Abstract
To study the relevance of density and background color to stress response, appetite, and growth in olive flounder, Paralichthys olivaceus, we reared two duplicate groups of juveniles (total length 4.46 ± 0.06 cm, body weight 0.77 ± 0.03 g) in flat-bottom aquaria with dark-green (control) and white backgrounds for 120 days. We measured cortisol and glucose levels in blood and calculated the daily food intake, food conversion efficiency, survival rate, and growth rate. To study the relevance of density and background color to malpigmentation (hypermelanosis) on the blind side, we also compared malpigmented ratios and prepro-melanin-concentrating hormone mRNA activities in the brain between the dark-green and white background groups, as well as between a relatively lower density (60 days) and higher density (120 days). Although we measured relatively higher levels of cortisol and glucose in the white background group and over 200 % of coverage area [PCA]), the bright background failed to induce an acute stress response of more than 20 ng/ml cortisol and 40 mg/dl glucose both in 60 days and 120 days, but did enhance appetite and growth. Also, a bright background color delayed hyperpigmentation only at a low density below 200 % PCA, but did not inhibit malpigmentation at a high density of more than 200 % PCA. In addition, below 200 % PCA, expression of MCH mRNA was significantly higher in the white group, but the level was reversed and was lower in the white group at more than 200 % PCA. In conclusion, although did not induce a high stress response over 200 % PCA, the bright background color resulted in a moderate increasing of cortisol level in blood below 20 ng/ml and enhanced appetite and growth. Moreover, at a density below 200 % PCA, the bright color inhibited hypermelanosis with high MCH mRNA activity, but at more than 200 % PCA did not inhibit malpigmentation, and the fish showed low MCH mRNA activity, indicating that the inhibitory effect of a bright background color on hypermelanosis is density dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olive flounder, Paralichthys olivaceus, is a heterosomatic pleuronectiformes. This fish is bilaterally symmetrical after hatching but becomes laterally asymmetrical after metamorphosis in the wild. The epidermal skin is asymmetrically pigmented only on the eyed side at the peak of metamorphosis (Seikai et al. 1987; Seikai 1992); the non-eyed (blind) side remains unpigmented because of the consistent cytolysis of chromatoblasts. The high commercial value of olive flounder makes it a promising species for aquaculture and resource enhancement in Korea. Only two or three decades have passed since the domestication of olive flounder began in earnest in China, Japan, and Korea. However, domestication in artificial facilities limits the genetic or instinctive traits of the fish, altering innate physiological phenomena and even causing morphological malformations, such as blind-skin malpigmentation (Venizelos and Benetti 1999; Bolker and Hill 2000).
The blind-side malpigmentation (hypermelanosis) exhibited by domesticated flounders is characterized by an excess of pigment cells on the blind side (Haga et al. 2002). However, many questions remain unanswered, such as (1) is this symptom manifested in the same way on the ocular side, (2) what factors initiate this process, (3) what mechanism is involved in the manifestation, and 4) is it hereditary? This symptom is rarely observed in wild olive flounder (Tomiyama et al. 2008) but is very common in domesticated flounder that are reared at high densities (Yamanome et al. 2005). It is thought that ambicolored fish in pleuronectiformes are influenced by various environmental factors, including burring substratum (Ottesen and Strand 1996; Iwata and Kikuchi 1998), density (Takahashi 1994), nutrition (Haga et al. 2002), and light (Iwata and Kikuchi 1998). Therefore, it should be possible to inhibit this symptom by manipulating these factors. However, it is unclear which environmental factor is the most important, and we do not know whether it is possible to efficiently control these factors during seedling production in artificial hatcheries.
Yamanome et al. (2005), using barfin flounder, Verasper moseri, and Yamanome et al. (2005), using olive flounder, proposed that bright background colors (white or yellow) could help inhibit hypermelanosis in pleuronectiformes by increasing the expression of melanin-concentrating hormone (MCH) mRNA in the brain. MCH, a cyclic peptide synthesized in the hypothalamus, consists of 17 amino acids and is related to physiological color changes through the aggregation of melanin granules (Kawauchi 1989; Kishida et al. 1989; Suzuki et al. 1995) as a functional antagonist of α-MSH (Baker 1993). Although a great deal of research has been conducted on the relationship between MCH and physiological color changes in fishes, few studies (Sugimoto 2002; Leclercq et al. 2010) have focused on the relationship between morphological color change and MCH activity. In addition, it is not yet clear whether morphological color changes, such as the differentiation and apoptosis of pigment cells in the skin, are affected by MCH. In flounder, hypermelanosis on the blind side, which is a morphological color change, can be inhibited by presenting a bright background color (Takahashi et al. 2004, 2007; Yamanome et al. 2005; Amiya et al. 2005, 2008), suggesting that endogenous MCH inhibits blind-side hypermelanosis in pleuronectiformes. However, because these studies on the effects of background color on hypermelanosis in pleuronectiformes were carried out in laboratory settings at low fish densities, we investigated whether bright background colors can be used as an inhibiting method in commercial hatcheries where fish are cultured at ultra-high densities.
Also, MCH is considered to be among the most downstream peptidergic neurons involved in the chain of hypothalamic signals that regulate food intake and energy homeostasis (Lin et al. 2000; Shi 2004). This hormone acts in the regulation of food intake and energy homeostasis, just as the two peptides are mutually antagonistic on pigment cells in fish (Pritchard et al. 2002). Initial observations of the relationship of MCH with food intake were made using rats and mice (Qu et al. 1996; Della-Zuana et al. 2002; Abbott et al. 2003; Ito et al. 2003), but, recently, a series of studies have established the role of MCH signaling in the regulation of food intake and energy balance in fish (Takahashi et al. 2004; Matsuda et al. 2006). However, although the effects of MCH on food intake and feeding behavior in fish have been intensively studied, its effect on skin melanophore production remains unexplored.
In the present study, we evaluated the possible relationships between density and background color with respect to stress response, appetite, growth, and blind-side hypermelanosis in olive flounder, Paralichthys olivaceus, in high-density farming. We also investigated the relevance of endogenous MCH to blind-side hypermelanosis and food intake in domesticated olive flounder.
Materials and methods
Experimental fish and rearing conditions
The experimental fish used in this study were 55th-day fry hatched from eggs from captive broodstock spawn in the hatching facility of the West Sea Fisheries Research Institute, NFRDI, Incheon, Republic of Korea. The fish were reared in gray, 20-m3 concrete tanks at a density of 200 fish/m2 (or fish/m3) using flowing natural seawater (mean temperature 18.3 ± 2.4 °C, salinity 30.7 ± 0.4 ‰, and dissolved oxygen [DO] 7.1 ± 0.5 mg/l) with artificial and natural lighting. To assess the effects of background color on hypermelanosis on the blind side in olive flounder, 800 size-matched fry (total length [TL] 4.5 ± 0.1 cm, body weight [BW] 0.77 ± 0.03 g) that were not entirely stained on the blind side were selected. The fry were randomly divided into two groups to create densities of 200 fish/m2 (percentage of coverage area, PCA, 40.6 ± 0.2 %: % = mean body surface area × fish number/base surface area; total biomass, 0.15 ± 0.01 kg/ton) per tank and were transferred in duplicate to 1,000-l fiberglass-reinforced aquaria that were either dark-green (control group) or white (white group) plastic. To prevent creating environmental stress for the fish, we reared the fish for 120 days with gentle aeration in indoor flow-through systems supplied with filtered seawater (onefold to fivefold water exchange rate per day depending on the biomass). We also fed the fish twice a day and cleaned the flat bottom of each tank by siphoning for 30 min after each feeding. The water in each tank was cleaned once a week by a complete exchange of seawater. The average water temperature was maintained at 19.9 ± 4.2 °C, with 30.4 ± 1.1 ‰ salinity and 7.7 ± 0.1 mg/l DO. The aquaria were exposed to artificial and natural lighting (2,000 lux, 12L/12D).
Rearing performance
Except on the sampling day, we fed the fry twice each day (10:00 and 16:00) to satiation (until most fish did not show feeding behaviors) with a diet of commercial pellets (SCF, Incheon, Korea; Table 1). The fish were not fed 24 h prior to sampling. The mass of food fed to each group was recorded, and the mass of food consumed was calculated using the following formula: food intake = total mass of fed diet—total mass of siphoned uneaten diet. On the last day, daily food intake (DFI: total mass of diet fed/[days × fish], mg/fish/day) and the food conversion ratio (FCE: body mass gain/mass of diet fed × 100, %) were determined. We kept records of daily mortalities and calculated the survival rate of each group. TL and BW of each animal were measured at 0, 60 (TL 10.5 ± 0.3 cm, BW 11.7 ± 0.7 g), and 120 days (TL 20.8 ± 0.5 cm, BW 91.7 ± 6.5 g) using a digimatic vernier calliper (CD-12”PSX; Mitytoyo, Kawasaki, Japan) and an electronic balance (DE/EW 4200; Kern & Sohn, Balingen, Germany), respectively. Condition factor (CF = final mass/(total length)3 × 103) was also calculated.
To assess the progression of abnormal pigmentation on the blind side, 30 flounder were randomly collected from each tank (n = 60 fish per group) at 0, 60, and 120 days using a net. The fish were euthanized after anesthesia for 10 min with 2-phenoxyethanol (1: 1,000 dilution, 0.3–0.4 mg/l). We rinsed the fish in distilled water to remove any salt and then preserved them in 5 % formalin. The blind side of the sampled flounder was photographed with a digital camera (Dimage A2; Konica Minolta, Tokyo, Japan). The ratio of the pigmented blind area (% = pigmented area/total blind area × 100) was calculated using a micro-imaging analysis system (QWin; Leica, Solms, Germany).
Because malpigmentation on the blind side is obvious using the naked eye when greater than 1 %, individuals with >1 % pigmentation were defined as ambicolored fish and were counted in each sample to calculate the proportion of ambicolored fish (% = ambicolored fish/total fish × 100).
Stress response
To assess differences between the dark-green and white background treatments with respect to stress responses, we selected size-matched flounders (mean TL 20.8 ± 0.5 cm, mean BW 91.7 ± 6.5 g) from the two groups at 120 days. The fish (n = 10/group) were not fed for 24 h prior to sampling. The fish were taken from the tanks and anesthetized with 2-phenoxyethanol, and blood was collected from 10 fish per group in 5-ml syringes coated with ammonium heparin. After centrifugation for 5 min at 12,000 rpm and 4 °C, plasma was collected for measurement of plasma glucose (GLU) and cortisol concentrations. Separate aliquots were frozen for each measurement. Total GLU was measured (n = 20) using the Fuji Dri-Chem 3500i system (Fujifilm, Tokyo, Japan) and commercial kits (Fujifilm). The GLU kit, used according to the manufacturer’s instructions, yielded measurement ranges of 10–600 mg/dl. The intra- and inter-assay coefficients of variation for GLU were 3.1 (n = 10) and 3.5 (n = 10), respectively. The total amount of cortisol in the heparinized plasma was measured (n = 10) after running a parallelism test by time-resolved fluoroimmunoassay (TR-FIA) using commercially available FIA kits (PerkinElmer, Turku, Finland). The FIA kit for cortisol was used according to the manufacturer’s instructions based on competition between plasma hormones and EU-labeled hormone tracers for binding to a specific anti-hormone antibody coated onto the well. The intra- and inter-assay coefficients of variation for cortisol were 4.31 (n = 10) and 3.64 (n = 10), respectively.
Prepro-MCH mRNA expression
To compare the effect of background color on MCH mRNA activity in the brain, we performed real-time qPCR using whole brains from juvenile olive flounder collected on days 60 and 120. Ten individuals were randomly collected from each group at 60 (TL 10.5 ± 0.3 cm, BW 11.7 ± 0.7 g) and 120 days (TL 20.8 ± 0.5 cm, BW 91.7 ± 6.5 g) using a net and were euthanized after anesthesia for 10 min with 2-phenoxyethanol (1:1,000 dilution, 0.3–0.4 mg/l). We rinsed the fish in distilled water to remove any salt and then sampled the whole brains. The brains were immersed in liquid nitrogen (−180 °C) immediately after extraction and stored at −75 °C until total RNA extraction. A total of 1 μg of total RNA from each brain was used for reverse transcription with an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Primers were designed from the sequence for olive flounder MCH (GenBank accession no. EU232720). The sequence of the forward primer [5′-TACCCATGGCCAAGACTGAA-3′] was based on nucleotides 152–171; the sequence of the reverse primer [5′-CTCCTCAGGATGGGGATGTT-3′] was based on nucleotides 412–431. For real-time PCR, we used IQ SYBR Green Supermix (Bio-Rad) with β-actin as a housekeeping gene. The mixture (10 μl) was transferred to a 0.2-ml thin-walled tube with 8 μl sterile water, 0.5 μl (250 nM) of each primer, and 1 μl cDNA. The mixture (20 μl) was then placed in a MiniOpticon Real-Time PCR Detection System (Bio-Rad). The experimental protocol was as follows: an initial period of 3 min at 95 °C for iTaq DNA polymerase activation followed by 50 cycles of 15 s at 95 °C, 20 s at 60 °C, and 30 s at 72 °C. We verified the specificity of the fluorescent signal after each run using melting curve analysis. The amount of fluorescence produced by SYBR Green in the reaction at the completion of each PCR cycle was recorded by a computer program (MJ Opticon Monitor 3.1.32; Bio-Rad). These data were used to produce real-time amplification curves for prepro-MCH by plotting the ∆ normalized reporter against the cycle number. Cycle threshold (cT) values, the cycle at which a statistically significant increase in reported fluorescence could be detected above the baseline (set at ∆ Rn = 0.2), were determined for prepro-MCH. The cT values were applied to the comparative cT method described in the User Bulletin (Bio-Rad) for relative quantification. We evaluated β-actin mRNA expression in each product as a control. The expression levels of the two tissues were normalized with respect to the β-actin signal and expressed as relative expression levels. The intra- and inter-assay coefficients of variation for pre-pro MCH were 6.1 (n = 10) and 5.5 (n = 10), respectively.
Statistical analyses
To determine differences between the mean values from each group, we used Student’s t tests (significant p value = 0.05) for TL, BW, the ratio of the pigmented blind area, and the proportion of ambicolored fish (n = 60/group). We used Mann–Whitney U tests (significant p value = 0.05) for DFI, FCE, survival (n = 2/group), cortisol, glucose, and MCH mRNA activity (n = 10/group). All tests were conducted using SPSS 7.0 K (IBM Corp., Armonk, NY, USA).
Results
Appetite, survival, and growth
DFI was significantly higher in fish reared with a white (0.64 ± 0.06 g/day) compared to a dark-green background (0.56 ± 0.05 g/day; p < 0.05). FCE and survival were not significantly different between the two groups (Table 2). Growth in all fish increased in both groups with time. However, on day 60, we did not find a significant difference in growth between the groups. Nevertheless, the fish in the white group were significantly heavier from day 90 onward and were significantly longer on day 120 than those in the dark-green group (p < 0.05; Fig. 1). The CF of the bright background group was significantly lower than that of the dark-green background group (p < 0.05; Table 2).
Stress response and pMCH mRNA activity
As for the stress level of the flounder, plasma cortisol was significantly higher in the white than in the dark-green treatment at both 60 and 120 days, as was GLU (p < 0.05; Fig. 2). Cortisol and glucose levels increased with time during the experimental period in both groups. Also, in both groups, stress was significantly higher after 120 days than on day 60 (p < 0.05; Fig. 2). However, during the entire experimental period, cortisol and glucose levels remained below 20 ng/ml and 40 mg/dl, respectively.
After 60 days of rearing, the relative expression of prepro-MCH mRNA compared with β-actin was significantly higher in the white group than in the dark-green group (p < 0.05). However, after 120 days of rearing, the relative activity of prepro-MCH mRNA compared with β-actin was significantly higher in the dark-green group than in the white group (p < 0.05; Fig. 3). Also, MCH mRNA activation decreased in both groups with time during the experimental period, and activation at 120 days in both groups was significantly lower than on day 60 (p < 0.05; Fig. 3).
Relative activities of prepro-MCH mRNA of olive flounders, Paralichthys olivaceus reared in dark-green and white background aquariums for 60 and 120 rearing days. One microgram of total RNA prepared from the brain was reverse transcribed and amplified using olive flounder MCH-specific primer by real-time PCR. We evaluated the expression of β-actin mRNA in each reaction product as a control. The expression level of two tissues was normalized with respect to the β-actin signal and expressed as relative expression level. Each value represents the mean ± SE. Statistical significance test was determined with a Mann–Whitney U test (n = 10; p value = 0.05)
Morphological features and ratios of blind-side malpigmentation
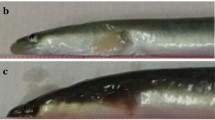
Blind-side skin features in both groups on days 60 (a) and 120 (b) are shown in Fig. 4. At 60 days in the dark-green group, the blind-side color was a distinctive white, but black pigmented spots composed of melanophores were evident and widespread around the trunk next to the rear dorsal fins, eye vestiges, and anus on the blind side of many fish. However, in the white group, black spots appeared only around the anus and the eye vestiges on the blind side of a few fish (Fig. 4a). On day 120 in all fish groups, the spots and pigmented area on the blind side had darkened and increased dramatically compared to day 60, and black spots were found around the pectoral fin and front trunk beside the dorsal fins, anus, and eye vestiges on the blind side (Fig. 4b).
The ratio of the pigmented blind-side area (starting value 0.04 ± 0.052 %) increased in both treatments by day 60, at which time the ratio was higher in the dark-green (4.44 ± 0.47 %) tank than in the white (0.37 ± 0.05 %) tank (p < 0.05). However, the ratios increased significantly in both groups from 60 to 120 days, during a period of rapid growth. On day 120, although the area ratio was higher with the white background (43.73 ± 1.84 %) than the dark-green background (38.69 ± 0.61 %), the ratio was not significantly different between the groups (p > 0.05; Fig. 5). The prevalence of ambicolored fish, none of which were observed at the start of the experiment, had increased in both groups by day 60 and was significantly higher with the dark-green (78.4 ± 1.2 %) than with the white background (23.4 ± 4.7 %) (p < 0.05). However, by day 120, the ratio in the white tank group had increased significantly; consequently, the ratio was not significantly different between the groups. In both groups, 100 % of the fish were ambicolored (Fig. 5).
Discussion
During our experiment, FCE and survival did not vary with background color. However, although the bright background caused a reduction in CF, possibly arising from an increase in skeletal growth relative to body weight increase, the white-group flounders ate more and grew faster than the fish in the dark-green group. This is consistent with the results of Takahashi et al. (2004), Amiya et al. (2005, 2008), and Yamanome et al. (2005, 2007), who examined the effects of background color on growth in flounder, which are heterosomatic teleosts. The effects of bright background colors on growth have also been reported in symmetric teleosts, including Atlantic salmon (McCormick et al. 1998); scaled carp (Papoutsoglou et al. 2000); red seabream, Pagrus pagrus (Rotllanta et al. 2003); Atlantic cod, Gadus morhua (Monk et al. 2008); and jundiá, Rhamdia quelen (Barcellos et al. 2009). Previous studies in mammals (Schwartz et al. 2000; Kauffman and Rissman 2004) and fishes (Matsuda et al. 2006; Amiya et al. 2008) have assumed that this effect is the result of complex interactions between various neural-hormonal responses (corticotrophin-releasing factor [CRF], cortisol, MCH, neuropeptide Y [NPY], proopiomelanocortin, etc.) that occur when bright color information is transmitted to the brain and that these interactions affect biochemical processes related to metabolism.
For fish growth, in particular, cortisol is recognized as one of the most critical substances involved in evaluating individual well-being (Pickering 1993; Bonga 1997; Papoutsoglou et al. 2000). Many studies have reported that cortisol can reduce fish growth through interactions with growth hormone, gonadal hormones, and others (Farbridge and Leatherland 1992; Foo and Lam 1993; Papoutsoglou et al. 2000). Papoutsoglou et al. (2000) proposed correlations among tank color, cortisol, and growth in carp. Background color can cause either positive or negative stress effects on food consumption and overall energy metabolism in teleosts by accelerating the synthesis of endogenous cortisol (Farbridge and Leatherland 1992; Foo and Lam 1993; Papoutsoglou 1998; Bernier et al. 2004). Cortisol in the blood is synthesized by outside factors, and when it is excessively released into the blood, it can negatively affect growth in teleosts despite normal food intake (De Boeck et al. 2001; Bernier et al. 2004). However, moderate releases and low cortisol levels can slowly promote food consumption by stimulating NPY (Bernier et al. 2004), inhibiting CRF (Cavagnini et al. 2000), inhibiting prolactin, and causing the release of growth hormone (Uchida et al. 2004) in several birds, mammals, and fishes. In the present study, although we did not find a serious stress response of flounder to background color, fish living among a white background showed significantly higher cortisol levels for the entire period, ate more, and grew faster than fish living among a dark-green background. The high appetite and faster growth rate of flatfish on a bright background was probably caused by the moderately high doses of cortisol in their blood (<20 ng/ml) due to the bright background color. Thus, bright colors and cortisol may have positive effects on the food consumption and growth of fish.
Growth performance could also be related to MCH interactions with growth, gonadal, and other hormones. In mammalian MCH, a functional antagonist of α-MSH plays a major role in the control of feeding behavior and energy homeostasis as an orexigenic molecule (Qu et al. 1996; Lin et al. 2000; Shi 2004). In teleosts, the role of MCH in appetite control has only recently been reported, but several studies have verified (Takahashi et al. 2004, Kawauchi and Baker 2004; Kawauchi 2006) that the hormone is also involved in improving appetite in fish. This appetite stimulation may follow a similar pathway as in mammals. In addition, in rainbow trout (Kishida et al. 1989; Suzuki et al. 1995) and tilapia (Gröneveld et al. 1995), MCH expression in the brain and in blood plasma is more active and growth is accelerated in white tanks compared to black tanks. These observations suggest that the MCH stimulated in the brain by white backgrounds enhances appetite and, in turn, ultimately accelerates growth in fish. This assumption is supported by Takahashi et al. (2004) who found that fasted flounder kept in black-colored tanks had greater numbers of detectable MCH-immunoreactive cell bodies in the hypothalamus than fed control animals; moreover, the level of MCH expression in the brains of the starved flounder was twofold greater than that in the control group. These observations support the hypothesis that an MCH signaling system is involved in regulating food intake in teleosts. Such a role might explain the markedly increased development of hypothalamic MCH neurons in lampreys following metamorphosis (Bird et al. 2001), when filter feeding is replaced by the ingestion of more abundant solid food. In addition, this hypothesis has been supported by the studies of Amiya et al. (2005) and Yamanome et al. (2005), in which barfin flounder reared in a bright-colored tank showed increased MCH mRNA expression in the brain (Amiya et al. 2005), more observable MCH-immunoreactive cell bodies in the hypothalamus (Yamanome et al. 2005), and faster growth than did individuals in a dark-colored tank, suggesting that MCH signaling is related to feeding control in pleuronectiformes. However, a few studies have reported that MCH may suppress food intake as an anorexigenic factor or may not play a critical role in the regulation of feeding in fish (Matsuda et al. 2006; Shimakura et al. 2008). Indeed, we did not find a distinct relationship between MCH and appetite (growth) in olive flounder cultured at a high density. Although appetite and growth were more accelerated in the white tank than in the dark-green one, significantly higher MCH activity was found in the white tank at 60 days and in the dark-green tank at 120 days, suggesting that MCH does not act as an orexigenic factor in cultured olive flounder. However, considering the type of MCH we studied, we cannot exclude the relevance of MCH to appetite in fish. Recently, Berman et al. (2009) found that zebrafish genomes encode two distinct MCH peptides, one (MCH1) similar or identical to salmonid MCH and the other (MCH2) bearing a striking resemblance to mammalian MCH. These two hormones take functional charge of different roles in the body: MCH 1 participates in skin pigmentation, whereas MCH 2 activates appetite. Indeed, MCH1 has been reported to regulate physiological color change in fishes (Kishida et al. 1989; Suzuki et al. 1995; Kawauchi 2006) and MCH 2 may be involved in food intake (behavior) and energy homeostasis in mammals (Lin et al. 2000; Pritchard et al. 2002; Shi 2004; Pissios et al. 2006). Therefore, we do not rule out the possibility that flounders have two forms of precursors that mature. As the MCH that we surveyed was the salmonid type, we hypothesize that the MCH in the present study participated only in skin color change, but did not act as an orexigenic peptide. Although MCH has been intensively studied for its role in appetite in mammals, the action of MCH on food intake and feeding behavior in fishes remains to be explored.
Our test results indicate that blind-side hypermelanosis develops with increasing total biomass (or PCA) among fish growing in a limited space, indicating that the increasing density caused by growth results in the spread and aggravation of hypermelanosis in both bright- and dark-background groups. The acceleration of bilateral body pigmentation of olive flounders induced by high density is partially consistent with results reported by Takahashi (1994) that suggested that abnormal pigmentation increases with stocking density. The apparent influence of density on the speed of malpigmentation on the blind side of flatfish may be due to a stress stimulus induced by increased PCA (crowding). Also, our results indicate that a bright background color fully inhibits blind-side hypermelanosis in olive flounder at low density under 200 % PCA but that the inhibitory ability of the bright background color is diminished by fish growth (or increased PCA), with relatively low and decreasing MCH mRNA expression in brain. Thus, we conclusively determined that blind-side hypermelanosis of flounder is density-dependent.
The background color of the living space is an important factor in determining the skin color of symmetrical teleosts (Fujii 2000; Papoutsoglou et al. 2000; Sugimoto 2002; Doolan et al. 2007, 2009; Leclercq et al. 2010). In asymmetrical flatfishes, the living space background color affects both skin color formation on the eyed side and the differentiation of pigment cells on the blind side. Amiya et al. (2005) and Yamanome et al. (2005, 2007) showed that, when flounders are reared in laboratory tanks at low densities, bright-colored tanks suppress hypermelanosis on the blind side by increasing MCH mRNA expression. However, the results of our study with juvenile olive flounder demonstrate that a bright background color did not continually inhibit blind-side hypermelanosis by increasing MCH mRNA expression. During the first 60 days of the experiment (under 200 % PCA), the bright background color suppressed blind-side pigmentation by increasing MCH mRNA expression, as shown by Yamanome et al. (2005, 2007), but the effect was not maintained until the end of the experiment (after an additional 60 days), as shown in Figs. 2, 3, and 5. However, the relative activity of MCH mRNA was consistently higher in the white group at 60 days and in the dark-green group at 120 days, which showed a low ratio of staining area on the blind side. This suggests that the expression of MCH in the brain of olive flounder is related to the inhibition of blind-side pigmentation, as was found in previous studies (Amiya et al. 2005; Yamanome et al. 2005). Our results also indicate that a relatively high biomass (density) following fast growth in the white group accelerated malpigmentation on the blind side of the flounder during the final 60 days of the study (Takahashi et al. 2004). Also, in the present study, the pigmented area on the blind side expanded with biomass and increased with time throughout the experimental period in all fish in both groups. We speculate that this is the result of increasing pigmentation on the blind side because of decreasing MCH activity to protect individuals from more frequent contact with other fish, as the density increased in each treatment. Additionally, by comparing our results with those of Yamanome et al. (2005, 2007), we found that the number of fish and total biomass per unit of bottom area was far higher in the present study than in their studies, which could account for the different results. Yamanome et al. (2005, 2007) showed that a bright background color suppressed aberration in cultivated juvenile barfin flounder (TL 8.5 ± 0.1 cm and BW 8.5 ± 0.4 g) and olive flounder (TL 5.6 ± 0.1 cm and BW 1.6 ± 0.1 g), but these flatfish were reared at a density of 15 fish/0.5 m3 for 9 and 5 months, respectively. In the present study, we cultivated juvenile olive flounder (TL 4.46 ± 0.06 cm, BW 0.77 ± 0.03 g) for 4 months at a high density of 200 fish/m3, which is similar to the rearing densities in commercial culture facilities. Takahashi (1994) and Kang et al. (2011) showed that density is a key factor in blind-side pigmentation in flatfish. Therefore, we hypothesize that the main reason for the difference between our results and those of Yamanome et al. (2005, 2007) is the difference in population density.
In conclusion, although a bright tank background color failed to induce strong stress of more than 20 ng/ml of cortisol and 40 mg/dl of glucose in flounder, we determined that a rearing tank with a bright background had a positive effect on appetite and growth, but did not effectively inhibit blind-side hypermelanosis of flounder at a commercially high density. Malpigmentation was inhibited by a bright background color during the initial 60 days, but the inhibitory action of the bright background vanished when self-protection from more frequent contact with other fish increased with density after 60 days. Therefore, we hypothesize that the bright background color did not affect hypermelanosis on its own. Instead, we partly attribute the effect to the density increase resulting from growth in body size and suggest that background color is not the only factor inducing hypermelanosis; rather, it is a subsidiary factor, and more than one environmental factor induces hypermelanosis in flounder. In addition, we found that a bright background significantly increased the expression of MCH mRNA in the brain during the initial 60 days, when the population was at a relatively low density, but that the effect of MCH mRNA activity was offset by increasing density following somatic growth during the final 60 days, indicating that MCH is not directly related to appetite and growth, but may be relevant to malpigmentation on the blind side in this teleost. Future studies of hypermelanosis in olive flounder should consider various environmental factors, including population density, tank bottom type, and light. In addition, experiments using exogenous MCH should be conducted to determine its effects on cultured olive flounder.
References
Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ (2003) Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology 44:3943–3949
Amiya N, Amano M, Takahashi A, Yamanome T, Kawauchi H, Yamamori K (2005) Effects of tank color on melanin-concentrating hormone levels in the brain, pituitary gland and plasma of the barfin flounder as revealed by a newly developed time-resolved fluoroimmunoassay. Gen Comp Endocrinol 143:252–256
Amiya N, Amano M, Yamanome T, Yamamori K, Takahashi A (2008) Effects of background color on GnRH and MCH levels in the barfin flounder brain. Gen Comp Endocrinol 155:88–93
Baker BI (1993) The role of melanin-concentrating hormone in colour change. Ann NY Acad Sci 680:279–289
Barcellos LJG, Kreutz LC, Quevedo RM, da Rosa JGS, Koakoski G, Centenaro L, Pottker E (2009) Influence of color background and shelter availability on jundiá (Rhamdia quelen) stress response. Aquaculture 288:51–56
Berman JR, Skarish G, Maro GS, Mignot E, Mourraini P (2009) Characterization of two melanin-concentrating hormone genes in zebrafish reveals evolutionary and physiological links with the mammalian MCH system. J Comp Neurol 517:695–710
Bernier NJ, Bedard N, Peter RE (2004) Effects of cortisol on food intake, growth, and forebrain neuropeptide Y and corticotropin-releasing factor gene expression in goldfish. Gen Com Endocrinol 135:230–240
Bird DJ, Potter IC, Sower SA, Baker BI (2001) The distribution of melanin concentrating hormone in the lamprey brain. Gen Comp Endocrinol 121:232–241
Bolker JA, Hill CR (2000) Pigmentation development in hatchery-reared flatfishes. J Fish Biol 56:1029–1052
Bonga SEW (1997) The stress response in fish. Physiol Rev 77:591–625
Cavagnini F, Croci M, Putignano P, Petroni ML, Invitti C (2000) Glucocorticoids and neuroendocrine function. Int J Obes 24:S77–S79
De Boeck G, Alsop D, Wood C (2001) Cortisol effects on aerobic and anaerobic metabolism, nitrogen excretion, and whole-body composition in juvenile rainbow trout. Physiol Biochem Zool 74:858–868
Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N (2002) Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obes Relat Metab Disord 26:1289–1295
Doolan BJ, Booth MA, Jones PL, Allan GL (2007) Effect of cage colour and light environment on the skin colour of Australian snapper Pagrus auratus (Bloch, Schneider, 1801). Aquac Res 38:1395–1403
Doolan BJ, Booth MA, Allan GL, Jones PL (2009) Changes in skin colour and cortisol response of Australian snapper Pagrus auratus (Bloch, Schneider, 1801) to different background colours. Aquac Res 40:542–550
Farbridge KJ, Leatherland JF (1992) Plasma growth hormone levels in fed and fasted rainbow trout (Oncorhynchus mykiss) are decreased following handling stress. Fish Physiol Biochem 10:67–73
Foo JTW, Lam TJ (1993) Serum cortisol response to handling stress and the effect of cortisol implantation on testosterone level in the tilapia Oreochromis mossambicus. Aquaculture 115:145–158
Fujii R (2000) The regulation of motile activity in fish chromatophores. Pigment Cell Res 13:300–319
Gröneveld D, Eckhardt ER, Coenen AJ, Martens GJ, Balm PH, Wendelaar Bonga SE (1995) Expression of tilapia prepromelanin-concentrating hormone mRNA in hypothalamic and neurohypophysial cells. J Mol Endocrinol 14:199–207
Haga Y, Takeuchi T, Seikai T (2002) Influence of all-trans retinoic acid on pigmentation and skeletal formation in larval Japanese flounder. Fish Sci 68:560–570
Ito M, Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H (2003) Chronic intracerebro-ventricular infusion of MCH causes obesity in mice. Am J Physiol Endocrinol Metab 284:E583–E588
Iwata N, Kikuchi K (1998) Effect of sandy substrate and light on hypermelanosis of the blind side in cultured Japanese flounder, Paralichthys olivaceus. Environ Biol Fish 52:291–297
Kang DY, Kim HC, Chang YJ (2011) Effects of stocking density on the blind-side hypermelanosis of cultured flounder, Paralichthys olivaceus. Fish Aquat Sci 14:135–141
Kauffman AS, Rissman EF (2004) The evolutionarily conserved gonadotropin-releasing hormone II modifies food intake. Endocrinology 145:686–691
Kawauchi H (1989) Melanin concentrating hormone II structure and biosynthesis of melanin-concentrating hormone. Life Sci 45:1133–1140
Kawauchi H (2006) Functions of melanin-concentrating hormone in fish. J Exp Zool 305A:751–760
Kawauchi H, Baker BI (2004) Melanin-concentrating hormone signaling systems in fish. Peptides 25:1577–1584
Kishida M, Baker BI, Eberle AN (1989) The measurement of melanin-concentrating hormone in trout blood. Gen Comp Endocrinol 74:221–229
Leclercq E, Taylor JF, Migaud H (2010) Morphological skin colour changes in teleosts. Fish Fish 11:159–193
Lin X, Volkoff H, Narnaware Y, Bernier NJ, Peyon P, Peter RE (2000) Brain regulation of feeding behavior and food intake in fish. Com Biochem Physiol A 126:415–434
Matsuda K, Shimakura SI, Maruyama K, Miura T, Uchiyama M, Kawauchi H, Shioda S, Takahashi A (2006) Central administration of melanin-concentrating hormone (MCH) suppresses food intake, but not locomotor activity, in the goldfish, Carassius auratus. Neurosci Lett 399:259–263
McCormick SD, Shrimpton JM, Carey JB, O’Dea MF, Sloan KE, Moriyama S, Björnsson BTh (1998) Repeated acute stress reduces growth rate of Atlantic salmon parr and alters plasma levels of growth hormone, insulin-like growth factor I and cortisol. Aquaculture 168:221–235
Monk J, Puvanendran V, Brown JA (2008) Does different aquarium bottom colour affect the growth, survival and foraging behaviour of Atlantic cod (Gadus morhua) larvae? Aquaculture 277:197–202
Ottesen OH, Strand HK (1996) Growth, development, and skin abnormalities of halibut (Hippoglossus hippoglossus L) juveniles kept on different bottom substrates. Aquaculture 146:17–25
Papoutsoglou SE (1998) In: Endocrinology of fishes. Stamoulis Press, Athens, p 599 (in Greek)
Papoutsoglou SE, Mylonakis G, Miliou H, Karakatsouli NP, Chadio S (2000) Effects of background color on growth performances and physiological responses of scaled carp (Cyprinus carpio L) reared in a closed circulated system. Aquac Eng 22:309–318
Pickering AD (1993) Growth and stress in fish production. Aquaculture 111:51–63
Pissios P, Bradley RL, Maratos-Flier E (2006) Expanding the scales: the multiple roles of MCH in regulating energy balance and other biological functions. Endocrinol Rev 27:606–620
Pritchard LE, Turnbull AV, White A (2002) Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signaling and obesity. J Endocrinol 172:411–421
Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E (1996) A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380:243–247
Rotllanta J, Tort L, Montero D, Pavlidisd M, Martinez M, Bongae SEW, Balme PHM (2003) Background colour influence on the stress response in cultured red porgy Pagrus pagrus. Aquaculture 223:129–139
Schwartz MW, Woods SC, Porte DJ, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404:661–671
Seikai T (1992) Process of pigment cell differentiation in skin on the left and right side of the Japanese flounder, Paralichthys olivaceus, during metamorphosis. Jpn J Ichthyol 29:85–92
Seikai T, Matsumoto J, Shimozaki M, Oikawa A, Akiyama T (1987) An association of melanophores appearing at metamorphosis as vehicles of asymmetric skin color formation with pigment anomalies developed under hatchery conditions in the Japanese flounder, Paralichthys olivaceus. Pigment Cell Res 1:143–151
Shi Y (2004) Beyond skin color: emerging roles of melaninconcentrating hormone in energy homeostasis and other physiological functions. Peptides 25:1605–1611
Shimakura S-I, Miura T, Maruyama K, Nakamachi T, Uchiyama M, Kageyama H, Shioda S, Takahashi A, Matsuda K (2008) α-Melanocyte-stimulating hormone mediates melanin-concentrating hormone-induced anorexigenic action in goldfish. Horm Behav 53:323–328
Sugimoto M (2002) Morphological color changes in fish: regulation of pigment cell density and morphology. Microsc Res Tech 58:496–503
Suzuki M, Narnaware YK, Baker BI, Levy A (1995) Influence of environmental colour and diurnal phase on MCH gene expression in the trout. J Neuroendocrinol 7:319–328
Takahashi Y (1994) Influence of stocking density and food at late phase of larval period on hypermelanosis on the blind body side in juvenile Japanese flounder. Nippon Suisan Gakkaishi 60:593–598
Takahashi A, Tsuchiya K, Yamanome T, Amano M, Yasuda A, Yamamori K, Kawauchi H (2004) Possible involvement of melanin-concentrating hormone in food intake in a teleost fish, barfin flounder. Peptides 25:1613–1622
Takahashi A, Kosugi T, Kobayashi Y, Yamanome T, Schioth HB, Kawauchi H (2007) The melanin-concentrating hormone receptor 2 (MCH-R2) mediates the effect of MCH to control body color for background adaptation in the barfin flounder. Gen Comp Endocrinol 151:210–219
Tomiyama T, Mizuno T, Watanabe M, Fujita T, Kawata G (2008) Patterns and frequency of hypermelanosis on the blind side in wild Japanese flounder. Nippon Suisan Gakkaishi 74:171–176
Uchida K, Yoshikawa-Ebesu JSM, Kajimura S, Yada T, Hirano T, Graua EG (2004) In vitro effects of cortisol on the release and gene expression of prolactin and growth hormone in the tilapia, Oreochromis mossambicus. Gen Comp Endocrinol 135:116–125
Venizelos A, Benetti DD (1999) Pigment abnormalities in flatfish. Aquaculture 176:181–188
Yamanome T, Amano M, Takahashi A (2005) White background reduces the occurrence of staining, activates melanin-concentrating hormone and promotes somatic growth in barfin flounder. Aquaculture 244:323–329
Acknowledgments
This research was supported by the research project (Conservation and Restoration of Fish Species for Aquaculture [12-AQ-23], Contribution no. RP-2012-AQ-061) from NFRDI, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, DY., Kim, HC. Influence of density and background color to stress response, appetite, growth, and blind-side hypermelanosis of flounder, Paralichthys olivaceus . Fish Physiol Biochem 39, 221–232 (2013). https://doi.org/10.1007/s10695-012-9693-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9693-2