Abstract
Fish suffer from anemia and hypovolemic hypotensive shock after in vivo exposure with microcystins. However, except for in vivo causes for anemia and hypotension, an in vitro study of fish erythrocytes exposed to MC is necessary. For a better understanding of hematology toxicity of MC, the main aim of the present study was to investigate the toxic effects of microcystin on fish erythrocytes in vitro. Crucian carp erythrocytes were incubated in vitro with microcystin-LR (MC-LR) at doses of 0, 1, 10, 100 and 1,000 nM. The level of lipid peroxidate significantly increased in MC-LR treatment groups. Glutathione decreased after exposure to MC-LR. The activities of antioxidative enzymes, including superoxide dismutase, catalase, glutathione peroxidase and glutathione-S-transferase, were significantly increased after exposure with MC-LR. The hemolysis was significantly increased, while the activities of acetylcholinesterase, Na+–K+-ATPase and Ca2+–Mg2+-ATPase were significantly decreased. In addition, pathological alterations in agglomerated and jagged erythrocytes were observed in blood smears. The findings indicate that damages to erythrocytes should also be responsible for anemia and hypotensive shock or even death.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of toxic cyanobacterial blooms in eutrophic lakes has become a worldwide problem (Paerl et al. 2001). Microcystins (MCs), a group of cyclic heptapeptide compounds with specific hepatotoxins produced by cyanobacterial species, have received worldwide concern in these years (Cohen 1989). Up to now, more than 80 different structural analogues of MCs have been identified, and it was reported that MC-LR was the most toxic (Fastner et al. 2002). MCs have been already demonstrated as potent inhibitors of protein phosphatases 1 (PP1) and phosphatases 2A (PP2A) (Nishiwaki-Matsushima et al. 1992). MCs cause damage to different organs, and liver is the main target (Robinson et al. 1989). One of the most important toxic mechanisms of microcystin is attributed as oxidative damage (Prieto et al. 2006).
Fish can be easily exposed to the toxins either by feeding or passively when the toxins pass through gills during breathing (Malbrouck and Kestemont 2006). MCs exert profound impacts on fish including behavior, physiological and biochemical processes as well as embryonic development (Zhang et al. 2007, 2008; Šetlíková and Wiegand 2009). Fish can use the antioxidant defense system to reduce the impairment of the oxidative stress, including radical-scavenging enzymes, i.e., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GR). And glutathione (GSH) is also an important antioxidant to clean out radicals (Prieto et al. 2006, 2007).
Blood plays an important role in the transportation and excretion of MCs in animals. MCs can be transported via the bile acid transport system or via the blood to the kidneys for excretion when MCs are absorbed and distributed into different organs (Cazenave et al. 2006). Several studies have demonstrated that MCs exposure could result in fish hematological changes, anemia as well as hypotension (Zhang et al. 2007; Li et al. 2009). Erythrocyte has been one of the important cellular models for toxicological study under laboratory condition (Anna et al. 1999). Paulina et al. (2006) have observed antioxidative responses of human erythrocytes in vitro, and they confirmed that damages to erythrocytes may be the result of direct covalent binding of MC-LR with –SH residues of proteins and indirectly be related with reactive oxygen species formation.
Some previous studies in vivo in our laboratory revealed that fish was suffered from anemia and hypovolemic hypotensive shock when crucian carp was injected intraperitoneally (i.p.) with extracted MCs (Zhang et al. 2007; Li et al. 2009). Red blood cell (RBC) counts, hematocrit (Ht) and hemoglobin (Hb) were significantly decreased, indicating the appearance of normocytic anemia of crucian carp (Zhang et al. 2007). Li et al. (2009) observed decreased circulating blood volume (BV). However, the underlying mechanisms of anemia and hypovolemic hypotensive shock still remain puzzling. Anemia generally results from blood loss, decreased RBC production, kidney failure, poor RBC maturation or increased RBC destruction (Greenburg 1996). Therefore, except for in vivo causes for anemia and hypotension, an in vitro study of fish erythrocytes after exposure to MCs is greatly necessary. The main goal of the present study was to assess the toxic effects of MC-LR on crucian carp erythrocytes by examining antioxidative response and biochemical parameters, as well as hemolysis. Our present work would provide a better understanding of anemia, hypotensive shock and even death in fish treated with MCs.
Materials and methods
Toxin and chemicals
MC-LR was purchased from Express Technology Co., Ltd, China, with a purity of 97%. All reagents were bought from various commercial sources and were of analytical grade.
Fish and erythrocytes
Healthy crucian carp (mean body weight 255.0 ± 21.5 g) were purchased from the local fish farm in Wuhan City, China. The fish were allowed to acclimate for 1 week prior to experimentation and were fed with commercial fish food at a rate of 4.0% of body weight. Feeding was terminated 2 days before the experiment. Experiments were performed according to the guidelines of Ethical Committee for Animal Experiments at Huazhong Agricultural University, Wuhan of China. Then, whole blood was taken by caudal puncture with heparinized syringes. The erythrocytes were isolated by centrifuging at 3,000 rpm for 10 min at 4°C and washed three times with ten volumes of an isotonic buffered (pH = 7.4) NaCl solution. After careful removal of the leukocytes, erythrocytes were incubated (RBC = 2.6 × 107 cells/ml) in the sterile incubation medium (90.5 mM NaCl, 3 mM KCl, 1.3 mM CaCl2, 0.5 mM MgSO4, 6 mM glucose, 1 mM pyruvate and 1 mM Tris–HCl, pH = 7.4). Each batch of erythrocytes was exposed for 1, 3, 12, 24 and 48 h with 1, 10, 100 and 1,000 nM MC-LR in a shaker at the temperature of 15°C. Control erythrocytes were simultaneously incubated with sterile incubation medium without any MC-LR.
Determination of lipid peroxidation and antioxidative response
Malondialdehyde (MDA), an end product of lipid peroxidation, was determined by measuring the formation of thiobarbituric acid–reactive substances (TBARS) according to the method described by Stock and Dormandy (1971). One milliliter of a freshly prepared solution (thiobarbituric reactant solution) containing 15% trichloroacetic acid (w/v), 0.375% TBA (w/v) and 2.5% hydrochloric acid (v/v) was added to supernatants and placed in a boiling water bath for 15 min. After cooling, absorbance was measured on a spectrophotometer at 532 nm.
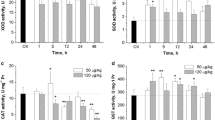
Total superoxide dismutase (SOD; EC 1.15.1.1) activity was measured using method as described by McCord and Fridovich (1969). The assay mixture contained 0.51 ml potassium phosphate–EDTA buffer (pH 7.8), 0.1 ml xanthine 50 μM, 0.1 ml cytochrome c 500 μM and 50 μl hemolysate. The reaction was initiated by the addition of 10 μl xanthine oxidase. SOD activity was determined spectrophotometrically at 505 nm. One unit of SOD activity is the amount of enzyme needed to inhibit 50% of cytochrome c reduction. Catalase (CAT; EC1.11.1.6) activity was measured according to the method of Aebi (1984). To 3 ml H2O2 (54 mM H2O2 in 50 mM phosphate buffer pH 7.0), 5 μl loaf catalase solution was added and the decrease in H2O2 was measured spectrophotometrically at 240 nm, at 25°C for 60 s. One unit of catalase activity was defined as the activity required to degrade 1 μmol hydrogen peroxide in 60 s. Glutathione peroxidase (GPx; EC 1.11.1.9) activity was assayed by method of Lawrence and Burk (1976), following the rate of NADPH oxidation at 340 nm by the coupled reaction with glutathione reductase. The assay mixture contained 600 μl buffer with 50 mM potassium phosphate + 1 mM EDTA + 1 mM NaN3 (pH 7.5), 100 μl 0.2 mM reduced glutathione (GSH), 100 μl 0.1 mM NADPH, 8 μl glutathione reductase and 20 μl hemolysate. After 5 min of preincubation (20–25°C), the reaction was initiated by the addition of 100 μl 0.25 mM H2O2. Glutathione-S-transferase (GST; EC2.5.1.18) activity was measured according to the method of Habig et al. (1974), using 1 mM glutathione (GSH), 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) and 0.1 M potassium phosphate buffer, (pH 6.5). The rate of increase in product concentration was monitored by measuring the absorbance at 340 nm at 25°C for 3–5 min in a spectrophotometer. Glutathione (GSH) in incubation solutions was determined according to the method described by Beutler (1975). Erythrocyte suspension was deproteinized by the addition of trichloroacetic acid (TCA) to a final concentration of 2%. To the supernatant (with glutathione) cleared by centrifugation, 5, 5′-dithio-bis (2-nitrobenzoic acid) (DTNB) was added to a final concentration of 0.47 mM. The formation of 5-thio-2-nitrobenzoic acid, which is proportional to the concentration of acid-soluble thiols mainly glutathione, was monitored at 25°C. The absorbance was measured at 412 nm in a spectrophotometer.
Measurement of biochemical analyses
Acetylcholinesterase (AChE) activity of erythrocytes was assayed by the method of Ellman et al. (1961). The assay mixture contained 0.45 mM ATCh as substrate and 0.3 mM DTNB as chromogen in 0.1 M phosphate buffer (pH 7.4) at 37°C. Na+–K+-ATPase and Ca2+–Mg2+-ATPase activities of cell membrane were determined by measuring the initial rate of release of Pi from ATP according to the method of Chauhan et al. (2002). The mixture contained 30 mM imidazole buffer (pH 7.0), membrane sample, 100 mM NaCl, 20 mM KCl, 3 mM MgCl2, 0.2 mM CaCl2, 0.1 mM EGTA and 0.1 mM ouabain. The enzymatic reaction was initiated by the addition of 3 mM ATP and stopped by the addition of 1 ml of 15% TCA 60 min later. The amount of inorganic phosphorus release was measured using KH2PO4 as a standard and determined at 660 nm. The protein content was determined by the method of Lowry et al. (1951). Hemoglobin in blood was estimated using Drabkin’s reagent by the method of Dacie and Lewis (1984).
Evaluation of hemolysis
The ratio of hemolysis was calculated from the equation:
The H% is the hemolysis of erythrocytes incubated with MC-LR; A pb is the absorbance of supernatant in all samples at 540 nm; A water is the absorbance of erythrocytes completely hemolysed in distilled water at 540 nm.
Observation of blood smears
At 12 h, blood smears were observed in 100- and 1,000-nM dose groups and control group. Air-dried smears were fixed for 1 min in methanol, stained with Wright–Giemsa for 1 min and washed twice in distilled water for 1 min. Blood smears were examined using an Olympus compound microscope at 400 total magnifications.
Statistical analysis
Every sample was measured in triplicates to get an average value for statistical use. All results expressed as mean ± SD were subjected to one-way analysis of variance (ANOVA) and Dunnett’s post-test using STATISTICA software package (Version 6.0, Statsoft, Inc.). Differences were measured against control values and considered to be statistically significant at P < 0.05.
Results
Lipid peroxidation and antioxidative response
Lipid peroxidation levels showed significant increases at 12 h in 100- and 1,000-nM dose groups and at 24 and 48 h in all treatment groups (Fig. 1).
In all MC-LR treatment groups, GSH levels significantly decreased at 12 and 24 h after MC-LR incubation. The GPx activities showed significant increases at 12 and 24 h after incubation of MC-LR in 1-, 10- and 100-nM dose groups. Enzymatic activity of GST exhibited marked increase at 12 h in 1, 10 and 100 nM, and prominent increases were observed at 24 and 48 h in all MC-LR treatment groups. CAT activity significantly increased at 12 and 24 h in 1-, 10- and 100-nM dose groups. The significant increases in SOD activity were observed at 3 h in 10- and 1,000-nM dose groups. After incubation at 12, 24 and 48 h, there were distinct increases in 1-, 10- and 100-nM dose groups (Fig. 2).
Biochemical determination
The activity of AChE significantly decreased at 12 h in 100-nM dose group and at 24 h in 10-, 100-, 1,000-nM dose groups, as well as 48 h in 100-nM dose group. The decrease in the activity of Ca2+–Mg2+-ATPase showed significant decreases at 12 and 24 h in 10-, 100- and 1,000-nM dose groups and at 48 h in 10- and 100-nM dose groups. The activity of Na+–K+-ATPase significantly decreased at 12 and 24 h in 10-, 100- and 1,000-nM dose groups and at 48 h in 100-nM dose group (Fig. 3).
Hemolysis examination
Hemolysis of fish erythrocytes is shown in Table 1. Compared with the control, significant increases were observed from 12 to 48 h in 10-, 100- and 1,000-nM dose groups.
Blood smear observation
Morphological changes were observed in erythrocytes at 12 h in 100- and 1,000-nM dose groups. Erythrocytes in control group exhibited round shape with pink-stained cytoplasm and central, oval nucleus (Fig. 4a). Significantly agglomerated erythrocytes were observed at 12 h in 100-nM group, with swelled, blebbing and notched cell membrane (Fig. 4b). Disrupted or dissolving cellular membrane was recorded at 12 h in 1,000-nM group, and some erythrocytes exhibited jagged margin (Fig. 4c).
Discussion
One previous study of our laboratory has demonstrated that MCs were detected mainly in blood, liver, gonad and kidney when crucian carp were injected i.p. with extracted MCs. And the highest concentration of MCs was found in blood (Lei et al. 2008). Therefore, in some extent, blood is one of the most important target organs in animals after MC exposure. Blood indices are useful and sensitive for monitoring the physiological status of fish exposed to toxicants (Adhikari et al. 2004). Some in vivo studies focused on hematological changes in fish treated with MC (Zhang et al. 2007). However, as so far as we know, no study has been conducted to investigate the toxic effects of MC on fish erythrocytes in vitro. In fact, erythrocyte in vitro has been one of the most important materials for mechanism investigating blood toxicology.
Many investigations demonstrated that various compounds exert toxic effect on erythrocytes via lipid peroxidation (Orhan and Sahin 2001; Bukowska and Kowalska 2004). Erythrocytes are prone to oxidative damage as a consequence of free radical attack, as they are exposed to high oxygen tension and are rich in iron and polyunsaturated fatty acids (Chiu and Lubin 1989). The spontaneous generation of superoxide, peroxide and hydroxyl radicals has been reported in RBC (Hebbel et al. 1982). Erythrocytes contain high concentrations of polyunsaturated fatty acids, molecular oxygen and ferrous ions in the ligand state; however, they contain cellular antioxidants and enzymes, GSH, CAT, SOD and GPx (Ertabak et al. 2004).
Lipid peroxidant is a significant membrane-damaging event (Valenzeno 1987), and the MDA is a measure of the extent of lipid peroxidative damage. In the present study, the significant increases in MDA levels indicated that marked lipid peroxide occurred in erythrocytes after exposure to MC-LR. Our results are consistent with earlier reports which hypothesized that the interaction of hemoglobin with redox drugs/xenobiotics to be the source of radical generation leading to membrane LPO and lysis (French et al. 1978; Clemens et al. 1984).
In the present study, significantly higher values of GPx, CAT and GST were observed in samples with lower concentration of MC-LR than higher ones; it seems that animals may have more time to metabolize the toxins or to form an adaptive response to reduce the adverse effects when exposed to the low dose of MCs. And the enzymatic activities increased in erythrocytes incubated with MC-LR; however, the level of GSH decreased. SOD catalyzes the dismutation of superoxide anions to form hydrogen peroxide and oxygen. The SOD activity behavior could be related to an increased oxidative stress (Maiti and Kar 1997; Kale et al. 1999). The consequence of the dismutation reaction of superoxide anion, catalyzed by SOD, is an increase in H2O2 level. The CAT, a soluble protein in erythrocytes, plays a role in the decomposition of hydrogen peroxide to give H2O. The presence of hydrogen peroxide could stimulate an increase in the CAT activity. GSH, one of the most potent biological molecules, affects scavenging of all functions including free radical reactions in the erythrocytes. One reason for the GSH decrease may be GSH oxidation to GSSG, and another reason is correlated with the increased activity of glutathione transferase (GST), which catalyzes the process of conjugation. In many aquatic organisms such as Artemia salina, fish and mussels, it was demonstrated that the metabolism of MCs begins with a conjugation reaction to GSH catalyzed by GST (Pflugmacher et al. 1998; Beattie et al. 2003). And this conjugation is mediated by the system of microsomal and cytosolic glutathione S-transferases. GST is considered to be an oxidative stress marker. The conjugation of electrophilic molecules with GSH is catalyzed by GST, and it is a part of the mechanism of detoxification of xenobiotics and peroxidation products (Neefjes et al. 1999). GPx is an enzyme capable of destroying the end products of ROS generation pathway involving H2O2 and organic peroxides (Ji 1999). Previous studies reported the changes in oxidative status in human erythrocytes under the influence of 2, 4-d and 2,4-DCP, and during oxidative stress, the increase in the activity of glutathione peroxidase decreases the concentration of GSH (Bartosz 1995). The action of GPx is strictly linked with the concentration of GSH because it catalyzes the reaction between glutathione and hydrogen peroxide, resulting in the formation of glutathione disulfide (Mates 2000).
Some biochemical indicators are widely used in toxicology and in clinical chemistry to diagnose erythrocyte damage (Wang et al. 2007). In this study, a significant decrease in the activity of AChE was observed. The inhibition in erythrocytes’ AChE activity was also found in erythrocytes treated with pyrethroid intoxication (Kale et al. 1999). They suggested that erythrocytes AChE can be a marker enzyme in pyrethroid toxicity due to the significant correlation of increased LPO level with the decreased AChE activity in erythrocytes. In the present study, the significant increased LPO levels should be responsible for the declined AChE activities. Some other studies also have demonstrated that there was a significant correlation between inhibition in AChE activity and increase in LPO level in erythrocytes (Su et al. 1971; Yang and Dettbarn 1996). In fact, some authors suggest that AChE inhibition induces cholinergic hyperactivity, initiating the accumulation of free radicals and leading to lipid peroxidation, which in turn may lead to cell injury (Yang and Dettbarn 1996; Üner et al. 2006). The membrane-bound Na+–K+-ATPase and Ca2+–Mg2+-ATPase activities in erythrocytes could be assessed as simple, safe and useful marker of intracellular damage (Ohta et al. 1989). It has been reported that the decrease in Na+–K+-ATPase and Ca2+–Mg2+-ATPase activity was relative to the increase in the level of LPO when erythrocytes exposed to heavy metals and chemical medication (Wang et al. 2007; Vaddi et al. 2009). In this study, the significant decrease in Na+–K+-ATPase and Ca2+–Mg2+-ATPase activity was observed in 10-, 100- and 1,000-nM dose groups from 12 to 24 h. Wang et al. (2007) found that Na+–K+-ATPase and Ca2+–Mg2+-ATPase activities in rat erythrocytes were significantly decreased when it exposed to butenolide. And they demonstrated that the inhibition of ATPase activity may be ascribed to lipid peroxidation and/or the decrease in the number of –SH groups induced by butenolide. Paulina et al. (2006) have demonstrated that damages to erythrocyte membrane and antioxidative enzymes may be the result of direct covalent binding of MC-LR with –SH residues of protein.
The lysis of cells and death of erythrocytes were ascribed to changes in the shape of erythrocytes when the bilayer structure was disturbed by peroxidation process and incorporation of xenobiotic into membrane in the study by (Paulina et al. 2006). In this study, we observed that MC-LR induces significant increase in hemolysis in 10-, 100-, 1,000-nM dose groups from 12 to 48 h. Anna et al. (1999) observed that the level of hemolysis of carp erythrocytes significantly increased when it exposed to zinc in vitro. And they suggested that the increase in hemolysis may be due to the lipid peroxidative damage. Metal ions affect the phospholipid arrangement in the membrane by phospholipase A2 action and finally increase the erythrocyte susceptibility to hemolysis (Anna et al. 1999). Paulina et al. (2006) also found that the level of hemolysis of human erythrocytes significantly increased when it exposed to MC-LR in vitro. They considered that the increase in hemolysis was ascribed to the lipid peroxidation that induced the membrane damage and hemolysis of erythrocytes.
Morphological changes in erythrocytes always occur in fish as a result of intoxication with inorganic or organic poisons (Malgorzata et al. 2005; Zeni et al. 2002). Malgorzata et al. (2005) observed that the share of cellular anomalies significantly increased when juvenile tench was exposed to cadmium, including abnormal cell shape, vacuolization, swelling, chromatin disintegration in the nucleus and nucleus indentation. Vosyliene (1999) considered that morphology of erythrocytes is one of the most specific and sensitive indicators when fish exposed to various environmental factors. In this study, we observed that the cell membrane was swelled, notched and disrupted at 12 h after MC-LR exposure. Paulina (2006) observed changes in the shape of erythrocytes and echinocytes when human erythrocytes were incubated with MC-LR. Echinocytes were formed when the chemical compounds were binding to the outer part of membrane monolayer or may be formed when membrane skeleton was treated with oxidative stress (Bukowska and Zatorska 2003).
In conclusion, the present study focused on in vitro damage to erythrocytes of crucian carp treated with microcystin-LR. The results of antioxidative response, alterations in other biochemical parameters, hemolysis examination and morphological observation all indicate damages to erythrocytes. And the findings also validate the previous research that anemia and hypotensive shock result in fish damage or even death.
References
Adhikari S, Sarkar B, Chatterjee A, Mahapatra CT, Ayyappan S (2004) Effects of cypermethrin and carbofuran on certain haematological parameters and prediction of their recovery in a freshwater teleost Labeo rohita (Hamilton). Ecotoxicol Environ Saf 58:220–226
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology, vol 105. Academic Press, Orlando, pp 121–126
Anna A, Zofia J, Teresa G, Roman G (1999) Effect of zinc on carp (Cyprinus carpio L.) erythrocytes. Comp Biochem Physiol C 123:209–215
Bartosz G (1995) The other face of oxygen: in Polish Copyright by PWN Sp. Z o.o, Warszawa, pp 200–204
Beattie KA, Ressler J, Wiegand C, Krause E, Codd GA, Pflug-macher S (2003) Comparative effects and metabolism of two microcystins and nodularin in the brine shrimp Artemia salina. Aquat Toxicol 62:219–226
Beutler E (1975) Red cell metabolism. A manual of biochemical methods, 2nd edn. Grune & Stratton, New York, pp 71–73
Bukowska B, Kowalska S (2004) Phenol and catechol induce prehemolytic and hemolytic changes in human erythrocytes. Toxicol Lett 152:73–84
Bukowska B, Zatorska A (2003) Prehemolytical changes in erythrocytes incubated with 2, 4-d. Curr Topics Biophys 27:11–15
Cazenave J, Bistoni MA, Pesce SF, Wunderlinm DA (2006) Differential detoxification and antioxidant response in diver se organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat Toxicol 76:1–12
Chauhan VPS, Tsiouris JA, Chauhan A, Sheikh AM, Brown WT, Vaughan M (2002) Increased oxidative stress and decreased activities of Ca2+/Mg2+-ATPase and Na+/K+-ATPase in the red blood cells of the hibernating black bear. Life Sci 71:153–161
Chiu D, Lubin B (1989) Oxidative hemoglobin denaturation and RBC destruction: the effect of heme on red cell membranes. Semin Hematol 26:128–135
Clemens MR, Remmer H, Waller HD (1984) Phenylhydrazine-induced lipid peroxidation of red blood cells in vitro and in vivo: monitoring by the production of volatile hydrocarbons. Biochem Pharmacol 333:1715–1718
Cohen P (1989) The structure and regulation of protein phosphatases. Annu Rev Biochem 58:435–508
Dacie JV, Lewis SM (1984) Practical haematology. Churchill Livingstone, New York, p 32
Ellman GL, Courtney KD, Andres VJ, Featherstone RM (1961) A new rapid colorimetric determination of acetylcholine esterase activity. Biochem Pharmacol 7:88–94
Fastner J, Codd GA, Metcalf JS, Woitke P, Wiedner C, Utkilen H (2002) An international intercomparison exercise for the determination of purified microcystin-LR and microcystins in cyanobacterial field material. Anal Biochem Chem 374:437–444
French JK, Winterbourn CC, Carrel RW (1978) Mechanism of oxyhaemoglobin breakdown on reaction with acetylphenylhydrazine. Biochem J 173:19–26
Greenburg AG (1996) Pathophysiology of anemia. Am J Med 101(Suppl. 2A):7S–11S
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase. J Biol Chem 249:7130–7139
Hebbel RP, Eaton JW, Balasingan M, Steinberg MH (1982) Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest 79:1254–1260
Ji LL (1999) Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med 222:283–292
Kale M, Rathore N, John S, Bhatnagar D (1999) Lipid peroxidative damage on pyrethroid exposed and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicol Lett 105:197–205
Lawrence A, Burk RF (1976) Glutathione peroxidase activity in selenium deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lei H, Xie P, Chen J, Liang G, Dai M, Zhang X (2008) Distribution of toxins in various tissues of crucian carp intraperitoneally injected with hepatotoxic microcystins. Environ Toxicol Chem 27:1167–1174
Li D, Xie P, Zhang X, Zhao Y (2009) Intraperitoneal injection of extracted microcystins results in hypovolemia and hypotension in crucian carp (Carassius auratus). Toxicon 53:638–644
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Maiti PK, Kar A (1997) Dual dose of testosterone in fenvalerate-treated mice with respect to thyroid function and lipid peroxidation. J Appl Toxicol 17:127–131
Malbrouck C, Kestemont P (2006) Effects of microcystins on fish. Environ Toxicol Chem 25:72–86
Malgorzata W, Barbara J, Jacek W (2005) Respiratory and hematological response of tench, Tinca tinca (L.) to a short-term cadmium exposure. Aquacult Int 14:141–152
Mates JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Neefjes VM, Evelo CT, Baars LG, Blanco CE (1999) Erythrocyte glutathione S transferase as a marker of oxidative stress at birth. Arch DisChild-Fetal 81:130–133
Nishiwaki-Matsushima R, Ohta T, Nishiwaki S, Suganuma M, Kohyama K, Ishikawa T, Carmichael WW, Fujiki H (1992) Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J Cancer Res Clin 118:420–424
Ohta A, Mohri T, Ohyashiki S (1989) Effect of LPO on membrane bound Ca2+ATPase activity of the intestinal brush border membrane. Biochim Biophys Acta 984:151–157
Orhan H, Sahin G (2001) In vitro effects of NSAIDS and paracetamol on oxidative stress-related parameters of human erythrocytes. Exp Toxicol Pathol 53:133–140
Paerl HW, Fulton RS, Moisander PH, Dyble J (2001) Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci World 1:76–113
Paulina S, Bozena B, Jaromir M, Wirgiliusz D (2006) Damage of cell membrane and antioxidative system in human erythrocytes incubated with microcystin-LR in vitro. Toxicon 47:387–397
Pflugmacher S, Wiegand C, Oberemm A, Beattie KA, Krause E, Codd GA, Steingerg CEW (1998) Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR : the first step of detoxication. Biochim Biophys Acta 1425:527–533
Prieto AI, Jos A, Pichardo S, Moreno IM, Caméan AM (2006) Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat Toxicol 77:314–321
Prieto AI, Pichardo S, Jos Á, Moreno IM, Caméan AM (2007) Time-dependent oxidative stress responses after acute exposure to toxic cyanobacterial cells containing microcystins in tilapia fish (Oreochromis niloticus) under laboratory conditions. Aquat Toxicol 84:337–345
Robinson NA, Miura GA, Matson CF, Lawrence WB, Pace JC (1989) Characterization of chemically tritiated microcystin-LR and its distribution in mice. Toxicon 27:1035–1042
Šetlíková I, Wiegand C (2009) Hepatic and branchial glutathione S-transferases of two fish species: Substrate specificity and biotransformation of microcystin-LR. Comp Biochem Physiol C 149:515–523
Stock J, Dormandy TL (1971) The autooxidation of human red cell lipid induced hydrogen peroxide. Br J Haematol 20:95–101
Su MQ, Kinoshita FK, Frawley JP, Dubois KP (1971) Comparative inhibition of aliesterases and cholinesterase in rats fed eighteen organophosphorus insecticides. Toxicol Appl Pharmacol 20:241–249
Üner N, Oruç EO, Sevgiler Y, Sahin N, Durmaz H, Usta D (2006) Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol 21:241–245
Vaddi DR, Pannuru P, Maturu P (2009) Modulatory role of Emblica officinalis against alcohol induced biochemical and biophysical changes in rat erythrocyte membrane. Food Chem Toxicol 47:1963–1985
Valenzeno DP (1987) Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem Photobiol 46:147–160
Vosyliene MZ (1999) The effect of heavy metals on haematological indices of fish (survey). Acta Zool Lituanica 9:76–82
Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY (2007) The oxidative damage of butenolide to isolation erythrocytes membranes. Toxicol In Vitro 21:863–869
Yang ZP, Dettbarn WD (1996) Diisopropylphosphorofluoridate induced cholinergic hyperactivity and lipid peroxidation. Toxicol Appl Pharmacol 138:48–53
Zeni C, Bovolenta MR, Stagni A (2002) Occurrence of echinocytosis in circulating RBC of black bullhead, Ictalurus melas (Rafinesque), following exposure to an anionic detergent at sublethal concentrations. Aquat Toxicol 57:217–224
Zhang X, Xie P, Li D, Shi Z (2007) Haematological and plasma biochemical responses of crucian carp (Carassius auratus) to intraperitoneal injection of extracted microcystins with the possible mechanisms of anemia. Toxicon 49:1150–1157
Zhang X, Xie P, Wang W, Li D, Li L, Tang R, Lei H, Shi Z (2008) Dose dependent effects of extracted microcystins on embryonic development, larval growth and histopathological changes of southern catfish Silurus meridionalis. Toxicon 51:449–456
Acknowledgments
We would like to express our sincere thanks to Dr. Patrick Kestemont and anonymous reviewers for their useful comments and suggestions on the manuscript. This study is funded by the National Natural Science Foundation of China (No. 30800160) and the Natural Science Foundation for Distinguished Young Scholars of Hubei Province (No. 2010CDA095).
Author information
Authors and Affiliations
Corresponding author
Additional information
Wenshan Zhou and Hualei Liang have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Zhou, W., Liang, H. & Zhang, X. Erythrocyte damage of crucian carp (Carassius auratus) caused by microcystin-LR: in vitro study. Fish Physiol Biochem 38, 849–858 (2012). https://doi.org/10.1007/s10695-011-9572-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-011-9572-2