Abstract
An adapted bench-scale Mass Loss Calorimeter (MLC) device is proposed for evaluating effective heat of rapid flaming combustion of fine Mediterranean forest fuels. The MLC apparatus uses a calibrated thermopile to quantify heat release rate (HRR) as an alternative to the classical oxygen consumption measurement. A porous holder was used to simulate rapid flaming combustion. Average effective heat of combustion (AEHC) during the flaming phase was related to the classical measurement of gross heat of combustion (GHC) obtained in oxygen bomb calorimeter. Results showed that the effective heat of combustion (oven-dry basis) was between 18% and 44% lower than the gross heat of combustion. A linear regression was obtained (r2 = 0.48; SEE = 1.25; p < 0.01; n = 26) to relate AEHC and GHC values. The simple model developed (AEHC = GHC − 6.75) suggests the possibility of reducing the heat of combustion values used in forest fire behaviour models for Mediterranean forest fuels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bench-scale tests are used as a first step in understanding the combustion process in forest fuels. Calorimetry studies to measure the amount of heat released from forest fuels have typically been carried out in oxygen consumption calorimeters [1, 2]. The main advantages of the oxygen consumption method are its standardized worldwide use and the availability of a large amount of data for all kind of materials. This methodology allows estimating the HRR directly from measurements, without knowing chemical composition or the combustion chemistry. Other systems such as the mass loss calorimeter (MLC) are also used to study heat released at bench-scale [3]. This device is the complete fire model of the cone calorimeter but the system is calibrated using known flow rates of methane gas and the heat release is then calculated using the change in air enthalpy. Its main advantage against the cone calorimeter is the simpler configuration because it is not necessary to analyze gas concentrations but, on the contrary, there is not information about convective gases to characterize the combustion process. In addition, this method is only appropriate for materials having radiative/convective heat release ratios similar to the calibration standard used.
Fire hazard assessment is carried out by bench-scale tests, to predict full-scale fire behaviour [4]. Physical models take into account the complex phenomena to relate combustion variables to fire front behaviour [5, 6].
Quantification of the fireline intensity of wildland fires, expressed as heat-release rate per unit length of the fire front, is usually estimated from the mass loss rate, by the Byram equation [7]:
where I is the fireline intensity (kW/m), H is the heat of combustion (kJ/kg), w is the fuel consumption on an area basis (kg/m2) and r is the rate of spread of the fire (m/s). In monitored experimental fires, forest fuel inventory before and after the test enables estimation of w. Several methodologies can be used to estimate r (e.g. visual estimation based on referenced points, imaging techniques, thermocouple measurements) [5]. This variable can also be monitored through infrared techniques with the aid of multispectral cameras and/or remote sensing [8]. Nevertheless, there is some controversy about the correct value of H used for forest fuels.
The BehavePlus fire modelling system for prediction of surface and crown fire behaviour [9] propose an average of 18 MJ/kg for ‘heat content’ as a characteristic value for forest fuels [10]. Other authors [11] propose a value of 15 MJ/kg, incorporating nominal radiation loss of 15% and additional heat loss due to evaporation of fuel moisture. This value is the upper limit obtained by Babrauskas [12] for flaming combustion of conifers in large scale experiments (12 MJ/kg to 15 MJ/kg), which demonstrated the importance of determining the heat of combustion during the flaming phase, which is much lower than the value obtained in the glowing phase. Forest modellers traditionally do not consider this variable because it is believed to introduce a small error into the energy calculation comparing r and w, and consequently is usually considered as a constant. Nevertheless there may be intraspecific variation in the gross heat of combustion depending on phenology (seasonal variation) and provenance, and also interspecific variation, depending on the chemical characteristics of the species considered [13]. In addition, heat of combustion data are not available for all species, and are particularly scarce for shrubs and herbaceous vegetation [13]. Calorimetry studies have revealed significant differences in HRR and H among species [13–15], so that the influence of these variables during forest fire must be clarified, because the reliability of the predictions may be compromised by uncertainty surrounding parameters such as heat of combustion [13]. In summary, the complexity of heat release estimation in forest fires is limited by correct measurement of the variables involved. On the other hand, the need to understand the complex forest fire combustion (rapid flaming combustion in porous fuel with a low bulk density along a dynamic fire front) has made necessary simulation of the phenomena at bench scale.
Several authors [see reviews in 13, 15] have considered measurement of the average effective heat of combustion (AEHC) of forest fuels by use of a cone calorimeter as a substitute for measurement of heat of combustion by use of an oxygen bomb calorimeter. The AEHC measured in the cone calorimeter reflects the heat of combustion due to combustion of the cellulose and hemicellulose contents of the vegetation, which allows complete combustion of pyrolysis products, except carbon monoxide and other products emitted before ignition. These combustion processes result in lower values of the AEHC than the heat of combustion obtained in an oxygen bomb calorimeter. There is therefore a need for studies that compare cone calorimeter and oxygen bomb calorimeter data, as previously suggested [13, 15].
Effective Heat of Combustion (EHC) of forest fuels is strongly dependent on fuel moisture content [12]. Nevertheless, there are other variables affecting EHC, like fireline intensity, oxygen supply, local heat balance and particular size and porosity characteristics of the fuel bed. Therefore, in order to compare results between different fuels at bench-scale, some of these variables must be fixed [13–15].
This study is a first bench-scale approach to relate effective heat of rapid flaming combustion emitted by fine forest fuel and the gross heat of combustion. An adapted mass loss calorimeter device was used to simulate rapid flaming combustion and an oxygen bomb calorimeter was used to determine the gross heat of combustion for 26 different Mediterranean forest fuels. Relationships between these two variables were explored and a relative comparison among fuels tested were conducted.
2 Materials and Methods
2.1 Materials
Series of tests were conducted in order to study 26 forest fuels. The characteristics of the forest fuels used in the tests are shown in Table 1. The species used are representative and very common in European Mediterranean forests, and the samples present a wide range of gross heat of combustion values, so it will be possible to extrapolate the results to a wide range of Mediterranean forest fuels.
As most forest fire behaviour models [5, 6, 9] as well as experimental estimates [10] assume that most of the fuel consumed during an active-flaming fire is of diameter <1 cm, fuel samples of this size were used in the present study.
2.2 Description of the Devices
2.2.1 Mass Loss Calorimeter (MLC)
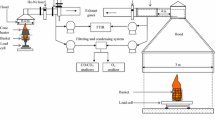
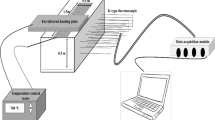
The Mass Loss Calorimeter (MLC) is manufactured by Fire Testing Technology Limited (FTT®). This apparatus (Figure 1a) is the complete fire model of the cone calorimeter, which has assumed a dominant role in bench-scale fire testing of building materials. A chimney (Figure 1b), manufactured from stainless steel (600 mm long × 114 mm inner diameter) and containing a thermopile of four mineral insulated inconel sheathed thermocouples (type K, 1.6 mm diameter), was added to the MLC (650 mm above the holder surface). The thermopile output is first calibrated by use of a methane burner (Figure 1c) and a flowmeter, and then used to quantify heat release [3, 14].
The MLC standard sample holder used for building materials contains low density ceramic wool to ensure correct positioning of the samples, 25 mm from the conical heater, and the sample is placed on aluminium foil. A specific porous holder adapted to forest fuel samples was designed [16] to simulate rapid flaming combustion. The holder (10 × 10 × 5 cm3) is made of stainless steel and has small uniformly sized holes over the entire outer surface (sides and bottom). These holes create an open space for inlet combustion gases to pass into the holder and through the fuel samples (Figure 1d).
The MLC device and the porous holder have been evaluated [14] and comply with the repeatability criteria established by different authors [2, 17, 18].
2.2.2 Oxygen Bomb Calorimeter
The oxygen bomb calorimeter manufactured by IKA® was used to obtain the gross heat of combustion. A description of the device is provided in ISO standard 1716 [19].
2.3 Experimental Methods
2.3.1 Mass Loss Calorimeter Tests
The characterization of forest fuels combustion was performed through a series of tests conducted with the selected forest fuels, in the mass loss calorimeter with the porous holder. The FMC was fixed on an oven-dry basis, after drying the samples at 60°C to constant weight. This conditioning protocol was selected because oven-drying of the samples at 100°C may result in loss of volatile substances before the test. The initial sample mass was 10 g and the resulting thickness of this mass was 5 cm. In accordance with the volume of the holder, these experimental conditions correspond to a bulk density of 20 kg/m3. The electric conical heater was regulated so that the samples were exposed to a heat flux of 50 kW/m2. Tests were repeated for each species so that at least two replicates that comply with the repeatability criteria were obtained (52 tests corresponding to 26 forest fuels). When twigs, needles or leaves were longer than the sample holder, they were trimmed to fill the holder, and uniformly covered its exposed surface area [20]. The initial mass of the sample was measured immediately before the sample was placed under the heater. The spark ignitor was used to provide the piloted ignition.
Prior to conducting the tests, and in order to determine the response of the MLC thermopile, a given flow of methane gas (99% purity) was burnt under the MLC chimney and thermopile signal recorded. For methane a HRR value of 1 kW is known to correspond to a 27.83 cm3/s volumetric flow rate.
The calibration is performed for 9 flows of methane corresponding to 5 kW, 4 kW, 3 kW, 2 kW, 1 kW, 0.75 kW, 0.5 kW, 0.25 kW and 0 kW. With these calibration values, the average value of the thermopile signal outputs (mV, °C) can be used to estimate heat release (kW). Calibration lines for a constant heat flux of 50 kW/m2 are shown in Figure 2.
2.3.2 Oxygen Bomb Calorimeter Tests
The gross heat of combustion (GHC) was calculated following the method outlined in ISO standard 1716 [19]. Analogous samples to those tested in the MLC were also oven-dried at 60°C and then tested in the oxygen bomb. Tests of the 26 forest fuels that comply with repeatability criteria (52 tests) were selected for comparison with the results of MLC tests.
The gross heat of combustion is the value that is directly obtained from the oxygen bomb test. The net heat of combustion (NHC) is considered the higher limit to the effective heat of combustion and it is a calculated adjustment that accounts for the fact that the water in the combustion product is vapor, rather than liquid [12]:
where [%H] is the percentage of hydrogen in fuel by unit mass. Alexander [21] proposed two categories of heat losses from GHC obtained in oxygen bomb must be taken, assuming complete combustion and absence of radiation loss: the first reduction for the latent heat absorbed when the water of reaction is vaporized (1.263 MJ/kg) and the second reduction is for fuel moisture content (24 kJ/kg per moisture content percentage point). Having in mind these differences between NHC and GHC and taking into account that tests were carried out with oven-dry fuels, GHC was selected as analysed variable.
2.4 Data Analysis
The MLC results are presented as curves of Heat Release Rate (HRR) and Mass Loss Rate (MLR) plotted against time (1 s frequency). The curves enable numerical calculation (from series of tests for each species) of: Peak Heat Release Rate (PHRR, kW/m2), Total Heat Release (THR, MJ/m2), Time to Ignition (TTI, s), Period of flaming combustion (Flame Duration FD, s) and Mass Loss Rate (MLR, g/s) for flaming combustion. The mass loss rate (MLR) can be used to determine a time-varying value of the Effective Heat of Combustion (EHC, MJ/kg) [3]:
As MLR requires numerical differentiation for its determination and is, therefore, noisier than measurements obtained directly from instrument readings, it is better to calculate average values of EHC. In addition, since the total mass loss is very small compared to the weight of the sample holder and the structure that supports it in the weighting scale, the signal is extremely noisy. To obtain such averages, the numerator in Eq. 3 must be averaged separately rather than calculating the average ratio. In this case Average Effective Heat of Combustion (AEHC, MJ/kg) over the flaming phase is obtained as:
where Δt is sampling time interval (in this case 1 s), TTI is time to ignition, EOF is time to end of flame, mTTI is the mass of specimen at time to ignition and mEOF is the mass of specimen at the time to end of flame.
The t-test parametric requirements were checked before comparison of mean values (AEHC and GHC) obtained with live (n = 20) and dead (n = 6) fine fuel. Kolomogorov–Smirnov tests were used to test the null hypothesis that the two samples can reasonably be assumed to come from the same distribution.
In order to explore correlations between GHC and MLC variables, the following steps were done: (i) A correlation matrix was calculated to detect relationships among variables (ii) Simple regression models were explored to correlate the AEHC obtained in the MLC device and the GHC obtained in the oxygen bomb calorimeter (iii) Linear regression models (least square method) were selected because linear model showed similar fit, lesser errors and better interpretation than other non-linear models like exponential or multiplicative models.
3 Results and Discussion
The typical progression of a test is shown in Figure 3: ignition is produced, the heat release rate rises quickly and the peak (PHRR) is reached, then the HRR decreases until the end of flame and finally the glowing phase starts and continues until the end of the test. MLC tests generated rapid flaming combustion with FD values comprised between 27 s and 104 s (59 ± 17 s, average ± standard deviation), MLR of 0.158 ± 0.038 g/s and peak MLR between 0.5 g/s and 1.6 g/s (0.88 ± 0.21 g/s). Average and range values of FD agree with residence time of flame (45 s) proposed by Stocks et al. [10] on crown fire experiments (an extremely rapid flaming fire). In addition, the mass flow rate for crown fire is approximately 3 kg/m2/min [22]. According to the experimental conditions (bulk density of 20 kg/m3) this flow rate corresponds to 0.5 g/s, which is the minimum value obtained. The results therefore indicate reasonably good simulation of a rapid flaming combustion during the bench-scale tests with forest fuels.
Heat release rate (HRR, grey line) and Mass Loss Rate (MLR, black line) for one of the tests (test no. 5) in the Pinus pinaster dead litter series tests (Heat flux of 50 kW/m2, bulk density of 20 kg/m3). Vertical lines show time-to-ignition (continuous) and time to end of flame (discontinuous). MLR has been smoothed by moving average method (5 s period)
During tests, the porous nature of both fuel and holder caused a variable effective heat of combustion, because the mass loss rate (g/s) changed greatly (Figure 3). The average EHC (AEHC) during the rapid flaming combustion was between 10.58 MJ/kg and 19.04 MJ/kg (14.25 ± 1.88 MJ/kg). The AEHC values (oven-dry basis) obtained for the selected Mediterranean fuels (10 MJ/kg to 19 MJ/kg) were consistent with the normal range for forest fuels [12, 13, 15, 20, 23].
The AEHC values for dead fuels are significantly higher than those for live fuels (t-test, p < 0.01; average 15.55 MJ/kg for dead fuels and 13.72 MJ/kg for live fuels). Results confirm the differences between dead and live fuels obtained by Madrigal et al. [14] in the adapted MLC device and the important implications of the presence of dead fuel during flaming combustion, taking into account that these differences were detected on a dry weight basis. The results therefore suggest that dead fuel may not only affect combustion process from the point of view of fuel moisture content. Nevertheless, the differences were not significant (p = 0.4534) for GHC (average 21.07 MJ/kg for dead fuels and 20.65 MJ/kg for live fuels). Madrigal et al. [14] detected differences between Pleurozium schereberi and Quercus coccifera in the AEHC values obtained with the MLC device, whereas no significant differences between GHC were detected with the oxygen bomb calorimeter. These results may confirm the effect of fuel structure in rapid flaming combustion properties. By contrast, the oxygen bomb calorimeter uses ground material to determine the GHC, and therefore overlooks the effect of the forest fuel structure on combustion characteristics [13]. Thus, the differences in GHC are caused by phytochemicals [24], and the values did not differ significantly between fuel types.
Correlation matrix (Table 2) shows the significant correlation between GHC (bomb calorimeter) and some of the considered combustion characteristics during the flaming phase (MLC device): PHRR, THR and AEHC. The variables most strongly correlated to GHC were AEHC (r = 0.7086, p < 0.001) and THR (r = 0.7956, p < 0.001). In addition both PHRR and THR presented significant correlation with AEHC (p < 0.01), TTI and FD (p < 0.05) showing the importance of those variables to describe the combustion process [4] and the strong autocorrelation among variables which describe the combustion during the flaming combustion phase. Simple linear and non-linear regression models were explored to relate GHC and AEHC values and linear regression models were finally developed (Figure 4a and b) because they presented lesser errors and easier interpretation. The regression analysis of all data (dead and live fuels, Figure 4a) revealed a moderate fit (r2 = 0.48; SEE = 1.25; p < 0.01; n = 26) but a simple model form (AEHC = GHC − 6.75). The AEHC (oven-dry basis) could therefore be predicted by subtracting a value of 6.75 MJ/kg from the GHC obtained in the oxygen bomb calorimeter. Separate regression analysis of the dead fuel (n = 6) and live fuel (n = 20) data revealed that dead fuel presents a better fit (r2 = 0.86; SEE = 0.78; p < 0.01; n = 6) and a stronger relationship between GHC and AEHC values than live fuel (r2 = 0.45; SEE = 1.11; p < 0.01; n = 20) (Figure 4b). The strong correlation for dead fuel (more data are necessary to confirm the result) suggests that GHC could be a good predictor for AEHC during an active-flaming fire. Nevertheless, only a moderate model fit was obtained (Figure 4a) because the AEHC of live fuel was less variable than the AEHC of dead fuel (Figure 4b), again suggesting the possibility of a greater influence of phytochemicals for dead fuel than for live fuel (i.e. dead fuels are more lignified than live ones). Some physical characteristics were fixed or the variability reduced by experimental configuration (fuel depth, fuel loading, fuel moisture content, bulk density). Nevertheless, there were differences among species in other forest fuel properties such as surface-area-to-volume ratio, density and packing ratio in the MLC tests. The variability in these important factors, in a configuration where natural diffusion of air is allowed, may strongly affect the combustion process and variability of AEHC among species. The moderate fit of the live fuel regression model shows that species with differences in GHC due to different chemical characteristics tend to have higher AEHC, but within a narrower range. The results therefore suggest that the differences in species for the estimated value of AEHC during rapid flaming combustion are lower than the GHC. Physical processes during the rapid flaming combustion probably absorb the variability due to the chemical characteristics of species. From this point of view, the AEHC (oven-dry basis) of most of Mediterranean fuels tested (Figure 4a and b) may be considered within a range of 12 MJ/kg to 16 MJ/kg (average 14 MJ/kg). Linear regression analysis of all data (Figure 4a) and of live and dead fuel data (Figure 4b) suggest that a reduction of 18% to 44% (average 30%) in the GHC is required in order to obtain AEHC on an oven-dry basis. This reduction is greater than the differences between GHC and AEHC obtained for Adenostoma fasciculatum (20% to 25%) in a cone calorimeter with a standard holder [15] which produces a lower rate of combustion than the porous holder tested in the present study [14]. Nelson and Adkins [11] proposed a nominal reduction of 15% for net heat of combustion with respect to GHC due to radiation losses and an additional heat loss due to evaporation of fuel moisture. Taking into account that MLC tests are carried out on an oven-dry basis, the results show that the reduction is greater than differences reported by these authors. Babrauskas [12] proposed a reduction of 30% for the net heat of combustion for flaming combustion of conifers based on the results of large-scale experiments, which are consistent with the results reported in the present study for oven-dry fuel.
a Linear regression relating AEHC (dependent variable) and GHC (independent variable) for all data (n = 26). b Linear regression relating AEHC (dependent variable) and GHC (independent variable) for series of dead fuels (n = 6) and live fuels (n = 20). Points indicate average value and bars indicate standard deviation limit of repeatability for the MLC device (8%, vertical bars) and oxygen bomb calorimeter (1%, horizontal bars)
4 Conclusions
This study is a first attempt to detect differences and correlations between effective heat of combustion for flaming combustion (AEHC) and gross heat of combustion (GHC) of Mediterranean forest fuels. Further research is needed in order to improve and generalize the results obtained to field conditions: different fuels must to be tested and the effect of fuel moisture content on effective heat of combustion during a rapid flaming combustion of live fuel must be explored. Linear regression model presents poor level of correlation showing the differences between the combustion in the rapid flaming mode and in the bomb calorimeter. Therefore results suggest that more physics should be taken account to develop future models. Nevertheless, the results show the need to reduce the heat of combustion used in forest fire behaviour models. The average reduction required is around 30%, although this may differ significantly depending on the vegetation growth forms and whether dead or live fuels are considered. Although heat of combustion of forest fuels may be considered as a constant or be comprised within a narrow range (10 MJ/kg to 19 MJ/kg according to the results of the present study), differences between species and fuel types were significant. Therefore modellers and end-users of forest fire behaviour models should take into account the species present in the field in order to improve simulations and prediction validations. Validation of the results in large-scale tests is also necessary in order to ratify the relationship between AEHC and GHC detected at bench-scale.
References
Babrauskas V (1984). Development of the cone calorimeter – a bench-scale heat release rate apparatus based on oxygen consumption. Fire Materials 8: 81-95. doi: 10.1002/fam.810080206
Babrauskas V (2002) The cone calorimeter. In: National Fire Protection Association, Society of Fire Protection Engineering (eds) SFPE handbook of fire protection engineering, 3rd edn. National Fire Protection Association, Quincy, MA, pp 3-63–3-81
International Organization for Standardization (2001) Simple heat release test using a conical radiant heater and a thermopile detector (ISO 13927). International Organization of Standardization, Geneva
Babrauskas V, Peacock RD (1992) Heat release rate: the single most important variable in fire hazard. Fire Safety Journal 18: 255-272.
Sullivan AL (2009) Wildland surface fire spread modelling, 1990–2007. 1: Physical and quasi-physical models. Int J Wildland Fire 18(4): 349–368 doi: 10.1071/WF06143
Linn RR (1997) A transport model for prediction of wildfire behavior. Technical Report LA13334-T. Los Alamos National Laboratory, Los Alamos, NM. doi:102172/505313
Byram GM (1966) Scaling laws for modeling mass fires. Pyrodinamics 4 (3): 271-284
Aranda JM, Meléndez J, de Castro AJ, López F (2008) Measurements of physical parameters of forest fires by infrared imaging methods. In: De las Heras J, Brebbia CA, Viegas D, Leone V (eds) Modelling, Monitoring and Management of Forest Fires. Wit Press, Southampton, Boston, pp 111-120
Andrews PL, Bevins CD, Seli RC (2005) BehavePlus fire modeling system Version 4.0 user’s guide. Gen. Tech. Rep. RMRS-GTR-106WWW. USDA Forest Service, Rocky Mountain Research Station. http://www.fs.fed.us/rm/pubs/rmrs_gtr106.pdf. Accessed 14 December 2009
Stocks BJ, Alexander ME, Wotton BM, Ctefner CN, Flanningan MD, Taylor SW, Lavoie N, Mason JA, Hartley GR, Maffey ME, Dalrymple GN, Blake TW, Cruz MG, Lanoville RA (2004). Crown fire behaviour in northern jack pine-black spruce forest. Can J For Res 34: 1548-1560. doi: 10.1139/X04-054
Nelson Jr RM, Adkins CM (1988) A dimensionless correlation for spread and wind-driven fires. Can J For Res 18: 391-397. doi: 10.1139/X88-058
Babrauskas V (2006) Effective heat of combustion for flaming combustion of conifers. Can J For Res 36: 659-663. doi: 10.1139/X05-253
Dibble AC, White RH, Lebow PK (2007) Combustion characteristics of north-eastern USA vegetation tested in the cone calorimeter: invasive versus non-invasive plants. Int J Wildland Fire 16: 426-443. doi: 10.1071/WF05103
Madrigal J, Hernando C, Guijarro M, Díez C, Marino E and De Castro A J (2009) Evaluation of forest fuel flammability and combustion properties with an adapted mass loss calorimeter device. J Fire Sci 27 (4): 323-342. doi: 10.1177/0734904109102030
Weise DR, White RH, Beall FC, Etlinger M (2005) Use of the cone calorimeter to detect seasonal differences in selected combustion characteristics of ornamental vegetation. Int J Wildland Fire 14: 321-338. doi: 10.1071/WF04035
Schemel CF, Simeoni A, Biteau H, Rivera JD, Torero JL (2008) A calorimetric study of wildland fuels. Exp Therm Flu Sci 32 (7): 1381-1389. doi: 10.1016/j.expthermflusci.2007.11.011
Janssens ML (2001) Heat release rate (HRR). In: Ohlemiller TJ, Johnson EL, Gann RG (eds) Measurement needs for fire safety, proceedings of an international workshop (NISTIR 6527). National Institute of Standard and Technology, Gaithersburg, pp 186–200
European Commission (1997) SBI round robins results. http://europa.eu.int/comm/enterprise/construction/internal/essreq/fire/sbiround/sbirep.htm. Accessed 1 September 2008
International Organization for Standardization (1995) Determination of the heat of combustion (calorific value) (ISO 1716). International Organization of Standardization, Geneva
White RH, Weise DR, Mackes K, Dibble AC (2002) Cone calorimeter testing of vegetation: an update. In: Proceedings of the 35th international conference on fire safety, July 22 to 24. Products Safety Corporation, Sissonville, pp 1–12
Alexander ME (1982) Calculating and interpreting forest fires intensities. Can J Bot 60: 349-357
Van Wagner CE (1977) Conditions of start and spread of crown fires. Can J For Res 7: 23-34. doi: 10.1139/X77-004
Etlinger MG, Beall FC (2004) Development of a laboratory protocol for fire performance of landscape plants. Int J Wildland Fire 13: 479-488. doi: 10.1071/WF04039
Sussot RA, DeGroot WF, Shafizadeh F (1975) Heat content of natural fuels. Journal of Fire Flammability 6: 311-325
Acknowledgments
This research was funded by the European Commission as part of the Integrated Project FIRE PARADOX, FP6-018505. We sincerely acknowledge the critical reviews of three anonymous referees who helped improve an early version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madrigal, J., Guijarro, M., Hernando, C. et al. Effective Heat of Combustion for Flaming Combustion of Mediterranean Forest Fuels. Fire Technol 47, 461–474 (2011). https://doi.org/10.1007/s10694-010-0165-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10694-010-0165-x