Abstract
Possession of a BRCA1/2 mutation increases risk of contralateral breast and ovarian cancer recurrence and may have an impact on health management decisions, such as imaging screening, preventive surgical interventions and systemic therapies. A hospital-based study was conducted to assess the frequency and spectrum of pathogenic germline BRCA1 and BRCA2 mutations in Polish women with familial and nonfamilial breast cancer. Genomic DNA was extracted from 1581 women with breast cancer and from 2225 healthy individuals. For genotyping BRCA1 (5382insC, T300G, 3819del5, 185delAG, C5370T, 3875del4, 3896delT, 4153delA, 4184del4, 4160delAG, G5332A) mutations and BRCA2 (G1408T, 5467insT, 6174delT, 6192delAT, 6675delTA, 8138del5, 9152delT, C9610T, 9630delC) mutations, a Custom TaqMan (Applied Biosystems) PCR-based technology was adopted. A BRCA1 mutation was found in 26 and 12.5 % of women with familial breast cancer and in 13 and 8.3 % nonfamilial (sporadic) breast cancer, diagnosed before or after 50 years of age, respectively. A much lower frequency of BRCA2 mutation was observed. The predominance of seven BRCA1 mutations (5382insC, T300G, 3819del5, 185delAG, C5370T, 3875del4, 4153delA) studied in the Masovian voivodeship population confirmed a strong founder effect for BRCA1 mutations in the Polish population, and the results of BRCA2 testing confirmed a high diversity in the studied pathogenic mutations in BRCA2 gene. We propose offering inexpensive testing for the presence of BRCA1 founder mutations to all Polish women at the time of initial breast cancer diagnosis, regardless of the patient’s family history or age of disease onset.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common type of cancer in the Polish population, and is a leading cause of cancer-related morbidity and mortality [1]. Most breast cancers are sporadic and their heritable risk may be explained by a combination of modest effects of inherited common low-penetrance polymorphisms of multiple genes [2, 3]. Highly penetrant hereditary breast cancers result from strong effect size mutations of a single “cancer gene”, and their risk can be recognized by family history, even if high-penetrance mutations are not identified [4].
Germline mutations in BRCA1 and BRCA2 genes confer a risk of breast cancer up to 30-times higher than the general population risk, and they account for up to 80 % of breast/ovarian cancer families [5, 6]. The lifetime risk of BRCA1/2 mutation carriers for developing breast and ovarian cancer is as high as 85 and 64 %, respectively [6]. According to the Breast Information Core (http://research.nhgri.nih.gov/bic/), more than 1700 BRCA1 mutations, polymorphisms and variants are deposited in the database [7]. The incidence and spectrum of the mutations vary among populations, and the carrier frequency in general populations ranges between 1/40 and 1/800 [8]. Some populations demonstrate a wide spectrum of mutations, while others are characterized by high prevalence of a small number of founder mutations [9]. In Poland, the frequency of BRCA1 mutations is estimated between 1/240 and 1/360, and the two founder mutations 5382insC and T300G (C61G) account for 70–90 % of the BRCA1 mutations found in the Polish population [8]. None of the BRCA1 founder mutations is unique to Poland.
Targeted genetic counseling and testing for the presence of BRCA1/2 mutations are mostly offered in the context of familial cancer genetics, and focus on high-risk factors, such as young age of diagnosis and the number of relatives affected by breast and/or ovarian cancers. However, since 30–50 % of women with a BRCA1/2 mutation have no known family history of breast or ovarian cancer [10, 11], and possession of a BRCA1/2 mutation may have an impact on health management decisions [12–14], other recommendations for testing of BRCA1/2 mutations are also possible.
In the study presented here, we asked whether the predictive testing guidelines in the Polish population could be readily limited to screening the most frequent BRCA1/2 mutations, and whether inclusion criteria should be based on the family history and age of screeners. To address this, we compared the incidence of selected BRCA1 and BRCA2 mutations screened in Polish women with familial and nonfamilial breast cancer. Selection of the most frequent mutations to be screened was based on the results of previous studies [7, 8, 15–19].
Materials and methods
Study population
A hospital-based study of Polish women with breast cancer was conducted at the Cancer Center-Institute of Oncology. Between 2003 and 2010, blood samples were collected from a total of 1581 women with newly-diagnosed breast cancer, predominately exhibiting early age of onset. Most of 906 cases who represented a family with at least one more breast or ovarian cancer diagnosis in a first- or second-degree relative were recruited at either the Department of Breast Cancer and Reconstructive Surgery or Genetic Counseling Unit when most of 675 cases with negative history for breast and/or ovarian cancer were recruit at the Department of Breast Cancer. The personal and familial cancer history was acquired by in-depth interviews in all patients. Patients from the both groups were treated mostly at the Warsaw Cancer Center. In addition, 2225 healthy individuals (1629 females and 596 males) exhibiting no known history of malignancy, normal results of screening colonoscopy, and normal mammography or PSA levels, were recruited, primarily from the National Colorectal Cancer Screening Program. The median age at diagnosis for women with breast cancer and healthy controls was 45 years (range: 17–85) and 58 years (range: 40–81), respectively. All patients and control subjects were Polish Caucasians recruited from the Masovian voivodeship population.
Mutation analysis
Genomic DNA was extracted from whole blood and treated with EDTA using the QIAamp DNA Mini Kit (Qiagen, Germany), following the manufacturer’s protocol. DNA samples that passed quality control were then brought to a final concentration of 50 ng/μl in Tris–EDTA buffer (pH = 8), with concentrations of Tris and EDTA not exceeding limits of 10 and 0.1 mM, respectively. Individual genotyping was carried out using Custom TaqMan SNP Genotyping Assays (Life Technologies, USA), a SensiMix™ II Probe Kit (Bioline Ltd, United Kingdom), and a 7900HT Real-Time PCR system (Life Technologies, USA). Samples with detected mutation were sequenced.
The BRCA1/2 mutation frequency disproportions between the specific groups of breast cancer cases and the group of control subjects were tested using the Fisher’s exact test implemented in PLINK v1.07 software (http://pngu.mgh.harvard.edu/purcell/plink/) [20]. The Bonferroni correction for multiple testing was used.
Results
To analyze the frequency and spectrum of pathogenic germline BRCA1 and BRCA2 mutations in Polish women with breast cancer, we selected 11 BRCA1 mutations (185delAG, T300G, 3819del5, 3875del4, 3896delT, 4153delA, 4160delAG, 4184del4, G5332A, C5370T, 5382insC) and 9 BRCA2 mutations (G1408T, 5467insT, 6174delT, 6192delAT, 6675delTA, 8138del5, 9152delT, C9610T, 9630delC) based on the results of previously reported research conducted on Polish subpopulations [7, 8, 15–19]. Most of the mutations tested introduce premature stop codons. The proportion of breast cancer patients with or without mutations was calculated for familial and nonfamilial instances and by age of onset.
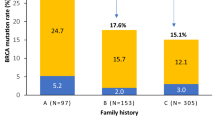
A total of 266 (16.8 %) BRCA1 mutations (Table 1) and 14 (0.89 %) BRCA2 mutations (Table 2) were found among 1581 cancer patients. Of these, the highest prevalence was found for the two Polish founder mutations, 5382insC and T300G, which accounted for 82.3 %, and the four most common mutations (5382insC, T300G, 3819del5, 185delAG) accounted for 91 % of all detected BRCA1 mutations (Table 1). Five BRCA1 mutations (5382insC, 3896delT, 185delAG, 4153delA, C5370T) were also detected in nine (0.4 %) of 2225 healthy controls, while no BRCA2 mutations were found in the control group.
Breast cancers were classified as familial with non-restrictive criteria when a proband represented a family with at least one breast or ovarian cancer diagnosis in a first- or second-degree relative. Information about cancers in relatives was obtained from the patient’s interview. As expected, the frequency of BRCA1 (Table 1) and BRCA2 (Table 2) mutations was higher in familial than in nonfamilial breast cancer patients. A BRCA1 and BRCA2 mutation was detected in 20.8 and 1.3 % patients with familial cancer and in 11.6 and 0.3 % patients with nonfamilial breast cancer, respectively. Notably, the high significance of association obtained for the dominant model of inheritance was particularly well pronounced in the nonfamilial breast cancer cases, and was predominantly constituted by the T300G and 5382insC BRCA1 gene mutations (Table 1).
Hereditary breast cancers are characterized by an early age of onset. In fact, in the screened breast cancer patients with a family history of breast and/or ovarian cancer, a BRCA1 mutation was found in 144 (26 %) of 553 and 44 (12.5 %) of 353 cases in which the disease was diagnosed before or after 50 years of age, respectively (Table 1). However, an unexpectedly high number of mutation positive women were also found among nonfamilial breast cancer patients. In this group, a BRCA1 mutation was detected in 61 (13 %) of 471 and 17 (8.3 %) of 204 patients with the disease onset before or after 50 years of age, respectively (Table 1).
Discussion
Specific founder mutations in BRCA1 are common in several populations. The study on relative contribution of founder and nonfounder BRCA mutations in all regions of Poland revealed that >60 % of breast and breast-ovarian cancer families with 3 or more cases of cancer carried BRCA mutation; of them 91 % was one of the 3 common founder mutations (5382incC, T300G, 4153delA) [7]. As reported recently, the most frequent mutations in unselected Polish breast and ovarian cancer patients were 5382incC and T300G [11]. The dominance of these two mutations, also found in our study, confirms a strong founder effect for BRCA1 mutations in the Polish population [15–18].
We found a BRCA1 mutation in 26 and 12.5 % of women with breast cancer diagnosed before or after 50 years of age, respectively, among whom at least one verified breast or ovarian cancer was also diagnosed in a first- or second-degree relative. In women diagnosed with sporadic breast cancer before or after 50 years of age, a BRCA1 mutation was found in 13 and 8.3 % patients, respectively (Table 1). Thus, unselected nonfamilial breast cancer patients, especially those who were diagnosed with breast cancer after 50 years of age, revealed unexpectedly high prevalence of the BRCA1 mutations. In contrast to the high prevalence of BRCA1 mutations found in both familial and sporadic breast cancers, even with late onset, a much lower frequency of BRCA2 mutations was observed.
According to roughly similar national guidelines and recommendations, genetic testing is preferentially offered to families with breast and/or ovary cancer history in the context of a familial cancer service [21]. Until now, Genetic Counseling Unit at the Warsaw Cancer Center-Institute of Oncology has offered adequate counseling and testing for the presence of BRCA1/2 mutations mostly to patients with familial breast and/or ovary cancer and their relatives. However, recently published studies revealed rather limited value of cancer family history for prediction of BRCA1 mutation presence in Polish breast and ovarian cancer patients [11]. In a consequence, it has been suggested the necessity for targeted genetic testing for T300G mutation in families with a single diagnosis of ovarian cancer [11].
The high frequency of BRCA1 mutations in nonfamilial breast cancers, as found in our study, may expand the indications for genetic testing of founder mutations to also include women with nonfamilial (sporadic) breast cancer. Such an expanded inclusion criteria would not significantly increase the cost of genetic testing, because testing limited to the sets of founder mutations represents a rather inexpensive and rapid analytical procedure. A cost-effective (internal costs, not including personnel and overhead, are around $20) PCR-based genotyping technology has proven to be adequately reliable in Polish population genetic screening. Unfortunately, a similar inexpensive genetic screening test for BRCA2 mutations could not be established.
Women affected with BRCA1/2-related breast cancer have a higher risk of developing independent, contralateral breast and ovarian cancer [5, 22]. To identify breast cancer at an early stage, an intensified surveillance program should be provided [21]. However, negative result of genetic testing is not informative for assessing the cancer risk. Therefore, genetic testing should not be offered without adequate genetic counseling that considers all biomedical, social and ethical challenges, as well as the implementation costs of surveillance programs offered to probands and their family members. To this end, while genetic testing integrated with breast cancer diagnosis can have an impact on prophylactic and therapeutic decisions [10, 22], it may not be universally accepted.
In summary, inexpensive testing for the presence of BRCA1 founder mutations should be offered to all Polish women at the time of initial breast cancer diagnosis, regardless of a patient’s family history or the age of disease onset.
References
Wojciechowska U, Didkowska J, Zatoński W (2010) Cancer in Poland in 2008. 1–124. http://www.onkologia.org.pl/doc/Nowotwory2008.pdf
Ostrowski J, Wyrwicz LS (2009) Integrating genomics, proteomics and bioinformatics in translational studies of molecular medicine. Expert Rev Mol Diagn 9:623–630. doi:10.1586/erm.09.41
Kenemans P, Verstraeten RA, Verheijen RHM (2004) Oncogenic pathways in hereditary and sporadic breast cancer. Maturitas 49:34–43. doi:10.1016/j.maturitas.2004.06.005
Foulkes WD (2008) Inherited susceptibility to common cancers. N Engl J Med 359:2143–2153. doi:10.1056/NEJMra0802968
Antoniou A, Pharoah PDP, Narod S, Risch HA, Eyfjord JE et al (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72:1117–1130. doi:10.1086/375033
Friedman E, Kotsopoulos J, Lubinski J, Lynch HT, Ghadirian P et al (2006) Spontaneous and therapeutic abortions and the risk of breast cancer among BRCA mutation carriers. Breast Cancer Res 8:R15. doi:10.1186/bcr1387
Górski B, Jakubowska A, Huzarski T, Byrski T, Gronwald J et al (2004) A high proportion of founder BRCA1 mutations in Polish breast cancer families. Int J Cancer 110:683–686. doi:10.1002/ijc.20162
Brozek I, Cybulska C, Ratajska M, Piatkowska M, Kluska A et al (2011) Prevalence of the most frequent BRCA1 mutations in Polish population. J Appl Genet 52:325–330. doi:10.1007/s13353-011-0040-6
Ferla R, Calò V, Cascio S, Rinaldi G, Badalamenti G et al (2007) Founder mutations in BRCA1 and BRCA2 genes. Ann Oncol 18(Suppl 6):vi93–vi98. doi:10.1093/annonc/mdm234
Trainer AH, Lewis CR, Tucker K, Meiser B, Friedlander M et al (2010) The role of BRCA mutation testing in determining breast cancer therapy. Nat Rev Clin Oncol 7:708–717. doi:10.1038/nrclinonc.2010.175
Brozek I, Ratajska M, Piatkowska M, Kluska A, Balabas A et al (2012) Limited significance of family history for presence of BRCA1 gene mutation in Polish breast and ovarian cancer cases. Fam Cancer 11:1–4. doi:10.1007/s10689-012-9519-5
Turner NC, Reis-Filho JS (2006) Basal-like breast cancer and the BRCA1 phenotype. Oncogene 25:5846–5853. doi:10.1038/sj.onc.1209876
Tilanus-Linthorst M, Verhoog L, Obdeijn I-M, Bartels K, Menke-Pluymers M et al (2002) A BRCA1/2 mutation, high breast density and prominent pushing margins of a tumor independently contribute to a frequent false-negative mammography. Int J Cancer 102:91–95. doi:10.1002/ijc.10666
Warner E, Plewes DB, Shumak RS, Catzavelos GC, Di Prospero LS et al (2001) Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol 19:3524–3531
Górski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J et al (2000) Founder mutations in the BRCA1 gene in Polish families with breast-ovarian cancer. Am J Hum Genet 66:1963–1968. doi:10.1086/302922
Skasko E, Paszko Z, Niwińska A, Kwiatkowska E, Kruczek A et al (2004) The presence of hereditary BRCA1 gene mutations in women with familial breast or ovarian cancer and the frequency of occurrence of these tumours in their relatives. Eur J Gynaecol Oncol 25:470–474
Grzybowska E, Siemińska M, Zientek H, Kalinowska E, Michalska J et al (2002) Germline mutations in the BRCA1 gene predisposing to breast and ovarian cancers in Upper Silesia population. Acta Biochim Pol 49:351–356
Skasko E, Kluska A, Niwińska A, Kwiatkowska E, Bałabas A et al (2009) Age at onset of bilateral breast cancer, the presence of hereditary BRCA1, BRCA2, CHEK2 gene mutations and positive family history of cancer. Onkologie 32:182–188. doi:10.1159/000200930
Balabas A, Skasko E, Nowakowska D, Niwinska A, Blecharz P (2010) Novel germline mutations in BRCA2 gene among breast and breast-ovarian cancer families from Poland. Fam Cancer 9:267–274. doi:10.1007/s10689-010-9338-5
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. doi:10.1086/519795
Gadzicki D, Evans DG, Harris H, Julian-Reynier C, Nippert I et al (2011) Genetic testing for familial/hereditary breast cancer-comparison of guidelines and recommendations from the UK, France, the Netherlands and Germany. J Community Genet 2:53–69. doi:10.1007/s12687-011-0042-4
Pharoah PDP, Antoniou A, Bobrow M, Zimmern RL, Easton DF et al (2002) Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet 31:33–36. doi:10.1038/ng853
Acknowledgments
This work was supported by PBZ-MNiSW-05/I/2007/01 grant from Polish Ministry of Science and Higher Education.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The study was approved by the local ethics committee (Medical Center for Postgraduate Education and Cancer Center, Warsaw, Poland), and all the participants provided appropriate consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pawel Gaj and Anna Kluska contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gaj, P., Kluska, A., Nowakowska, D. et al. High frequency of BRCA1 founder mutations in Polish women with nonfamilial breast cancer. Familial Cancer 11, 623–628 (2012). https://doi.org/10.1007/s10689-012-9560-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-012-9560-4