Abstract
Juvenile stellate sturgeon Acipenser stellatus were intraperitoneally injected with estradiol-17β (E2; 0 and 5 mg/kg fish) to investigate the possibility of sex reversal and also determine the changes in biochemical parameters. Five-month-old fish (40.9 ± 1.1 g) were injected every 3-week interval during a 190-day trial. At the termination of the experiment, final weight and other growth parameters including weight gain and specific growth rate, hepatosomatic and viscerosomatic indices were not affected by repetitive injection of E2. Hematological features of E2-treated fish showed significant reductions in number of red blood cells, hemoglobin concentration, hematocrit value and mean corpuscular hemoglobin (P < 0.05), but no significant changes were observed in number of white blood cells, mean corpuscular volume and mean corpuscular hemoglobin concentration (P > 0.05). Calcium, phosphorus, glucose, triacylglycerol, cholesterol, total protein and estradiol concentrations were significantly increased in fish injected with E2 (P < 0.001). Plasma progesterone and testosterone levels were noticeably lower in fish injected with 5 mg/kg E2 rather than the control fish (P < 0.001). Histological observations of gonads showed that all fish injected with 5 mg/kg E2 apparently feminized, while 66.6 % of the control group was female. These results revealed that the injection of E2 is an effective method for feminization of stellate sturgeon without having significant inhibitory effects on growth and survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are many different ways to do sex control and manipulation in fish. Both genetic and endocrine manipulations in fish are used to create monosex population for aquaculture (Devlin and Nagahama 2002). Hormone treatment is relatively easy to use and has good efficiency and low price to produce all the same gender, compared with genetic manipulation. In this case, aquaculture has based its planning attributes on higher growth rate and short-term maturity (for broodstock and roe production). The effectiveness of hormone concentration depends on fish species, age and physiological condition, type of hormone used and duration of exposure.
Of the sex steroids, estradiol-17β (E2) is known to promote vitellogenesis and induce feminization in fish population (Omoto et al. 2002; Schafhauser-Smith and Benfey 2003; Wang et al. 2008). Although E2 has been utilized for controlling sex differentiation in various species of teleosts, information on sex reversal in chondrosteans is extremely rare and even there are none about stellate sturgeon, Acipenser stellatus. Some previous researches on sturgeons revealed that different hormonal treatments could skew sex ratios. For instance, Akhundov and Fedorov (1994) examined the effect of estradiol dipropionate on ovarian development in sterlet, Acipenser ruthenus, and showed that both sexes were present in all treatments. Juvenile paddlefish, Polyodon spathula, implanted with 17-methyltestosterone (MT) showed changes in sex ratio (Shelton and Mims 1998). Omoto et al. (2002) applied E2 and MT in hybrid sturgeon (Huso huso ♀ × A. ruthenus ♂) and found that sex reversal can be induced by both hormones. Also, dietary E2 feminized all shortnose sturgeon, Acipenser brevirostrum, after a 9-month trial (Flynn and Benfey 2007). However, some studies have demonstrated the deleterious effect of high doses of dietary E2 (50–100 mg/kg feed) on survival and growth (Flynn and Benfey 2007; Meknatkhah et al. 2012).
The wild populations of sturgeons have dramatically declined because of overfishing, destruction of natural spawning grounds and environmental pollution (Falahatkar et al. 2009). Therefore, commercial sturgeon aquaculture grows fast (FAO 2012) and has the potential to fill the gap of markets with producing meat and caviar. In sturgeon culture, females are more valuable than males because of their precious caviar (Omoto et al. 2002; Falahatkar et al. 2011). Therefore, commercial sturgeon farms are more interested in all female populations (Van Eenennaam et al. 1996). There is no information regarding the usage of E2 in its injection method in fish. Thus, it has been hypothesized that E2 injection method can be an alternative and effective method for feminizing the fish without having adverse impacts on growth performances. Therefore, the present study assesses the effectiveness of E2 injection to determine sex ratio, growth performance and physiological changes in stellate sturgeon.
Materials and methods
Experimental animals and rearing conditions
“One hundred and twenty” 5-month old, juvenile stellate sturgeon reared at Dr. Yousefpour Fish Hatchery Center in Siahkal, Guilan, Iran, with an average weight of 40.9 ± 1.1 g were used for this study. They were randomly distributed into six circular concrete tanks (20 fish per tank) containing a water volume of 806 l and supplied with flow-through freshwater at 17 ± 0.5 l/min. During the experimental period, water was aerated continuously with compressed air delivered through an air stone in each tank. The fish was kept under natural photoperiod (12 h light/12 h dark at start and 13 h light/11 h dark at final) and fed 2–6 times a day depending on water temperature according to their satiation with a commercial diet (Biomar® 0.8–3, Nersac, France) during the experimental time. The fish were kept and reared under these conditions for approximately 7 months from October to April. Throughout the trial, the average water temperature and dissolved oxygen concentrations which recorded daily were 12.4 ± 0.3 °C and 8.9 ± 0.2 mg/l, respectively.
Experimental design
Two experimental groups with three replicates for each treatment were considered in this study. Twenty fish were introduced to each tank. The fish were intraperitoneally injected with either 0.9 % sterilized sodium chloride solution (as a control group) or estradiol-17β (5 mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA) every 3 weeks, from October 13 to April 19. E2 injection solution was prepared by dissolving E2 in 100 % ethanol and then in 0.9 % sterilized sodium chloride solution as a vehicle.
Growth performance
Body weight (g) and total length (cm) of all fish were individually measured to the nearest of 0.01 g and 0.1 cm, respectively, every 3 weeks. The individual fish from each tank was anesthetized by 300 mg/l clove powder and then weighted. Recording body weight was coincident with the hormonal injection. Fish were food-deprived for 24 h before each weighing and injecting. At the end of the experiment, specific growth rate (SGR), body weight increase (BWI), weight gain (WG), feed conversion ratio (FCR), condition factor (CF), voluntary feed intake (VFI), viscerosomatic index (VSI) and hepatosomatic index (HSI) were calculated using the following formulas to compare growth performance between two experimental groups (Biswas et al. 2008; Falahatkar et al. 2013):
Blood and tissue sampling
All the procedure and sampling during the experiment were conducted in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. At the termination of the experiment, five fish from each tank were quickly captured and sampled after being anesthetized. Then, approximately 2 ml of blood samples was collected from the caudal vasculature using a 5-ml heparinized syringe equipped with a 25-gauge needle. Blood samples were divided into two portions. Half of the blood was used for separating plasma, and the remaining blood was used for hematological analysis. Plasma was separated by centrifugation (1,500×g for 10 min) and stored at −70 °C until subsequent analysis.
Steroid hormone concentrations (E2, testosterone and progesterone) were measured by a specific radioimmunoassay (RIA) using available kits (Immunotech, Marseille, France) according to the method provided by Kubokawa et al. (1999) with some modification. Plasma glucose was analyzed based on the colorimetric glucose oxidase–peroxidase reaction (Bayunova et al. 2002). Plasma cholesterol and triacylglycerol levels were determined by the CHOD-PAP and GPO-PAP methods, respectively (Chatzifotis et al. 2010), using commercial available kits (Pars Azmun, Karaj, Iran). Total protein levels were measured by the Biuret method (ZiestChem Diagnostics, Tehran, Iran). Total plasma calcium was determined by the colorimetric method (Sigma-Aldrich procedure no. 587). Plasma phosphorus was measured using an endpoint colorimetric assay (Sigma-Aldrich procedure no. 360). All biochemical and hormonal parameters were measured in triplicate.
Hematocrit (Hct) value was determined by the standard microhematocrit method and expressed in percentage. Hemoglobin (Hb) concentration was determined using the cyanmethemoglobin method. The number of white blood cells (WBC), red blood cells (RBC), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were calculated according to Ranzani-Paiva et al. (2004).
After bleeding, the fish were killed by a sharp blow to the head. Then, the livers and right gonads were removed for determining hepatosomatic index and sex, respectively. Histological analysis was conducted on the gonads according to the method of Hurvitz et al. (2007). Briefly, a subsample of gonad was fixed in Bouin’s solution for 48 h and then stored in the 70 % ethanol until analysis was performed. Fixed gonadal tissue was dehydrated, embedded in paraffin, sectioned at 6 μm and then stained with hematoxylin and eosin. Two slides were prepared for each sample. Histological slides were examined under a light microscopy (Olympus BX51, Tokyo, Japan) to evaluate the sex and gonadal stage of each fish according to the terminology used by Mojazi Amiri et al. (1996).
Statistical analysis
Prior to the analysis, all data form each individual fish were examined for normality and homogeneity of variances based on Kolmogorov–Smirnov and Levene’s tests, respectively. A two-way nested analysis of variance, wherein replicates were nested within the experimental variable, was used to determine the effect of the different doses of E2. There were no significant differences among the replicates (tanks). Therefore, an independent sample t test was used for statistical comparison between control and E2-injected fish. Comparison of sex ratio between control and E2-treated fish was made according to chi-square test. All statistical analyses were performed using SPSS (version 13, Chicago, IL, USA). A P value of 0.05 was used to determine statistical significance for all comparison. Data are presented as mean ± standard error (SE) within the text.
Results
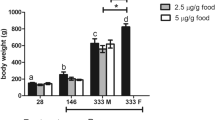
Growth indices are presented in Table 1. Results show that the long-term injection of E2 had no significant effect on WG, BWI, SGR, CF and VFI. Although the control group exhibited higher survival and greater final body weight than those in the E2-treated fish, there were no significant differences between them (P > 0.05, Table 1). The long-term treatment with E2 did not cause significant variations in HSI and VSI, although a slight increase was observed as compared with the control group (P > 0.05, Fig. 1).
Lower levels of Hct and Hb were detected in fish treated with E2 at the end of experiment (P < 0.05, Table 2). Significantly lower RBC and MCH were also obtained from fish injected with E2 (P < 0.05). No significant variations were observed in WBC and other blood indices between two groups of fish (P > 0.05).
Fish injected with E2 had significantly elevated plasma glucose concentration compared with the control fish (P < 0.001, Table 3). Plasma cholesterol and triacylglycerol levels were significantly higher than those of the control group (P < 0.001). Total protein level in fish treated with E2 was about five times more than the level in the control group (P < 0.001). A 15-fold increase in plasma calcium and a sevenfold increase in plasma phosphorus were observed in E2-treated fish (P < 0.001, Table 3).
The plasma concentrations of progesterone and testosterone are presented in Fig. 2a, b, respectively. Plasma progesterone and testosterone levels in the fish injected with E2 were significantly lower than those of the control group (P < 0.001). The E2-treated fish had noticeably higher concentrations of E2 ranging 4.3–38 ng/ml compared with the control group (P < 0.001, Fig. 2c).
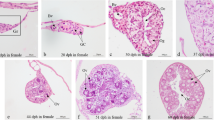
Histological analysis showed that a 100 % female population of stellate sturgeon was produced by injections of 5 mg E2/kg body weight, while 66 % of our control group was female. The fish treated with E2 had small ovaries consisting of oogonia nest and oocytes, which were in the pre-vitellogenic stage and in the perinucleus substage (Fig. 3a, b).
Discussion
Although the growth performance, HSI and VSI were not affected by repeated E2 injections, the present study revealed that the used dose and method of applying E2 appear to be effective in feminization of stellate sturgeon. Reversing the gonadal sex by giving estrogens has been reported in many fish species with very long periods of gonadal differentiation such as sturgeon. Omoto et al. (2002) revealed that E2 is an effective hormone in feminization of bester (a hybrid between Huso huso ♀ and A. ruthenus ♂) if given before gonadal differentiation. Flynn and Benfey (2007) reported that all juvenile shortnose sturgeon fed E2 at different dosages for a 7- or 9-month period were feminized, but doses above 10 mg E2/kg resulted in increased mortality and pathologies. Meknatkhah et al. (2012), who applied feeding experiment (25 and 50 mg E2/kg) for 7 months in order to sex reverse juvenile stellate sturgeon, showed that E2 treatments induced oocyte development and feminization, but had the negative effects on feed intake and subsequent growth and resulted in weakness in fish. In the current study, repeated injections of E2 caused feminization without significant or profound impacts on growth performance. This conflicts with the results of Hendry et al. (2003) in Atlantic halibut (Hippoglossus hippoglossus), Schafhauser-Smith and Benfey (2003) in triploid brook trout (Salvelinus fontinalis), Flynn and Benfey (2007) in shortnose sturgeon and Wang et al. (2008) in bluegill sunfish (Lepomis macrochirus). Such discrepancy may depend on species, the dosage and method of application of hormone and experimental condition.
In this study, the long-term administration of E2 caused a significant decrease in most hematological indices (Hct, Hb, RBC and MCH). Similarly, Schafhauser-Smith and Benfey (2003) reported that the administration of 30 mg E2/kg feed to female triploid brook trout led to a decline in both Hct and Hb levels. Similar results have also been found from our previous study on stellate sturgeon treated by oral administration of various doses (25 and 50 mg E2/kg feed) of E2 (Meknatkhah et al. 2012). It seems that E2 has an inhibitory effect on erythropoiesis in stellate sturgeon as it has been previously reported in teleosts (Pickering 1986), but the mechanism of action is still unknown.
Our results revealed that plasma lipid (cholesterol and triacylglycerol) concentrations are highly affected by repeated injections of E2. Hyperlipidimic effects of E2 have been reported in other fish species in short- or long-term exposure. For instance, circulating cholesterol and triacylglycerol concentrations have been increased in response to E2 exposure for 24 h in rainbow trout Oncorhynchus mykiss (Wallaert and Babin 1992). Goldfish (Carassius auratus) exposed to 10 μg/g E2 via silastic implant for a period of 5 months also experienced elevations in plasma cholesterol and triacylglycerol concentrations (Sharpe and MacLatchy 2007). High cholesterol and triacylglycerol concentrations may be associated with the growth and development of gonads, as demonstrated in fish (Wallaert and Babin 1992; Sharpe and MacLatchy 2007).
Because of lower water temperature during the winter season (~10 °C), feed intake and growth rate were not as high as in fall and spring. Although the growth was not affected by repeated injections of E2 or physiological saline solution, a slight increase (not significant) in the liver of E2-treated group was observed. This suggests an elevated lipogenic activity or vitellogenin synthesis, because liver is a site for vitellogenin and lipid synthesis. Higher water temperature during winter season or longer period of experiment would be a suitable condition for observation of such significant increase in HSI in fish treated with E2. The exposure with exogenous E2 has been shown to increase hepatic lipogenesis and vitellogenesis in fish (Wallaert and Babin 1992; Schafhauser-Smith and Benfey 2003; Sharpe and MacLatchy 2007). Overall, our findings suggest that E2 exposure interferes with endogenous lipid dynamics and even alters hepatic lipid storage in stellate sturgeon, as has been demonstrated in mammals (Bertolotti and Spady 1996; Dodge et al. 1996; Trautwein et al. 2003). However, further studies would be required to clarify the precise mechanism of lipid dynamics in sturgeon after exposure to E2.
E2 caused a significant increase in plasma concentration of total protein. Similar effects of E2 on blood total protein have been reported in rainbow trout (Leatherland 1985) and red sea bream Chrysophrys major (Woo et al. 1993). An increase in total protein levels of E2-treated fish is presumably related to the vitellogenic process which has been accompanied by hyperlipidemia and increased liver size. In addition, it may also be due to its presence in protein complex (such as high-density and low-density lipoprotein) for the transport of cholesterol and triacylglycerol in the circulation (Kocaman et al. 2005; Sharpe and MacLatchy 2007).
A significant increase in glucose was observed in response to repeated E2 administration. This result indicated that E2 affects carbohydrate mechanism in stellate sturgeon. The potential of E2 to induce hyperglycemia has previously been demonstrated by Teles et al. (2004) for sea bass (Dicentrarchus labrax) and Lerner et al. (2007) for Atlantic salmon (Salmo salar). E2 presumably induced hyperglycemia through either gluconeogenesis process in liver or an elevation in the activities of intestinal enzymes, which are responsible for glucose transmission or absorption (Woo et al. 1993; Mommsen et al. 1999). Since glucose is an important fuel for most metabolism activities, its production is a useful pathway for providing energy to cope with the increased metabolic demands.
In the current study, E2 treatment significantly elevated plasma concentrations of both calcium and phosphorus. It has been established that E2 treatment induces vitellogenesis in fishes, which is accompanied by an increase in blood plasma calcium and phosphate (Woo et al. 1993; Persson et al. 1998; Guerreiro et al. 2002; Linares-Casenave et al. 2003), because vitellogenin is a calcium-binding lipophosphoprotein. E2 may be involved in the regulation and elevation of blood plasma calcium through an increase in its uptake from the environmental water or its mobilization from internal sources of calcium, e.g., cartilage or scutes in sturgeons.
In the current investigation, a significant reduction in both plasma progesterone and testosterone was concomitant with increased estradiol levels in fish treated with E2. Similarly, Yuan et al. (2011) showed that oral administration of E2 for 15 months declined serum testosterone levels and enhanced serum estradiol concentrations in a dose-dependent manner in Asian swamp eel (Monopterus albus). The long-term administration of E2 to female brook trout led to a decrease in plasma testosterone and an increase in plasma E2 concentration (Schafhauser-Smith and Benfey 2003). Lower concentrations of progesterone and testosterone in E2-treated fish suggested that repeated E2 injections may affect the steroid biosynthesis process by changing the enzyme activities in the steroidogenic pathway, which convert pregnenolone to progesterone and progesterone to testosterone. Maximum E2 in E2-injected fish was likely related to the vitellogenic process. It is well established in fish species that E2 regulates vitellogenin production by the liver and its level is correlated with vitellogenin synthesis in the liver and with the female reproductive cycle (Ding 2005; Babin et al. 2007). Furthermore, exogenous E2 probably affects the metabolic clearance rate, so that plasma E2 levels remain significantly high 3 weeks after each treating with this hormone. This hypothesis is supported by consideration of previous study in which plasma E2 was poorly metabolized in immature rainbow trout exposed to E2 (Atteke et al. 2003). On the other hand, elevated plasma E2 may be caused by a positive feedback response to hormone therapy on the hypothalamus–pituitary–gonad axis for the growth and development of gonads.
Histological observations of gonads suggested that oogenesis was induced in all the fish treated with exogenous estradiol, so that fish had ovaries with a lot of oocytes at the perinucleus stage. In this study, 66 % of control group were also female. Therefore, it seems that other factors such as artificial propagation and some environmental factors before the beginning of experiment are also involved in skewing sex ratio toward female in the control group.
In conclusion, this study revealed that, for the first time, it is possible to use E2 through injection method for sex reversal in stellate sturgeon without having adverse effects on the growth and survival. According to previous studies on sturgeon species, it seems that the dosage of 5 mg/kg is an optimal dosage for feminization in stellate sturgeon. Further investigations are needed to clearly determine the best dose and exact time of E2 injection and full elucidate its mechanism of action on physiological system through cellular and molecular studies.
References
Akhundov MM, Fedorov KE (1994) Effects of exogenous estradiol on the formation of ovaries in juvenile sterlet Acipenser ruthenus. J Ichthyol 35:109–120
Atteke C, Vetillard A, Fostier A, Garnier D, Jego P, Bailhache T (2003) Effects of progesterone and estradiol on the reproductive axis in immature diploid and triploid rainbow trout. Comp Biochem Physiol A 134:693–705
Babin PJ, Carnevali O, Lubzens E, Schneider WJ (2007) Molecular aspects of oocyte vitellogenesis in fish. In: Babin PJ, Cerdà J, Lubzens E (eds) The fish oocyte: from basic studies to biotechnological applications. Springer, Amsterdam, pp 39–76
Bayunova L, Barannikova I, Semenkova T (2002) Sturgeon stress reactions in aquaculture. J Appl Ichthyol 18:397–404
Bertolotti M, Spady D (1996) Effect of hypocholesterolemic doses of 17 alpha-ethinyl estradiol on cholesterol balance in liver and extrahepatic tissues. J Lipid Res 37:1812–1822
Biswas AK, Seoka M, Ueno K, Yong ASK, Biswas BK, Kim Y, Takii K, Kumai H (2008) Growth performance and physiological responses in striped knifejaw, Oplegnathus fasciatus, held under different photoperiods. Aquaculture 279:42–46
Chatzifotis S, Panagiotidou M, Papaioannou N, Pavlidis M, Nengas I, Mylonas CC (2010) Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 307:65–70
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Ding JL (2005) Vitellogenesis and vitellogenin uptake into oocyte. In: Melamed P, Sherwood N (eds) Hormones and their receptors in fish reproduction. World Scientific Publishing, Singapore, pp 254–276
Dodge JA, Glasebrook AL, Magee DE, Phillips DL, Sato M, Short LL, Bryant HU (1996) Environmental estrogens: effects on cholesterol lowering and bone in the ovariectomized rat. J Steroid Biochem Mol Biol 59:155–161
Falahatkar B, Poursaeid S, Shakoorian M, Barton B (2009) Responses to handling and confinement stressors in juvenile great sturgeon Huso huso. J Fish Biol 75:784–796
Falahatkar B, Tolouei MH, Falahatkar S, Abbasalizadeh A (2011) Laparoscopy, a minimally-invasive technique for sex identification in cultured great sturgeon Huso huso. Aquaculture 321:273–279
Falahatkar B, Akhavan SR, Efatpanah I, Meknatkhah B (2013) Effect of feeding and starvation during the winter period on the growth performance of young-of-year (YOY) great sturgeon, Huso huso. J Appl Ichthyol 29:26–30
FAO (2012) Yearbooks of fishery and aquaculture statistics. www.fao.org. Downloaded on 3 March 2013
Flynn SR, Benfey TJ (2007) Effects of dietary estradiol-17β in juvenile shortnose sturgeon, Acipenser brevirostrum, Lesueur. Aquaculture 270:405–412
Guerreiro PM, Fuentes J, Canario AVM, Power DM (2002) Calcium balance in sea bream (Sparus aurata): the effect of oestradiol-17β. J Endocrinol 173:377–385
Hendry CI, Martin-Robichaud DJ, Benfey TJ (2003) Hormonal sex reversal of Atlantic halibut (Hippoglossus hippoglossus) L. Aquaculture 219:769–781
Hurvitz A, Jackson K, Degani G, Levavi-Sivan B (2007) Use of endoscopy for gender and ovarian stage determinations in Russian sturgeon (Acipenser gueldenstaedtii) grown in aquaculture. Aquaculture 270:158–166
Kocaman EM, Yanik T, Erdoğan O, Çıltaş AK (2005) Alteration in cholesterol, glucose and triglyceride levels in reproduction of rainbow trout (Onchorhynchus mykiss). J Anim Vet Adv 4:801–804
Kubokawa K, Watanabe T, Yoshioka M, Iwata M (1999) Effects of acute stress on plasma cortisol, sex steroid hormone and glucose levels in male and female sockeye salmon during the breeding season. Aquaculture 172:335–349
Leatherland JF (1985) Effects of 17β-estradiol and methyl testosterone on the activity of the thyroid gland in Rainbow trout, Salmo gairdneri Richardson. Gen Comp Endocrinol 60:343–352
Lerner DT, Björsson BT, McCormick SD (2007) Aqueous exposure to 4-nonylphenol and 17β-estradiol increases stress sensitivity and disrupts ion regulatory ability of juvenile Atlantic salmon. Environ Toxicol Chem 6:1433–1440
Linares-Casenave J, Kroll KJ, Van Eenennaam JP, Doroshov SI (2003) Effect of ovarian stage on plasma vitellogenin and calcium in cultured white sturgeon. Aquaculture 221:645–656
Meknatkhah B, Falahatkar B, Khara H (2012) Effects of dietary estradiol 17-β on growth, survival and physiological performance in stellate sturgeon (Acipenser stellatus). Aqua 2012, September 1–5, Prague, Czech Republic
Mojazi Amiri B, Maebayashi M, Adachi S, Yamauchi K (1996) Ovarian development and serum sex steroid and vitellogenin profiles in the female cultured sturgeon hybrid, the bester. J Fish Biol 48:1164–1178
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanism of action and metabolic regulation. Rev Fish Biol Fish 9:211–268
Omoto N, Maebayashi M, Mitsuhashi E, Yoshitomi K, Adachi S, Yamauchi K (2002) Effects of estradiol-17β and 17α-methyltestosterone on gonadal sex differentiation in the F2 hybrid sturgeon, the bester. Fish Sci 68:1047–1054
Persson P, Sundell P, Björnsson KTh, Lundqvist H (1998) Calcium metabolism and osmoregulation during sexual maturation of river running Atlantic salmon. J Fish Biol 52:334–349
Pickering AD (1986) Changes in blood cell composition of the brown trout, Salmo trutta L., during the spawning season. J Fish Biol 29:335–347
Ranzani-Paiva MJT, Ishikawa CM, das Eiras AC, da Silveira VR (2004) Effects of an experimental challenge with Mycobacterium marinum on the blood parameters of Nile Tilapia, Orechromis niloticus (Linnaeus, 1757). Braz Arch Biol Technol 6:945–953
Schafhauser-Smith D, Benfey TJ (2003) The effects of long-term estradiol-17β treatment on the growth and physiology of female triploid brook trout (Salvelinus fontinalis). Gen Comp Endocrinol 131:9–20
Sharpe RL, MacLatchy DL (2007) Lipid dynamics in goldfish (Carassius auratus) during a period of gonadal recrudescence: effects of β-sitosterol and 17β-estradiol exposure. Comp Biochem Physiol C 145:507–517
Shelton WL, Mims SD (1998) Induced sex reversal in gynogenetic paddlefish. Aquaculture ‘98 Book of Abstracts
Teles M, Gravato C, Pacheco M, Santos MA (2004) Juvenile Sea bass biotransformation, endocrine and genotoxic responses to β-naphthoflavone, 4-nonylphenol and 17β-estradiol: comparison of individual and combined chemical exposures. Chemosphere 57:147–158
Trautwein EA, Duchateau GSMJE, Lin Y, Mel’nikov SM, Molhuizen HOF, Ntanios FY (2003) Proposed mechanisms of cholesterol-lowering action of plant sterols. Eur J Lipid Sci Technol 105:171–185
Van Eenennaam AL, Van Eenennaam JP, Medrano JF, Doroshov SI (1996) Rapid verification of meiotic gynogenesis and polyploidy in white sturgeon (Acipenser transmontanus Richardson). Aquaculture 147:177–189
Wallaert C, Babin PJ (1992) Effects of 17β-estradiol and starvation on trout plasma lipoproteins. Lipids 27:1032–1041
Wang HP, Gao Z, Beres B, Ottobre J, Wallat G, Tiu L, Rapp D, O’Bryant P, Yao H (2008) Effects of estradiol-17β on survival, growth performance, sex reversal and gonadal structure of bluegill sunfish Lepomis macrochirus. Aquaculture 285:216–223
Woo NYS, Chung ASB, Ng TB (1993) Influence of oral administration of estradiol-17β and testosterone on growth, digestion, food conversion and metabolism in the underlying read sea bream, Chrysophrys major. Fish Physiol Biochem 10:377–387
Yuan HW, Xu QQ, Gong SY, Yuan YC, Chu ZJ, Yang DQ (2011) Effects of different exogenous estradiol contents on steroid hormones, GSI, survival rate and sex reversal in the Asian swamp eel. Adv Mat Res 382:481–485
Acknowledgments
This project was supported by the provided grant form Islamic Azad University (Lahijan Branch) to HK. Great acknowledge is given to the staff at the Dr. Yousefpour Fish Hatchery Center for the helps during holding the fish.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falahatkar, B., Poursaeid, S., Meknatkhah, B. et al. Long-term effects of intraperitoneal injection of estradiol-17β on the growth and physiology of juvenile stellate sturgeon Acipenser stellatus . Fish Physiol Biochem 40, 365–373 (2014). https://doi.org/10.1007/s10695-013-9849-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9849-8