen consumption measurements, the flush pump was turned off while the re-circulation pump remained on, thus making the system operate as a closed respirometer. After a designated period of time, the computer-controlled flush pump turns on and flushes out the water from the respirometer, by replenishing it with ambient tank water (i.e. having oxygen levels that are ~100 % saturation). Using the automatic readings taken during each measuring phase, the program calculates the mass-specific oxygen rates (mg O2 kg−1 h−1).
Metabolic rate determination at various acclimation temperatures
Juvenile shortnose sturgeon (~11 g) were acclimated for >10 days to one of four experimental temperatures (10, 15, 20, or 25 °C; N = 6 for each temperature) in the large holding tanks. On the day of the measurements, fish were randomly selected in the early morning, quickly weighed in water, and individually placed into a respirometer (see above). A black plastic curtain covered each respirometer to prevent fish from seeing one another in an adjacent respirometer, which could affect their activity patterns and, thus, oxygen consumption. Water temperatures within the respirometer and ambient tank were maintained at the respective experimental temperatures using a heating/chilling unit. Fish were allowed to recover from the transfer for 4 h prior to beginning the oxygen consumption measurements. This recovery period was determined by ensuring the fish’s oxygen consumption was observed to be at a stable level. Previous experiments from our lab (Kieffer et al. 2001; Penny and Kieffer submitted) also showed that juvenile sturgeon returned to resting oxygen consumption levels within 30–240 min following handling/exercise stress. Following this 4 h adjustment period, oxygen consumption measurements were collected over a 4–5 h period. Since the fish were able to move freely around inside the respirometer (thus routine metabolism), three measurements were selected over the 4–5 h measurement period for each temperature condition: lowest (minimum), highest (maximum), and overall mean oxygen consumption rates.
Temperature quotient calculations (Q 10) and statistics
Temperature quotients (Q 10) were calculated using the formula provided by Prosser (1991): Q 10 = (k 2/k 1)10/(t2 − t1), where k 1 and k 2 are the rates of reaction (rate constants) at temperatures t 1 and t 2, respectively. All values are presented as mean ± SEM (N = 6 fish per group). A one-way ANOVA was conducted for each rate of oxygen consumption measurement across the temperature conditions (N = 6 at each temperature). When the ANOVA revealed significance (P < 0.05), Tukey’s tests were used to test for where these differences between pairs of temperatures occurred. Alpha in all cases was 5 % (P < 0.05).
Metabolic rate determination in fish acutely exposed to various temperatures
Juvenile shortnose sturgeon (~11 g; N = 6 for each temperature) acclimated to 10 °C in the large holding tanks were randomly removed in the early morning and weighed underwater before being individually placed into an intermittent flow respirometer (1 L) that were immersed within the ambient tank of water. Following the same recovery period as noted above for acclimated fish, the software program was initiated and measurements over a 1 h period were automatically taken. A 1 h period for each temperature condition was chosen in order to keep within the same 5 h window that was used for the acclimation study.
Upon completion of the 1 h 10 °C measurement period, the flush pump exchanged the 10 °C respirometer water with 15 °C water. The fish were held at 15 °C for 1 h while their metabolic rate was determined. Following the metabolic rate determination at 15 °C, the fish were then exposed to 20 °C water for 1 h, and the procedure was repeated. Finally, the fish were exposed to 25 °C for 1 h, and their metabolic rate was determined. Once the experiment was completed, the fish were then removed from the respirometer and placed in a holding tank at 25 °C. Because of the short-time duration at each “new” temperature, only the average routine rates of oxygen consumption were taken.
Temperature quotient calculations (Q 10) and statistics
Q 10 values were calculated using the formula provided by Prosser (1991): Q 10 = (k 2/k 1)10/(t2 − t1), where k 1 and k 2 are the rates of reaction (rate constants) at temperatures t 1 and t 2, respectively. All data are represented as mean ± SEM A one-way repeated measures ANOVA was conducted to test for significant differences in metabolic rate across the acute exposure temperature conditions. Following this, a Tukey’s post hoc test was completed to compare each of the temperatures back to the 10 °C reference point, as well as amongst each other. Alpha in all cases was 5 % (P < 0.05).
Results
Metabolic rate determination at various acclimation temperatures
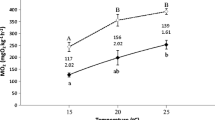
Figure 1a–d depicts the routine metabolic rates that were obtained over the measurement period for juvenile shortnose sturgeon (N = 6 per temperature condition) from which the mean minimum, maximum, and overall mean values were determined (see Fig. 2).
Routine metabolic rates (±SEM) obtained over a 5 h period for juvenile shortnose sturgeon (N = 6 per temperature condition) from which the mean minimum (lowest), mean maximum (highest), and overall mean routine metabolic rates were determined. See text for details of which values are different from each other
There were clear differences between individual fish, and the variability between fish was lowest at 10 °C and increased as temperature increased (see Fig. 1a–d). From these 5 h tracings, the lowest (minimum routine metabolic rate), the mean (the mean of all the data over the measurement period), and the highest (maximum routine metabolic rate) were determined for each fish at each temperature. These data are summarized in Fig. 2.
The mean routine metabolic rates (mg O2 kg−1 h−1) at each of the four temperature conditions were found to be influenced by temperature (Fig. 2; P = 0.005), and differences occurred between 10 and 15 °C (P = 0.042), 10 and 20 °C (P = 0.009), and 10 and 25 °C (P = 0.011). Similarly, there was a significant effect of temperature on the minimum routine metabolic rates experienced for the shortnose sturgeon (P = 0.004). A Tukey’s post hoc test revealed that the differences were only significant between 10 and 20 °C (P = 0.005) and between 10 and 25 °C (P = 0.012). Maximum routine metabolic rates were also affected by temperature (P = 0.008); metabolic rates significantly differed between 10 and 15 °C (P = 0.041), 10 and 20 °C (P = 0.013), and 10 and 25 °C (P = 0.017). Overall, metabolic rates (for mean, minimum, and maximum values) increased from 10 to 15 °C and then began to plateau as temperature increased to 20 and 25 °C (Fig. 2).
Q 10 values
For mean metabolic rates, the temperature coefficient values that reflected typical biological process (Q 10 of ~2.0 or greater) were obtained for all temperature comparisons with 10 °C (Table 1). The highest Q 10 value obtained was for the comparisons between the 15 and 10 °C conditions (Q 10 = 4.3). In contrast, temperature had little effect on metabolic rate (i.e. Q 10 values ~1.0) in fish acclimated between 15, 20, and 25 °C.
The effects of an acute temperature change on metabolic rate determination in sturgeon
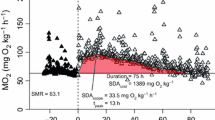
For this experiment, 10 °C acclimated fish were acutely exposed, in sequential order, for 1 h to 15 °C, then to 20 °C, and finally to 25 °C. The metabolic rates during each hour were measured, and the mean values are presented in Fig. 3. At 10 °C, the average routine metabolic rate was ~200 mg O2 kg−1 h−1. Immediately following exposure to 15 °C, the metabolic rate increased significantly to ~350 mg O2 kg−1 h−1. This rate further increased to 389 mg O2 kg−1 h−1 following exposure to 20 °C, and finally to 437.8 mg O2 kg−1 h−1 at 25 °C. A one-way repeated measures ANOVA (α = 0.05) determined that significant differences (P < 0.001) amongst treatment groups existed, and a Tukey’s post hoc test revealed that these significant differences occurred between 25 and 10 °C (P < 0.001), 20 and 10 °C (P < 0.001), 15 and 10 °C (P = 0.006). All increased temperatures for which the fish were exposed to cause a significant change in their metabolic rate when being compared to the initial temperature they were held at.
Mean routine metabolic rates (±SEM) for 10 °C acclimated juvenile shortnose sturgeon. These fish (N = 6) were subsequently acutely exposed for an hour to 15 °C, then 20 °C, and finally 25 °C. The routine metabolic rates are presented for each temperature. An asterisk indicates a significant (P < 0.05) difference from the 10 °C condition
Q 10 values
For the overall mean values, it was determined that routine metabolic rate of sturgeon was temperature-dependent for most temperature comparisons. In particular, the strongest relationship existed when 10 °C fish were compared to 15, 20, and 25 °C conditions. Table 1 depicts the Q 10 values obtained between all temperature conditions.
Discussion
The Intergovernmental Panel on Climate Change (2007) has reported that the mean global surface temperature over land and oceans has increased by 0.7 °C over the past 100 years (IPCC 2007, in Lassalle et al. 2010). By the end of the twenty-first century, it is expected that this increase in global surface temperature could range between 1.1 and 6.4 °C, which could have significant impacts on the distribution and survival of many species (Lassalle et al. 2010). Regardless of the temperature threat that summer months may impose on populations of shortnose sturgeon, there are currently very few studies that have examined their temperature limits (Ziegeweid et al. 2008) and how metabolic rates are affected by temperature. Therefore, it is important to examine the relationship between metabolic rate and environmental temperatures in order to assess how other traits will be affected by thermal changes (Cano and Nicieza 2006).
In the current study, the overall mean routine oxygen consumption of the shortnose sturgeon acclimated to 10, 15, 20, or 25 °C reflect rates that have been shown for some species of sturgeon. For example, a study by Secor and Gunderson (1998) on juvenile (~200 g) Atlantic sturgeon reported that metabolic rates were 228 ± 28 mg O2 kg−1 h−1 at 19 °C and 307 ± 20 mg O2 kg−1 h−1 at 26 °C, which are quite similar to what was obtained for juvenile shortnose sturgeon in this experiment at 20 and 25 °C. Other studies, however, have reported rates of routine metabolism for different species of sturgeon that were quite dissimilar to values obtained in this study. For example, a study by McKenzie et al. (1995) reported that routine rates of oxygen consumption for larger (~800 g) Adriatic sturgeon in normoxic conditions were 140.3 ± 13.5 mg O2 kg−1 h−1 at 23 °C, compared to 309 ± 33 mg O2 kg−1 h−1 at 25 °C for shortnose sturgeon (11 g) in the current study. Lastly, Mayfield and Cech (2004) showed that metabolic rates increased exponentially with increases in temperature (11, 19, and 24 °C) in 0+ green sturgeon, Acipenser medirostris.
It is apparent that overall mean routine metabolic rate increased from 10 to 15 °C trials, but then these values reach a plateau (Fig. 2). Statistically, this trend is also supported, as significant differences in routine metabolism were obtained for all temperature comparisons against 10 °C. This trend is reflective of the true effects that temperature had on the sturgeon’s metabolism, as the plateau also occurs upon examination of the overall minimum and maximum routine metabolic rate data. The plateau in routine metabolism was an unexpected result, since it was hypothesized that increases in temperature would have pronounced effects (i.e. increased effects) on metabolism, as has been observed in other species of fish (e.g. salmonids: Lee et al. 2003; sharks: Carlson and Parsons 1999; largemouth bass: Cooke et al. 2001). The Q 10 values obtained from the acclimated portion of the experiment further illustrate the idea that gradual increases in temperature did not significantly increase routine metabolic rate in juvenile shortnose sturgeon, with the exception of the 10 °C compared to the 15 °C group. When examining the Q 10 values in Table 1, the only values that appear to reflect true biological processes (approximately ≥2.0) are those compared to 10 °C. The remaining comparisons between temperatures have Q 10 values of approximately 1.0, implying that the increases in temperature were not having a considerable influence on the fish’s metabolism. The highest Q 10 value (4.3) was obtained for the comparison between 15 and 10 °C, which implies that the metabolic rate of the sturgeon was most affected by the initial increase in temperature from 10 °C.
While the temperatures examined in the acute portion of the experiment were the same as for the acclimated experiment, it was expected that acute temperature variations would result in significant increases in metabolic rate, as the sturgeon would not be able to fully adapt to their temperature environments after being acclimated to the 10 °C starting point. Overall, mean routine metabolic rates of the fish were higher at each respective temperature condition compared to the acclimated experiment. Yet, routine metabolism failed to significantly increase exponentially as temperature was increased (Fig. 3); however, when examining the data from Fig. 3, it appears as though oxygen consumption is steadily increasing. Nevertheless, significant differences were obtained between the same temperatures as the acclimated experiment (i.e. between 15 vs. 10, 20 vs. 10, and 25 vs. 10 °C), and no others. This implies that regardless of the type of temperature exposure, the mean routine metabolic rate of juvenile shortnose sturgeon appears to plateau above 15 °C (up to 25°) and their oxygen consumption does not continue to increase significantly (i.e. at least linearly or exponentially).
While it was expected that the Q 10 values obtained for the acute temperature comparisons would be higher compared with the acclimated animals, they were somewhat lower (Table 1). Since Q 10 values that were greater than or approximately equal to 2.0 were obtained for the same temperature comparisons (25 vs. 10, 20 vs. 10, and 15 vs. 10 °C), it can be inferred that a true biological relationship exists between temperature and routine metabolism at these conditions. For the acute temperature exposures, it was expected that a dependency on temperature would exist for all temperature comparisons made, since the sturgeon were abruptly exposed to 15, 20, and 25 °C for 1 h increments without being given a longer length of time to adjust. This illustrates the idea that the sturgeon were able to withstand the thermal increases without having to significantly increase their oxygen intake, or they had resorted to using anaerobic processes at this point.
The failure of both the acclimated and acute increases in temperature exposure to produce an increase in routine metabolism is noteworthy. The plateau in oxygen consumption after 15 °C in both experiments has several possible implications. Firstly, it could imply that the shortnose sturgeon has accumulated physiological characteristics that have permitted it to be extremely tolerant and efficient in consuming oxygen over a wide range of temperatures. For example, our laboratory has recently demonstrated that the oxygen consumption rates in shortnose sturgeon plateau with increases in temperature during critical thermal maximum tests (Zhang and Kieffer, unpublished results). Additionally, juvenile shortnose sturgeon may not have the capability to increase their routine metabolic rate beyond a certain point, even if desired. Thus, to maintain active metabolism (i.e. maintain aerobic scope) across a broad range of temperatures, for example, the routine (resting) metabolic rate of shortnose sturgeon may be capped. To support this line of thought, research from our laboratory has shown that shortnose sturgeon can maintain nearly identical critical swimming speeds (UCrit) between 15 and 25 °C (Deslauriers and Kieffer 2012). In fact, the shape of the Ucrit to temperature curve is very similar to the resting metabolic rate to temperature curve noted in the present study. However, since their metabolism plateaus above 15 °C in both forms of temperature exposure, it is unclear whether this response is inherently adaptive and will persist in response to further temperature increases, or if it acts as a signal that any further increase will result in the plummeting of metabolism and lead to death. Also, whether fish are using cost-saving measures (i.e. reducing activity and thus reducing their routine metabolic rate) at higher temperatures is not known and cannot be discerned from the current data. However, it is clear that some individuals decrease their metabolic rates at some points during the trials at warmer temperatures, and the variability in this response appears to be highest at the warmer temperatures (see Fig. 1). Future experiments should determine activity levels at various temperatures in shortnose sturgeon. In contrast, the findings might suggest that juvenile shortnose sturgeon can maintain similar oxygen consumption rates over a wide array of warmer temperatures (15 °C and higher). As noted above, our recent studies show that sturgeon can maintain oxygen consumption rates up to 31 °C during critical thermal maximum tests (Zhang and Kieffer, unpublished). Alternatively, it might only be when temperatures are reduced (i.e. 10 °C or lower) that significant effects on metabolic rate are noted. For example, if the 10 °C data are removed, any relationship between temperature and metabolic rate disappears.
In general, the relationship between temperature and metabolic rate is not clear across sturgeon species. For example, in other species of sturgeon, including the 0+ green sturgeon, a different relationship exists between temperature and metabolic rate. Between 10 and 20 °C, 0+ fish do not modify their metabolic rates. It is only once the temperatures exceed 20 °C that 0+ green sturgeon modify their metabolic rates (Mayfield and Cech 2004). This contrasts with what is observed for 1+ green sturgeon. For example, in 1+ fish, a significant increase in resting metabolic rate was noted with subsequent increases in temperature (Mayfield and Cech 2004). Thus, the metabolic patterns of different sizes of green sturgeon appear to reflect different temperature sensitivities. Future experiments should consider the importance of ontogeny on these relationships. Additional experiments are also required to address the impacts of low (below 10 °C) and high (above 30 °C) temperatures on routine metabolic rates in sturgeon. Thus, it is probable that the degree of the temperature range used in the present study was simply not great enough to demonstrate a more accurate relationship between temperature and metabolic rate.
Conclusions
Presently, there is an absence of the literature that examines the effects of temperature on routine metabolism in sturgeon, which is important in providing a more holistic view of sturgeon physiology. Since most of the current research has focused on the relationship between temperature and other variables such as physical growth (e.g. Allen et al. 2006), much of their oxygen consumption responses still remain unknown. The purpose of this study was to examine routine metabolism for juvenile shortnose sturgeon exposed to acclimated and acute temperature conditions, at 10, 15, 20, and 25 °C, which reflects relevant natural variation. Additionally, the differences in metabolism during the two forms of exposure were explored to determine if one form was more temperature-dependent than the other. Overall, upon completion of this study, it was determined that (1) an increase in temperature, whether acclimated or acute, initially produced a significant increase in routine metabolic rate, but exhibited a plateau thereafter, (2) this relationship is markedly dissimilar to other fish, including some sturgeon species, and (3) the relationship between routine metabolic rate and temperature increase was strongest when comparing 15, 20, and 25 °C with 10 °C, in both acclimated and acute experiments, but failed to mirror typical dependency of metabolism on temperature when comparing temperatures above 15 °C.
References
Allen PJ, Nicholl M, Cole S, Vlazny A, Cech JJ (2006) Growth of larval to juvenile green sturgeon in elevated temperature regimes. Trans Am Fish Soc 135:89–96
Beitinger TJ, Lutterschmidt WI (2011) Measures of thermal tolerance. In: Farrell AP, Stevens ED, Cech JJ, Richards JG (eds) Encyclopedia of fish physiology—from genome to environment. Academic Press, Waltham
Billard R, Lecointre G (2001) Biology and conservation of sturgeon and paddlefish. Rev Fish Biol Fisher 10:355–392
Birstein VJ, Bemis WE (1997) How many species are there within the genus Acipenser? Environ Biol Fish 48:157–163
Brett JR (1971) Energetic responses of salmon temperature. A study of thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka). Am Zool 11:99–113
Brett JR, Glass NR (1973) Metabolic rates and critical swimming speeds of sockeye salmon (Oncorhynchus nerka) in relation to size and temperature. J Fish Bd Can 30:379–387
Brett JR, Groves TDD (1979) Physiological energetics. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology, vol 7. Academic Press, New York, pp 279–352
Cano JM, Nicieza AG (2006) Temperature, metabolic rate, and constraints on locomotor performance in ectotherm vertebrates. Funct Ecol 20:464–470
Carlson JK, Parsons GR (1999) Seasonal differences in routine oxygen consumption rates of the bonnethead shark. J Fish Biol 55:876–879
Cooke SJ, Kassler TW, Philipp DP (2001) Physiological performance of largemouth bass related to local adaptation and interstock hybridization: implications for conservation and management. J Fish Biol 59:248–268
Dadswell MJ, Taubert BD, Squiers TS, Marchette S Buckley J (1984) Synopsis of biological data on shortnose sturgeon, Acipenser brevirostrum LeSuer 1818. National Oceanographic and Atmospheric Administration Technical Report NMFS 14, Washington, D.C
Deslauriers D, Kieffer JD (2012) The effects of temperature on swimming performance of juvenile shortnose sturgeon (Acipenser brevirostrum). J Appl Ichth 28:176–181
Fry FEJ (1971) The effect of environmental factors on the physiology on fish. In: Hoar WS, Randall DJ (eds) Fish physiology, vol VI. Academic Press, New York, pp 1–98
Fry FEJ, Hart JS (1948) The relation of temperature to oxygen consumption in the goldfish. Biol Bull 94:66–77
Kieffer JD, Cooke SJ (2009) Physiology and organismal performance of centrarchids. In: Cooke SJ, Philipp DP (eds) Centrachid fishes: diversity, biology and conservation. Oxford Press, Wiley, pp 207–263
Kieffer JD, Wakefield AM (2009) Oxygen consumption, ammonia excretion and protein use in response to thermal changes in juvenile Atlantic salmon Salmo salar. J Fish Biol 74:591–603
Kieffer JD, Alsop D, Wood CM (1998) A respirometric analysis of fuel use during aerobic swimming at different temperatures in rainbow trout (Oncorhynchus mykiss). J Exp Biol 201:3123–3133
Kieffer JD, Wakefield AM, Litvak MK (2001) Juvenile sturgeon exhibit reduced physiological responses to exercise. J Exp Biol 204:4281–4289
Kynard B (1997) Life History, latitudinal patterns, and status of the shortnose sturgeon, Acipenser brevirostrum. Environ Biol Fishes 48:319–334
Lassalle G, Crouzet P, Gessner J, Rochard E (2010) Global warming impacts and conservation responses for the critically endangered European Atlantic sturgeon. Biol Cons 143:2441–2452
Lee CG, Farrell AP, Lotto A, MacNutt MJ, Hinch SG, Healey MC (2003) The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol 206:3239–3251
Mayfield RB, Cech JJ (2004) Temperature effects on green sturgeon bioenergetics. Trans Am Fish Soc 133:961–970
McKenzie DJ, Piraccini G, Steffensen JF, Bolis CL, Taylor EW (1995) Effects of diet on spontaneous locomotor activity and oxygen consumption in Adriatic sturgeon (Acipenser maccarii). Fish Physiol Biochem 14:341–355
Prosser CL (1991) Environmental and metabolic animal physiology. Wiley, New York, pp 109–166
Scott WB, Crossman EJ (1973) Freshwater fishes of Canada, 184th edn. Fisheries Research Board of Canada Bulletin, Canada
Secor DH, Gunderson TE (1998) Effects of hypoxia and temperature on survival, growth, and respiration of juvenile Atlantic sturgeon, Acipenser oxyrinchus. Fish Bull 96:603–613
Wilson JA, McKinley RS (2004) Distribution, habitat and movements. In: LeBreton GTO, Beamish FWH, McKinley RS (eds) Sturgeons and paddlefish of North America. Kluwer Academic Publishers, Netherlands, pp 40–72
Ziegeweid JR, Jennings CA, Peterson DL (2008) Thermal maxima for juvenile shortnose sturgeon acclimated to different temperatures. Environ Biol Fish 82:299–307
Acknowledgments
Funding for this research was provided by a Natural Science and Engineering Council Grant of Canada (NSERC) discovery grant to J.D.K. Support was also provided by the MADSAM fish group and the Marguerite and Murray Vaughan Graduate Fellowship in Marine Science to F.M.P. All procedures followed the guidelines of animal use set out by the Canadian Council of Animal Care and were approved by the Institutions Animal Care Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kieffer, J.D., Penny, F.M. & Papadopoulos, V. Temperature has a reduced effect on routine metabolic rates of juvenile shortnose sturgeon (Acipenser brevirostrum). Fish Physiol Biochem 40, 551–559 (2014). https://doi.org/10.1007/s10695-013-9865-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9865-8