Abstract
Thyroid cancer (TC) is a known extra-intestinal manifestation and contributes to the mortality and morbidity in patients with familial adenomatous polyposis (FAP). Its exact prevalence is not well established and recent studies have shown an increasing number of TC in this patient population. The prevalence of benign thyroid masses and endocrinologic thyroid disorders are also poorly described. We conducted a systematic review and meta-analysis by using a random-effects model to characterize TC and estimated the prevalence of thyroid diseases in FAP patients. Twelve studies (n = 9821) were included. Pooled prevalence of TC, benign thyroid masses, and endocrinologic thyroid disorders in FAP were 2.6% [95% confidence interval (CI) 1.3–4.8], 48.8% [95% CI 33.8–64.0], and 6.9% [95% CI 4.5–10.3] respectively. Subgroup analyses revealed higher prevalence of TC in studies with fewer participants, studies that used screening ultrasound to diagnose TC, and studies that were published after 2002. TC diagnosis preceded the diagnosis of FAP in 34% of the patients. The means age at diagnosis of FAP and TC were 29 and 31 years, respectively. 95% of the patients were female and the most common pathology was of papillary subtype (83.3%). Most mutations (79.2%) were located at the 5′ end of APC gene. In summary, benign thyroid disorders are common in FAP, yet, TC is an uncommon phenomenon. Certain patient subset, such as young female with APC mutation at the 5′ end, might benefit from routine surveillance ultrasound.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial adenomatous polyposis (FAP, OMIM #175100) is an autosomal dominant inherited syndrome with an incidence of 1 in 6850 to 1 in 23,700 [1]. It is caused by a germline mutation in Adenomatous Polyposis Coli (APC) gene, a tumor suppressor gene located in the 5q21 region, and has a high penetrance for colorectal cancer. Almost every mutation carrier develops colorectal cancer by 40 years of age [1]. Other gastrointestinal malignancies in FAP patients include duodenal ampullary adenocarcinoma and gastric adenocarcinoma. Improved life expectancy in patients with FAP in recent years has shifted the prevalence of its extra-intestinal manifestations, including pancreatic cancer, hepatoblastoma, brain tumor, benign tumors such as adrenal adenoma, osteoma, desmoid tumor, and dental abnormalities—all of which contribute to the morbidity and mortality in FAP patients to various degrees [1].
Thyroid cancer (TC) was first described as an extra-intestinal malignancy of FAP in Crial’s report in 1949 [2]. Subsequent reports estimated the prevalence of TC in FAP between 0.4 and 1.2% [3, 4] whereas the prevalence of TC in general population was reportedly 0.14% [5]. The recent studies however suggested that its prevalence may be as high as 11.8% [6]. This would have an implication for cancer screening in FAP patients if it reflected a true prevalence. The histologic characteristics of FAP-associated TC were not described until the 1990s. In 1994, Harach et al. first reported an unusual feature of cribriform pattern and solid areas with spindle cell component in thyroid pathology [7]. This was later known as cribriform-morula variant of papillary thyroid cancer (CMV-PTC) and was found to occur in 0.16% of the patients with papillary TC [8]. While a proportion of CMV-PTC in FAP-associated TC had been approximated to 0–12.5%, some authors estimated its prevalence to be as high as 80% and suggested this pathologic variant as a surrogate marker for the diagnosis of FAP [3, 9].
Despite the fact that TC has occurred in the background of underlying benign thyroid disorders in FAP [7, 10], the prevalence of benign thyroid disorders in this patient population has been poorly described. Characterizing the prevalence of the benign thyroid lesions will expand the syndrome’s phenotypic spectrum. FAP, once known for just a cancer predisposition syndrome, is virtually multisystem disorder with both nonmalignant and malignant conditions of various organs; the familiarity of treating physicians to these extra-intestinal manifestations can therefore alleviate the patients’ morbidity and mortality. In conclusion, the purpose of this study is to evaluate the prevalence of thyroid diseases in FAP and describe the clinical, pathological, and genetic characteristics of FAP-associated TC by reviewing existing cohort studies.
Methods
Search strategy
In consultation with a medical librarian (JY), we used PubMed and EMBASE databases to search literatures that were published before 11 July 2016. Search terms were provided in Supplementary Text 1. We limited the search only to human study published in English. Duplicated references were removed by reference manager software (EndNote®X7 Thomson Reuters, Philadelphia, PA, USA). The reference lists of all included studies were examined to identify additional studies not retrieved through online searches. A citation search was also conducted through Web of Science to identify articles referencing any of the included studies.
Eligibility criteria and data extraction
Cohort studies which conducted in cancer registries involving FAP patients and demonstrated the prevalence of TC and/or benign thyroid disorders were eligible for inclusion. Both observational cohort studies and screening cohort studies were included. Cross sectional studies, case reports, case series, letters, conference abstracts, systematic reviews, studies that included at-risk family members for the analysis, and studies with unknown pathology of TC were excluded. If one cancer registry was investigated for more than one study, we included only the latest publication. Studies that focused exclusively on pediatric population will be excluded given the possible unrepresentativeness of general FAP patients due to an age-dependent penetrance of the disorder. Studies that TC was diagnosed by autopsy will also be excluded since it is known that postmortem evaluation commonly detects incidental thyroid cancer in asymptomatic individuals [11].
Three reviewers (JC, TP, and AA) independently screened the titles and abstracts and obtained full manuscripts for relevant studies. They also read the full text of selected articles and excluded studies that did not meet eligibility criteria. Disagreements among them were resolved by consensus. When they did not reach the consensus, the fourth author (SK) was involved to make a final decision. One author (SO) subsequently extracted the following data: first author, publication year, origin of cancer registry, definition of FAP, study design, duration of follow-up, and patients’ age and gender. Numbers of patients with the following diagnoses were recorded: FAP, TC, benign thyroid masses diagnosed by prospective surveillance screening ultrasound, and endocrinologic thyroid disorders including hypothyroidism, hyperthyroidism, and thyroiditis. The following details regarding TC were obtained: methods of TC diagnosis, age at diagnosis of FAP and TC, bilaterality, multicentricity, and pathology. The location of APC mutation was classified with reference to the mutation cluster region (MCR; codon 1286–1513): 5′ end (proximal to the MCR), within the MCR, and 3′ end (distal to the MCR) [12]. Another author (SK) cross-checked all data and, again, disagreements between them were resolved by consensus. The third author (TP) became involve, if necessary, to make a final decision.
Assessment of risk of bias within studies
Two authors (SO and SK) assessed the risk of bias by utilizing Newcastle–Ottawa Scale for observational study (Supplementary Text 2) [13]. With a maximum score of 9, the scale assesses risk of bias in three domains: (1) selection of the participants; (2) comparability of study groups; and (3) ascertainment of outcomes of interest. Studies with scores ≥ 7 were considered as having low risk of bias, scores of 4–6 as having a moderate risk of bias, and scores of ≤ 3 as having high risk of bias. Where discrepancies arose, articles were re-examined and consensus was reached by discussion and the opinion of a third reviewer (TP).
Statistical analysis
Given the expected considerable heterogeneity between studies, we employed a random-effects model with Logit transformation to estimate the pooled prevalence of TC, benign thyroid masses, and endocrinologic thyroid disorders with 95% CI. I2 index and Cochran’s Q test were utilized to measure the heterogeneities across studies [14]. Using a mixed-effects model [15], we further explored the potential sources of heterogeneity by analyzing the pre-specified subgroups with following moderators: study design (retrospective vs. prospective), use of screening ultrasound for TC diagnosis, number of the study population (N < 200 vs. ≥ 200), and the publication year (before or during 2000 vs. after 2000). TC characteristics were presented as means ± standard deviations (SD) or crude percentages. Sensitivity analysis was performed using one-study remove method to detect the impact of each study on the combined effect. Publication bias was assessed by funnel plot inspection, Egger’s test, and Begg’s test [16, 17]. Analyses were performed by using Comprehensive Meta-Analysis software version 3.3.070 (https://www.meta-analysis.com).
Results
Characteristics of included studies
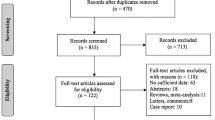
9821 FAP patients from 12 cohort studies published between 1987 and 2016 were included. Steps of literature search were illustrated in Fig. 1. The characteristics and Newcastle–Ottawa Scales of individual studies were provided in Table 1 and Supplementary Table 1, respectively. According to the Newcastle–Ottawa Scales, most studies had moderate risk of bias while one study had high risk of bias [3]. The median Newcastle–Ottawa Scales score was 4. The details of each registry and their respective definition of FAP were further provided in Supplementary Table 2.
Thyroid disease in FAP
From a total of 120 patients who had TC, the crude unweighted mean of TC prevalence was 1.2% (ranging 0.4–11.8%). Meta-analytic pooled prevalence of TC in FAP (Fig. 2A) was 2.6% (95% CI 1.3–4.8%). Characteristics of patients with TC were provided in Table 2. Female was presented in 95 ± 3.9% of the affected patients. In FAP patients who had TC, the mean age of FAP diagnosis was 29 ± 11.4 years old, whereas that of TC diagnosis was 31 ± 11.8 years old. Approximately one-third (34 ± 9%) of TC patients had presented with TC prior to the diagnosis of FAP. 45.6 ± 12.9% of TC was bilateral and 58.8 ± 9.6% was multicentric.
Papillary TC was the most common pathology (100/120; 83.3%); 26 out of 100 specimens (26%) were reported as CMV-PTC. The remaining specimens were reported as follicular type (4/120; 3.3%), medullary type (1/120; 0.8%), and mixed type (6/120; 5%). The pathology was unavailable in 9 patients (7.5%). Data on the location of APC mutation in TC were available in 24 patients (data not shown): 19 patients (79.2%) had mutation at the 5′ end; 4 patients (16.6%) at the 3′ end; and only one patient (4.2%) harbored the mutation in the MCR.
Five studies (n = 411) proactively performed screening thyroid ultrasound [9, 22,23,24,25]. In 368 individuals who received ultrasound, 179 of them had benign thyroid nodules–making a pooled prevalence of 48.8% (95% CI 33.8–64.0%), as shown in Fig. 2B. Three studies (n = 321) reported the prevalence of endocrinologic thyroid disorders, with a subsequent pooled prevalence of 6.9% (95% CI 4.5–10.3%; Fig. 2C) [6, 23, 24].
Heterogeneity and subgroup analysis
When evaluating the prevalence of TC, there was a high level of heterogeneity across all studies (Q = 117.23, df = 11, P < 0.001, I2 = 90.61). The results of subgroup analyses were provided in Table 3. We found that the prevalence of TC was significantly higher in (a) studies that used screening ultrasound (6.9%, I2 = 0) compared to the non-use group (1.4%, I2 = 85.6); (b) studies with study population < 200 (6.3%, I2 = 0) compared to those with study population ≥ 200 (1.2%, I2 = 92.3%); and (c) studies published after 2002 (5%, I2 = 83%) as opposed to those published before or during 2002 (1%, I2 = 83.8%). The difference in the prevalence was not observed when classifying studies by study type.
Sensitivity analysis and publication bias
Pooled prevalence was recalculated iteratively after each study was removed; subsequent pooled prevalence was not significantly different from one another and ranged between 2.2 and 3.0%, indicating robustness of the included studies. The funnel plot depicted in Fig. 3 was almost symmetric, indicating the absence of publication bias. This was confirmed by the Egger’s test (P = 0.10) and Begg’s test (P = 0.15).
Discussion
To our knowledge, this present meta-analysis is the largest group of pooled cases of thyroid disease in FAP patients, including 9821 individuals from 12 cohort studies. It confirmed a higher prevalence of TC, up to 19-fold, in this patient population when compared to the prevalence of TC in the United States (2.6 vs. 0.14%, respectively) [5]. Our results corroborated earlier observation that TC in FAP tended to occur in young female, presented with papillary TC as the most common pathology, had a high rate of mulcentric and bilateral diseases, and preceded the diagnosis of FAP in approximately one-third of the cases [3, 9, 18, 21].
Generally, TC shows a male-to-female ratio of 1:3–1:4 and has a mean age at diagnosis of 49 years for women and 53 years for men [5, 26]. We found that FAP-associated TC had a more pronounced gender discrepancy (male-to-female ratio of 1:19) and occurred at a younger age (mean age at diagnosis of 31 years). It was postulated that female aged less than 35 years with FAP had a 160-time higher risk of developing TC [18], and that papillary TC in FAP and in sporadic cases are, in fact, different disease entities [27]. Nevertheless, the decision to refer all young female patients diagnosed with papillary TC for colonoscopy remains inconclusive [28]. According to previous literature, CMV-PTC histologic subtype was considered a frequent and specific finding in FAP and its presence should alert clinicians to perform a colonoscopy and/or APC gene analysis [29, 30]. A recent study however showed that only 39% of the published CMV-PTC cases harbored germline APC mutation [29], whereas at least 26% of all patients with FAP-associated TC in our study demonstrated CMV-PTC subtype. We therefore hypothesized that the sensitivity and specificity of CMV-PTC variant for diagnosing FAP may not be as high as previously thought. Additionally, CMV-PTC is rare and its diagnosis is challenging even for experienced cytologists [31]. Considering the multicentricity and bilaterality, intraoperative multicentric and bilateral diseases were found respectively in 50 and 32% among all papillary TC cases in a study by Gerfo et al. [32], while our analysis showed that the proportions of multicentricity and bilaterality in FAP-associated TC were respectively 58.8 and 45.6%. Hence, our results support younger age at diagnosis and almost exclusive occurrence in female as major different characteristics of FAP-associated papillary TC, when comparing to sporadic cases. On the other hand, the diagnostic utility of histopathologic features (multicentricity, bilaterality, and the presence of CMV-PTC variant) to differentiate the sporadic and familial forms of TC is uncertain and requires further investigations.
In non-selected cases of FAP, more than 60% of mutations in APC gene are found in the MCR, between codons 1284 and 1580 [33]. Mutation at the 5′ end of APC gene (i.e. proximal to the MCR) is common in patients with FAP-associated TC [33, 34]. Our result showed that out of 24 mutations, 19 of them located in the 5′ end proximal to the MCR. Septer et al. recently analyzed the largest pool of APC mutation data (n = 48) in FAP-associated TC cases, emphasized an increasing risk of TC if the patient harbors a mutation in codon 1061 or any mutation proximal to codon 528, and concluded that annual surveillance ultrasound should be performed from age 18 onwards if the patient has these mutations [33]. By combining our data to those of Septer et al., genotypes of FAP-associated TC were available in 52 patients; 44.2% of them were localized at the 5′ end, proximal to codon 938, whereas only 17.5% of the mutations in the reference population with FAP occurred in this region [33]. This, therefore, stresses a high frequency of the mutations in FAP-associated TC in the 5′ end of APC gene and confirms its genotype–phenotype correlation. The pathomechanism of this phenomenon is unclear, but there has been a suggestion that an activation of RET/PTC1 oncogene in thyroid tissue may play an additional role in the carcinogenesis of TC in FAP patients [35].
We found a high pooled prevalence of benign thyroid masses (48.8%) in FAP patients who underwent screening thyroid ultrasound. Without using routine ultrasound, the occurrence of benign thyroid masses in FAP were only 0.2–6% [6, 36]. This discrepancy is similar to that of general population when using thyroid ultrasound results in higher incidence of thyroid masses when compared to manual palpation [37]. Few studies have reported the occurrence of thyroiditis, hypothyroidism, and hyperthyroidism in FAP [6, 10, 23, 24] while we found that 6.9% of FAP patients had endocrinologic thyroid disorders. Although the number is not justified for regular thyroid function testing, the treating physicians should be aware of this phenotypic spectrum. In normal population, the link between TC and benign thyroid disorders is not negligible [38, 39]. Unfortunately, the disease trajectory and clinical outcome of these benign conditions in FAP are unknown. The occurrence of FAP-associated TC in the background of lymphocytic thyroiditis, as well as Grave’s disease, has been characterized [7, 10]. Herraiz et al. have described ultrasonographic changes in two FAP patients after a follow-up at an interval of 27 months: one with an enlargement of a pre-existing nodule and another one with the development of two new benign nodules [9]. Their data may confer the slow rate of growth of the nodules; however, a larger, prospective study is necessary to describe their natural history and potential associations with TC, and an appropriate interval for follow-up. Until the risk is better delineated and guideline is established, periodic ultrasound and/or laboratory follow-up to monitor these benign thyroid disorders are crucial. It was suggested that an interval of greater than one year is possibly safe for a follow-up ultrasound once thyroid nodules are detected [9].
Increasing diagnosis of TC has been well recognized while the mortality from TC has remained stable [26]. Most new cases can be attributed to the diagnosis of papillary TC with small or non-palpable lesions due to advanced diagnostic techniques and increasing surveillance in young or middle-aged population [26, 40]. Our study corroborates with this finding by showing that the publication year after 2002 and the use of surveillance thyroid ultrasound are associated with a higher prevalence of TC [40]. Papillary TC is the least aggressive histologic subtype and most patients will never develop clinically significant symptoms [26, 40]. Despite its often multicentricity and nodal involvement in FAP-associated TC, the condition shows an indolent course [27]; in fact, recent study has even suggested a possibility of spontaneous regression of CMV-PTC in FAP after the age of 35 years [31]. While the observed-to-expected morbidity ratio (i.e. standardized morbidity ratio) of TC in FAP was reportedly 19.2 in one study [41], various studies indicated that mortality of FAP-associated TC is extremely low and patients tended to die from another causes [41,42,43].
To detect FAP-associated TC, current recommendations controversially include both annual clinical examination and a referral, if nodules are present [1], and routine use of screening ultrasound [6, 9, 22]. The American College of Gastroenterology recommended FAP patients to undergo annual screening thyroid ultrasound [44]; however, this recommendation has a low quality of evidence. The uncertainty was re-addressed by the American Thyroid Association that the use of ultrasound may lead to an early diagnosis of FAP-associated TC, and there is insufficient evidence that these interventions would reduce morbidity and mortality [30]. In general population, the United States Preventive Services Task Force found inadequate evidence on the benefits of screening and recommended against TC screening in asymptomatic adults. The sensitivity and specificity of thyroid ultrasound to detect one or more malignant features were reportedly 94.3 and 55.0%, respectively [45]. Hence, universal thyroid ultrasound approach may lead to not only a burden and significant anxiety to the patients undergoing the screening procedure, but also false positive results due to its low specificity [36]. Among few studies investigating the consequences of thyroid ultrasound screening in FAP, Feng et al. have shown that TC in patients undergone thyroid ultrasound (n = 15) may have a more favorable morbidity profile when compared to those in nonscreening-detected cases (n = 18) [24]. However, the study may be too small to draw a conclusion. Based on aforementioned findings, the current evidence on the overdiagnosis and overtreatment of TC (particularly unaggressive papillary subtype), and the lack of cost-effectiveness analysis for the use of ultrasound surveillance in FAP-associated TC, we recommend against the use of surveillance ultrasound to detect any thyroid abnormalities in every FAP patients. Until a larger study is conducted, screening thyroid ultrasound among FAP patients with high risk of TC, such as young female with the APC mutation proximal to the 5′ end, seems reasonable.
Several limitations should be considered when interpreting our results. First, risk of bias across studies exists because all recruited studies are heterogeneous. Apart from the use of thyroid ultrasound, sample size of the study, and publication year, which affect the prevalence of TC according to the subgroup analyses, other possible factors include demographic difference in each study and operator dependency of ultrasound. Furthermore, although most studies used routine thyroid ultrasound to diagnose TC [9, 22,23,24,25], a study by Steinhagen et al. detected TC cases from palpable nodules and non-screening ultrasound [6]. Second, the included articles were observational studies and risk of biases within studies exist—both selection bias and information bias. Most of them had moderate risk of bias and none of them complied all items in the Newcastle–Ottawa Scales. Third, two studies conducted during 1990s did not provide details on FAP definition [3, 4]; however, the fact that all selected studies were derived from either hospital-based or population-based cancer registries is a strength of our study due to presumptive robust methodology and systematic data collection as exact cancer identification are generally required [46]. However, one must consider the referral bias in each cancer registry since not all patients with FAP end up in a registry and it is more likely that only complicated patients were recruited. The definition of FAP in each registry and inclusion of other similar phenotypes (such as attenuated FAP and MUTYH-associated polyposis syndrome) may further complicate between-study heterogeneity, as shown in Supplementary Table 2. Fourth, not all included papers reported the occurrence of CMV-PTC especially the older ones; thus, the prevalence of this particular histology may be underestimated. Fifth, the present study did not include unpublished studies and studies published in non-English languages. This may under- or overestimate the actual prevalence of TC-associated FAP. Finally, the number of studies and overall number of patients when analyzing the prevalence of benign thyroid disorders were relatively small.
In conclusion, benign thyroid disorders are common in FAP but their clinical significance and an appropriate follow-up interval are yet undetermined. We described the characteristics of FAP-associated TC and concluded that younger age at diagnosis and almost exclusive occurrence in female are major differentiating characteristics of FAP-associated and sporadic forms of TC. We confirmed the genotype–phenotype correlation of TC in FAP patients and stressed a high frequency of the mutations in the 5′ end of APC gene. We demonstrated that the prevalence of TC in FAP patients is 2.6%, which is 19-fold higher than general population; however, the absolute prevalence is still very low. Its increased prevalence in recent years can also be explained by the detection method bias—this is consistent with the global trend of overdiagnosis and overtreatment of TC. Based on the controversies from current guidelines, a low absolute prevalence, a low specificity of thyroid ultrasound to detect TC, a high proportion of papillary TC with resultant low morbidity and mortality, a lack of cost-effectiveness analysis, and a potential burden for patients if the universal screening approach is employed, we suggest that only a selective subgroup of FAP patients with a high risk of developing TC, such as young female with APC mutation at the 5′ end, might benefit from routine surveillance ultrasound.
References
Groen EJ, Roos A, Muntinghe FL et al (2008) Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol 15(9):2439–2450. https://doi.org/10.1245/s10434-008-9981-3
Crail HW (1949) Multiple primary malignancies arising in the rectum, brain, and thyroid; report of a case. U S Nav Med Bull 49(1):123–128
Perrier ND, van Heerden JA, Goellner JR et al. (1998) Thyroid cancer in patients with familial adenomatous polyposis. World journal of surgery 22(7):738–742 (discussion 43)
Bulow C, Bulow S (1997) Is screening for thyroid carcinoma indicated in familial adenomatous polyposis? The leeds castle polyposis group. Int J Colorectal Dis 12(4):240–242
Howlader N, Noone AM, Krapcho M et al (2016) SEER Cancer Statistics Review, 1975–2013. National Cancer Institute, Bethesda
Steinhagen E, Guillem JG, Chang G et al (2012) The prevalence of thyroid cancer and benign thyroid disease in patients with familial adenomatous polyposis may be higher than previously recognized. Clin Colorectal Cancer 11(4):304–308. https://doi.org/10.1016/j.clcc.2012.01.006
Harach HR, Williams GT, Williams ED (1994) Familial adenomatous polyposis associated thyroid carcinoma: a distinct type of follicular cell neoplasm. Histopathology 25(6):549–561
Tomoda C, Miyauchi A, Uruno T et al (2004) Cribriform-morular variant of papillary thyroid carcinoma: clue to early detection of familial adenomatous polyposis-associated colon cancer. World J Surg 28(9):886–889
Herraiz M, Barbesino G, Faquin W et al (2007) Prevalence of thyroid cancer in familial adenomatous polyposis syndrome and the role of screening ultrasound examinations. Clin Gastroenterol Hepatol 5(3):367–373. https://doi.org/10.1016/j.cgh.2006.10.019
Herrera L, Carrel A, Rao U, Castillo N, Petrelli N (1989) Familial adenomatous polyposis in association with thyroiditis. Report of two cases. Dis Colon Rectum 32(10):893–896
Furuya-Kanamori L, Bell KJ, Clark J, Glasziou P, Doi SA (2016) Prevalence of differentiated thyroid cancer in autopsy studies over six decades: a meta-analysis. J Clin Oncol. https://doi.org/10.1200/JCO.2016.67.7419
Miyoshi Y, Nagase H, Ando H et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1(4):229–233
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to meta-analysis. Wiley, Chichester
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Plail RO, Bussey HJ, Glazer G, Thomson JP (1987) Adenomatous polyposis: an association with carcinoma of the thyroid. Br J Surg 74(5):377–380
van der Linde K, Vasen HF, van Vliet AC (1998) Occurrence of thyroid carcinoma in Dutch patients with familial adenomatous polyposis: an epidemiological study and report of new cases. Eur J Gastroenterol Hepatol 10(9):777–781
Ho JW, Chu KM, Tse CW, Yuen ST (2002) Phenotype and management of patients with familial adenomatous polyposis in Hong Kong: perspective of the Hereditary Gastrointestinal Cancer Registry. Hong Kong medical journal = Xianggang yi xue za zhi/Hong Kong. Acad Med 8(5):342–347
Truta B, Allen BA, Conrad PG et al. (2003) Genotype and phenotype of patients with both familial adenomatous polyposis and thyroid carcinoma. Familial Cancer 2(2):95–99
Martayan A, Sanchez-Mete L, Baldelli R et al (2010) Gene variants associated to malignant thyroid disease in familial adenomatous polyposis: a novel APC germline mutation. J Endocrinol Investig 33(9):603–606
Steinhagen E, Hui VW, Levy RA et al (2014) Results of a prospective thyroid ultrasound screening program in adenomatous polyposis patients. Am J Surg 208(5):764–769. https://doi.org/10.1016/j.amjsurg.2014.03.012
Feng X, Milas M, O’Malley M et al (2015) Characteristics of benign and malignant thyroid disease in familial adenomatous polyposis patients and recommendations for disease surveillance. Thyroid 25(3):325–332. https://doi.org/10.1089/thy.2014.0107
Casellas-Cabrera N, Diaz-Algorri Y, Carlo-Chevere VJ et al (2016) Risk of thyroid cancer among Caribbean Hispanic patients with familial adenomatous polyposis. Familial Cancer 15(2):267–274. https://doi.org/10.1007/s10689-015-9862-4
Davies L, Welch HG (2014) Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140(4):317–322. https://doi.org/10.1001/jamaoto.2014.1
Cetta F, Ugolini G, Barellini L, Civitelli S, Carmellini M (2011) FAP associated cribriform morular variant of PTC: striking female prevalence and indolent course. Endocr J 58(9):817–818
Harb WJ, Sturgis EM (2009) Differentiated thyroid cancer associated with intestinal polyposis syndromes: a review. Head Neck 31(11):1511–1519. https://doi.org/10.1002/hed.21156
Pradhan D, Sharma A, Mohanty SK (2015) Cribriform-morular variant of papillary thyroid carcinoma. Pathol Res Pract 211(10):712–716. https://doi.org/10.1016/j.prp.2015.04.011
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133. https://doi.org/10.1089/thy.2015.0020
Uchino S, Ishikawa H, Miyauchi A et al (2016) Age- and gender-specific risk of thyroid cancer in patients with familial adenomatous polyposis. J Clin Endocrinol Metab 101(12):4611–4617. https://doi.org/10.1210/jc.2016-2043
Gerfo PL, Chabot J, Gazetas P (1990) The intraoperative incidence of detectable bilateral and multicentric disease in papillary cancer of the thyroid. Surgery 108(6):958–962 (discussion 62-3)
Septer S, Slowik V, Morgan R, Dai H, Attard T (2013) Thyroid cancer complicating familial adenomatous polyposis: mutation spectrum of at-risk individuals. Hered Cancer Clin Pract 11(1):13. https://doi.org/10.1186/1897-4287-11-13
Cetta F, Montalto G, Gori M, Curia MC, Cama A, Olschwang S (2000) Germline mutations of the APC gene in patients with familial adenomatous polyposis-associated thyroid carcinoma: results from a European cooperative study. J Clin Endocrinol Metab 85(1):286–292. https://doi.org/10.1210/jcem.85.1.6254
Cetta F, Chiappetta G, Melillo RM et al (1998) The ret/ptc1 oncogene is activated in familial adenomatous polyposis-associated thyroid papillary carcinomas. J Clin Endocrinol Metab 83(3):1003–1006. https://doi.org/10.1210/jcem.83.3.4614
Ghorbanoghli Z, Bastiaansen BA, Langers AM et al (2017) Extracolonic cancer risk in Dutch patients with APC (adenomatous polyposis coli)-associated polyposis. J Med Genet. https://doi.org/10.1136/jmedgenet-2017-104545
Vanderpump MP (2011) The epidemiology of thyroid disease. Br Med Bull 99:39–51. https://doi.org/10.1093/bmb/ldr030
Staniforth JU, Erdirimanne S, Eslick GD (2016) Thyroid carcinoma in Graves’ disease: a meta-analysis. Int J Surg 27:118–125. https://doi.org/10.1016/j.ijsu.2015.11.027
Lee J-H, Kim Y, Choi J-W, Kim Y-S (2013) The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol 168(3):343–349. https://doi.org/10.1530/eje-12-0903
Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L (2016) Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 375(7):614–617. https://doi.org/10.1056/NEJMp1604412
Iwama T, Mishima Y, Utsunomiya J (1993) The impact of familial adenomatous polyposis on the tumorigenesis and mortality at the several organs. Its rational treatment. Ann Surg 217(2):101–108
Gibbons DC, Sinha A, Phillips RK, Clark SK (2011) Colorectal cancer: no longer the issue in familial adenomatous polyposis? Familial Cancer 10(1):11–20. https://doi.org/10.1007/s10689-010-9394-x
de Campos FG, Perez RO, Imperiale AR, Seid VE, Nahas SC, Cecconello I (2010) Evaluating causes of death in familial adenomatous polyposis. J Gastrointest Surg 14(12):1943–1949. https://doi.org/10.1007/s11605-010-1288-6
Syngal S, Brand RE, Church JM et al. (2015) ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110(2):223–262. https://doi.org/10.1038/ajg.2014.435
Force USPST, Bibbins-Domingo K, Grossman DC et al (2017) Screening for thyroid cancer: US preventive services task force recommendation statement. JAMA 317(18):1882–1887. https://doi.org/10.1001/jama.2017.4011
Mohammadzadeh Z, Ghazisaeedi M, Nahvijou A, Kalhori SR, Davoodi S, Zendehdel K (2017) Systematic review of hospital based cancer registries (HBCRs): necessary tool to improve quality of care in cancer patients. Asian Pac J Cancer Prev 18(8):2027–2033. https://doi.org/10.22034/APJCP.2017.18.8.2027
Acknowledgements
We would like to express our gratitude to Marian MacMaster and Joan Yanicke, our lovely librarians at the Medical Library, Metrowest Medical Center, for checking the relevant search terms and helping us retrieve all the relevant articles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chenbhanich, J., Atsawarungruangkit, A., Korpaisarn, S. et al. Prevalence of thyroid diseases in familial adenomatous polyposis: a systematic review and meta-analysis. Familial Cancer 18, 53–62 (2019). https://doi.org/10.1007/s10689-018-0085-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-018-0085-3