Abstract
Identification of mutations in the BRCA2 gene and estimation of their clinical consequences for women and men treated in the Maria Sklodowska-Curie Memorial Cancer Center Warsaw, Poland in the years 1998–2008. The probands (97 women and 8 men) had a family history of breast and ovarian cancer (median age 46). The presence of molecular changes was examined in DNA isolated from peripheral blood lymphocytes. Germline mutations in 27 exons of the BRCA2 gene were screened by ‘touchdown’ PCR amplification, DHPLC and sequencing. Missense mutations were classified by multiple-sequences alignments of orthologous BRCA2 protein sequences with T-Coffee software. 39 molecular changes (8 novel) were identified in the BRCA2 gene in 105 investigated patients. In 12 patients the following pathogenic mutations were identified: 5467insT, 6174delT, 6192delAT, 6675delTA, 8141del5, 9152delT, 9326insA, 9631delC, IVS23-2A > G and E394X. The presence of 10 missense type mutations was detected including the following: D1420O, T1915 M, N3124I. The determination of pathogenic status of molecular variants detected in BRCA2 gene, described in the BIC mutation database as ‘UV’ depends on many parameters. Important is the assessment of the evolutionary conservation of their protein sequences and studying of the frequency of molecular variants detected in breast cancer patients and in population. A high diversity was found of the pathogenic mutations detected in BRCA2 gene in the Polish population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germline mutations in BRCA2 gene located in 13q12-13 chromosome are connected with 45–85% risk of breast cancer development and 11–27% risk of ovarian and pancreatic cancers occurrence [1–3]. BRCA2 is a nuclear protein containing 3,418 amino acids, which works together with RAD51 protein. Both proteins are very important for homologous recombination, DNA repair and genome stability preservation. BRCA2 protein cooperates with RAD51 through the region containing eight strongly conserved BRC repeats in its middle region, a helical domain HD and three oligonucleotid domains OB, and also two nuclear localization signal (NLS) domains located in the C-terminal region of BRCA2 protein [4] (Fig. 1). The OB domains form a linear and elongated structure, however the greatest alfa-helical domain OB2 forms a protruding region described as ‘the tower’ [5]. The presence of normal BRCA2 protein influences cellular processes i.e. maintenance of genome stability and regulation of transcription activity. Deletions, nucleotide insertions, and nonsense mutations, which are the cause of translation termination and synthesis of a truncated protein, undoubtedly demonstrate the pathogenic effect that accompanies breast cancer development.
The studies published as yet suggest that most hereditary breast and ovarian cancers are caused by mutations in BRCA1 gene, however BRCA2 gene mutations are the cause of breast cancer in males [6]. Forty-three percent of all mutations detected in BRCA2 gene, excluding common polymorphisms, cause no synthesis of abnormal protein. Those changes include mainly missense mutations identified in 13% of women with breast cancer, described as ‘unclassified variants’; i.e. their biological effect has not been elucidated yet [7]. The so-called “UVs” changes constitute a significant problem in the interpretation of the degree of their pathogenicity and possible influence on malignant process [8, 9].
Thompson et al. [10] reported that mutations in exon 11 of BRCA2 gene significantly increased the risk of ovarian cancer development. That fragment bordered on its sides by nucleotides in 3,035–6,629 position has been regarded as particularly predisposing to ovarian cancer development, describing it as ‘ovarian cancer cluster region’.
The aim of our study was an assessment of the spectrum of BRCA2 gene mutations and their frequency in women and men with familial breast cancer and ovarian cancer, in whom no mutations were found in BRCA1 gene. All missense mutations detected in BRCA2 gene were classified by means of analysis of evolutionary conservation of BRCA2 protein sequence. The purpose of the study included a comparison of the frequency of changes of indefinite biological effect detected in BRCA2 gene in patients with the incidence of such changes in control DNA derived from healthy subjects. The study could help to assess, whether so slight molecular changes, i.e. variants of unknown clinical significance, increase the risk of breast and ovarian cancer development, or whether they exert no effect on BRCA2 protein function.
Materials and methods
Patients
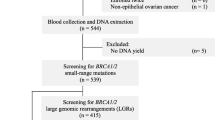
In order to search for BRCA2 mutations, 105 persons were selected, in whom no mutations in BRCA1 gene were found, coming from families with numerous aggregations of breast, ovarian, pancreatic, prostatic and other cancers. Those patients were treated in the Oncology Center in Warsaw and the Oncology Center–Branch in Cracow in the years 1998–2008 and remain in care of the Genetic Counselling Unit, Oncology Center in Warsaw.
Each patient gave written consent for peripheral blood sampling and performing of genetic tests.
The selection of patients for BRCA2 molecular studies was carried out according to the following criteria:
-
1.
Women with breast or ovarian cancer with a family history of breast, ovarian, prostatic or pancreatic cancer (age at onset 17–67).
-
2.
Men with sporadic breast cancer.
-
3.
Healthy subjects in the families of whom breast cancer occurred in first-grade male relatives below the age of 40 years.
Four hundred control DNA samples were obtained from anonymous blood samples of newborns from seven provinces of Poland.
Methods
The genomic DNA was isolated from peripheral blood lymphocytes using Genomic Midi AX Kit (A&A Biotechnology, Poland). A variant of polymerase chain reaction, the so-called ‘touchdown PCR’ was applied. For PCR reactions, primer sequences were used, proposed by Wagner et al. [11].
The PCR product was studied using denaturation liquid chromatography (dHPLC) technique. That method uses TEAA (triethylammonium acetate) buffer with acetonitrile linear gradient (54.3–63.3%) at 0.9 ml/min flow rate. DNA is divided in acetonitrile gradient, depending on the size of the fragments or presence of heteroduplexes. The melting point optimal for individual exons was established using software for the Wave system. The separation was conducted in DNASep, Transgenomic column containing alkylated polystyrenedivinylbenzene as bed. The conditions of PCR and separation in DNASep column are presented in detail in Table 1.
The DNA fragments suspected of mutation presence were purified (Centrifugal Filter Devices) and then were sequenced with fluorescence-labelled terminators—Big Dye Terminator v.3.1 Cycle Sequencing Ready Reaction Kit using 5% denaturating polyacrylamide gel. The sequencing was done using ABI PRISM 377 (Applied Biosystems) apparatus.
The missense mutations detected during screening tests were classified by comparative analysis of evolutionary conservation of BRCA2 protein sequence in fish (two species) and mammals (five species), which was conducted using T-Coffee (version 5.72) software [12]. The software made possible to determine the grade of phylogenetic kinship through identification of common motifs.
For the analysis of the conservation, DNA sequences of the following species were used:
-
Tetraodon nigroviridis—fish
-
Danio rerio—fish
-
Mus musculus—domestic mouse
-
Rattus norvegicus—Norwegian rat
-
Felis catus—domestic cat
-
Canis familiaris—domestic dog
-
Homo sapiens—man
In the result of the analysis of conservation, evolutionarily strongly conserved BRCA2 protein fragments of a high degree of similarity were detected.
Statistical analysis
The statistical analysis was carried out to compare the frequency of the detected molecular variants in breast cancer patients and in the control group (400 DNA samples). Fisher test was used for the statistical analysis, calculating the probability factor for the relation between two independent features in ‘two-by-two’ arrangement.
Results
Germline mutations in BRCA2 gene were searched for in 103 patients with familial breast cancer and ovarian cancer and in two healthy subjects. In that group, breast cancer developed in 91 women and eight men, three probands had ovarian cancer and in one patient breast and ovarian cancer syndrome was diagnosed. That group included also two healthy women in the family of whom breast cancer developed in their first-grade male relatives below the age of 40 years. The patients were aged 17–67 years; the median was 46 years.
Thirty-nine molecular variants were detected in the study group, including eight changes determined for the first time (five pathogenic mutations and three variants of indefinite biological effect). Ten frame shift mutations were detected, the result of which was production of truncated protein. They included: 5467insT, 6174delT, 6192delAT, 6675delTA, 8141del5, 9152delT, 9326insA and 9631delC (Table 2). The 8141del5 mutation was detected in three patients.
The 5467insT mutation, the consequence of which was occurrence of stop codon 1747, was detected in a man with breast cancer. Another change, 6174delT, causing protein synthesis termination in codon 2003 developed in a female breast cancer patient with a family history of five breast cancers, three cases of lung cancer and leukaemia. The 6192delAT mutation, the effect of which was occurrence of a stop codon in 2001 position, was found in a female patient, in whom breast cancer was diagnosed at the age of 54 years, while in her first-grade relatives bilateral breast cancer at the age of 48 years and prostate cancer were diagnosed. The group of pathogenic mutations not included in the BIC database involved also deletion of two bases in 6,675 position, leading to protein synthesis termination in codon 2174. It was disclosed in a female patient with bilateral breast cancer, in whom the first disease occurred below the age of 40 years. A first-grade female relative of that patient developed breast cancer at the age of 45 years and ovarian cancer at the age of 62 years.
The 8141del5 mutation was found in exon 17, which caused formation of stop codon 2368. That change was detected in three non-related female breast cancer patients. In one of them the disease occurred at the age of 40 years, in another at the age of 58 years, and in the third one—at the age of 47 years. In first- and second-grade relatives of those patients breast cancer were also disclosed. Another mutation detected for the first time was 9152delT. It occurred in a 62-year-old man, the first-grade relatives of whom had ovarian cancer and oesophageal cancer.
An insertion of adenine in position 9,326 was found in exon 23, causing formation of stop codon 3042. The carrier of that mutation was a female breast cancer patient, in the family of whom three-first- and second-grade relatives had breast cancer and pancreatic cancer.
The last of the mentioned frame shift mutations was 9631delC. That change was detected in a woman, who, at very young age (33 and 39 years) had sporadic bilateral breast cancer. Besides that, in exon 23 of BRCA2 gene a IVS23-2A > G change was disclosed at exon splice site. That change also exerted a pathogenic effect and was not earlier included into the BIC database. It was detected in a 40-year-old female patient with a family history of breast, prostate and gall bladder cancers.
The group of pathogenic mutations detected by us was completed with the nonsense change E394X. It was found in a female breast cancer patient, the first-grade male relative of whom also had breast cancer. For genetic counselling purposes we confirmed the carrier state of 6192delAT mutation in four healthy relatives, E394X mutation in two healthy relatives and 8141del5 mutation in healthy daughter of the patient. The pattern of location of pathogenic mutations detected in BRCA2 gene is presented in Fig. 1.
Ten missense changes were found among 105 studied subjects (Table 3).
The frequency of changes was compared between breast cancer patients and the control group of healthy subjects for three detected missense mutations, the biological effect of which has not been elucidated yet. The incidence of N991D change in exon 11 of BRCA2 gene in breast cancer patients (10/105;9.5%) was not significantly different from that in healthy subjects (34/400;8.5%) OR = 1.1331 (95% CI: 0.5405 < O.R. < 2.3757), p = 0.7007. A weak evolutionary conservation of that change was found in BRCA2 protein sequence, thus we suggest that it was of polymorphous character. Another change, D1420O, moderately conserved evolutionarily in mammals in BRCA2 protein sequence, occurred with a higher frequency in breast cancer patients (6/105;5.7%) than in healthy subjects (7/400;1.7%); OR = 3.4026 (95% CI: 1.1186 < O.R. < 10.3505); p = 0.0339. The missense N3124I change occurred with 2.85% (3/105) incidence in patients, while it was not found in the DNA of healthy subjects (0/400), p = 0.0087. The N3124I mutation was located in BRCA2 protein domain bound to DNA and was characterised by the highest evolutionary conservation (in fish and mammals). The family history of female breast cancer patients, carriers of D1420O and N3124I changes is shown in Table 4.
Among the remaining mutation that lead to amino acid exchange in the encoded protein, four demonstrated a very good evolutionary conservation. They included: N289H in exon 10 defined in the BIC as clinically insignificant, and G602R, K1690 N and I2627F of indefinite biological effect. The female carriers of N289H mutation had positive family history of breast cancer occurrence in young relatives, and prostatic and breast cancer development in males. A G602R mutation developed in a female breast cancer patient with a family history of two breast cancer and hepatic cancer. The exchange of lysine for asparaginic acid in 1690 position was found in two probands. In one of them the disease occurred below the age of 40 and she had a positive family history of breast, pancreatic and lung cancer and Hodgkin’s disease. In the other proband ovarian cancer developed at the age of 48 years and two cases of gallbladder cancer were found in her family. The presence seems also to be significant of a I2627F change in a woman, in whom breast cancer was diagnosed at the age of 32 years and breast cancer was also found in her first-degree male relative.
In 12 study subjects C5972T mutation was also detected, leading to T1915 M exchange. That mutation was disclosed in a very young female patient (18 years) with multifocal lobular breast cancer, while four other patients developed bilateral breast cancer. The patient, in whom breast and ovarian cancer syndrome was diagnosed, and three female ovarian cancer patients were carriers of T1915 M mutation.
Eight ‘silent’ changes were also disclosed, although not leading to amino acid exchange in the encoded protein, but their clinical importance could not be excluded (Table 3). The frame shift mutation 10323delCins11 occurring in exon 27 also should be regarded as belonging to changes of indefinite biological effect (according to BIC). The group of detected mutations is completed with nine intron changes in exons 8, 11, 12, 14, 15, 17, 22 and a G203A change in the non-encoding 5′UTR segment of exon 2.
Discussion
Twenty-seven exons of BRCA2 gene, i.e. all encoding regions of BRCA2 and the so called splice sites were examined in 103 patients with familial breast or ovarian cancer and two healthy subjects, finding 12/105 (11.4%) mutations of pathogenic importance. Among them ten frame shift mutations, one terminal change and one splice site change were detected. The effects of the mutations disclosed included truncated protein synthesis or function impairment of produced BRCA2 protein.
Five pathogenic mutations were detected for the first time in the study group. They included: G1408T, 6192delAT, 6675delTA, 9152delT and IVS23-2A/G.
The nonsense G1408T (E394X) mutation is located in the N-terminal region of BRCA2 protein before the region containing eight strongly conserved repeats of BRC (Fig. 1). The 6192delAT mutation, similarly as two pathogenic mutations 5467insT and 6174delT, are located within the strongly conserved BRC repeats. BRCA2 protein is directly connected with RAD51, which is particularly important for damage repair of double DNA strand through recombination between homologous DNA sequences. Interaction between BRCA2 and RAD51 occurs mainly in the region of BRC repeats in BRCA2 protein, and the sequence of the repeats is characterised by high evolutionary conservation (in birds and mammals) [12]. BRCA2 protein can control both joint and separate actions of RAD51 in DNA repair. Therefore, 5467insT, 6174delT and 6192delAT mutations exert a particular effect on double DNA strand repair function and strongly predispose to breast cancer development. The 6174delT mutation included in the BIC [13] database is the change occurring most frequently in the population of Ashkenazi Jews, both women and men with breast cancer Codick et al. [14] and also Distelman-Menachem et al. [15].
The 6675delTA mutation, detected for the first time, is located just behind the region of strongly conserved BRC repeats. That change causes synthesis of impaired protein deprived of helical domain HD and three oligonucleotide domains OB located in the C-terminal region of BRCA2 protein.
Two new pathogenic mutations: 9152delT and splice site change IVS23-2A/G, and one previously known mutation 9326insA were found within the greatest alpha-helical domain OB2, which forms a protruding region described as ‘the tower’, and which is located in the C-terminal region of BRCA2 protein. The consequence of those mutations is disturbance of double DNA strand repair functions. Csokay et al. [6] and Haraldsson et al. [16] also described the 9326insA change in men with breast cancer who had no family history of breast cancer.
Another mutation located within the helical domain HD, namely 8141del5, detected by us in three patients, led to synthesis of a vestigial protein deprived of helical domain HD and three oligonucleotide domains OB located in the C-terminal region of BRCA2 protein [17]. The 9631delC mutation was found in the region of oligonucleotide domain OB3 and led to formation of stop codon 3162. That mutation was described by Grzybowska et al. [18] in three patients with breast and ovarian cancers and a family history of those tumours. We think that the mutation is characteristic of patients from the Polish population.
A number of missense changes were also detected in our study material. The G4486T mutation caused an affinity change of hydrophilic asparaginic acid for hydrophobic tyrosine (D1420O); the frequency of that mutation was significantly higher in patients with familial breast cancer than in healthy subjects. That mutation is located in limiting BRC3 sequence in the region of strongly conserved BRC repeats. That sequence plays an important role in RAD51-BRCA2 interaction, therefore that mutation significantly increases the risk of breast cancer development [19]. That mutation was also detected by Kwiatkowska et al. [20] in a man with breast cancer.
Many authors think that mutations leading to changes in the region of BRC4 to BRC7 repeats are connected with a predisposition to breast cancer development [21]. Two changes were found in that region: A5298C causing an exchange of hydrophobic lysine for hydrophilic asparagine taking place within BRC5 sequence, and C5972T mutation, in which a change of polarity of amino acids also occurred. Górski et al. [22] described that molecular variant in BRCA2 gene as strongly predisposing to breast cancer development in young women. The authors studying a large group of patients (over 3,600) observed that the carriers of T1915 M variant in BRCA2 gene more frequently developed bilateral and multifocal breast cancer, although the differences between the groups failed to reach statistical significance. That confirmed our observations, since out of 12 female patients with C5972T mutation, in four cases bilateral breast cancer occurred and in one proband breast cancer developed at the age of 18 years.
The effect of A9599T change was an exchange of hydrophilic asparaginic acid for hydrophobic isoleucine (N3124I). The N3124I mutation is located in the BRCA2 sequence evolutionarily strongly conserved in fish and mammals, therefore we can conclude that it increases the risk of breast cancer development.
In our study material two mutations: N372H and K1690 N were detected, which were described in the BIC database as non-pathogenic changes, however a homozygous N372H change was found in one female patient with breast cancer diagnosed at the age of 37 years, with a family history of breast cancer in her first- and second-grade relatives (21 and 39 years). Spurdle et al. [23], Healey et al. [24] think that HH genotype N372H change is connected with increased risk of breast cancer development in the Australian and British populations. Palli et al. [25] reported that homozygous N372H mutation in BRCA2 gene significantly increased the risk of breast cancer development in males.
A missense K1690 N change, the protein sequence of which was characterised by strong evolutionary conservation in fish and mammals, was detected in a breast cancer patient with a family history of ovarian cancer (48 years) and gastric cancer in two relatives (31 and 57 years). We can suppose that change increases the risk of breast cancer and also gastric cancer development. Jakubowska et al. [26] described a higher incidence of mutations in BRCA2 gene in patients belonging to families with breast cancer and gastric cancer aggregations.
In our study material 10323delCins11 mutation was also disclosed in exon 27 of BRCA2 gene, the consequence of which was stop codon 3329. That mutation was described in BIC as the change with non-pathogenic effects.
Searching for mutations in BRCA2 gene in patients with familial breast cancer and ovarian cancer is very important for genetic counselling and also for the selection of adequate adjuvant therapy. Easton et al. [8] and Goldgar et al. [9] found that new mutations identified in high-risk families can be suspected of pathogenicity if they reveal a high degree of evolutionary conservation in the functional domains of BRCA2 protein. In order to broaden the knowledge about the changes of indefinite biological effect found in BRCA2 gene, we intend, in co-operation with the Genetic Counselling Unit, to carry out an analysis of co-segregation of the coexistence of detected molecular variants with breast cancer cases among relatives.
Conclusions
The determination of pathogenic status of molecular variants detected in BRCA2 gene, described in the BIC mutation database as ‘UV’ depends on many parameters. Important is the assessment of the evolutionary conservation of their protein sequences and studying of the frequency of molecular variants detected in breast cancer patients and in population.
A high diversity was found of the pathogenic mutations detected in BRCA2 gene in the Polish population. There is a tendency in genetic counseling to search for mutations developing most frequently in the Polish population, however most mutations are only determined in one family. Therefore, in breast cancer patients with a strong positive family history of breast, ovarian, pancreatic and prostatic cancer, a screening strategy is needed for the whole BRCA2 gene.
References
Hansen TVO, Bisgaard ML, Jonson L et al (2008) Novel de novo BRCA2 mutation in a patient with a family history of breast cancer. BMC Med Genet 9:58
Couch FJ, Jonson MR, Rabe KG et al (2007) The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomark Prev 16:342–346
Antoniou A, Pharoah PD, Narod S et al (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130
Chen PL, Chen CF, Chen Y et al (1998) The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment. Proc Natl Acad Sci USA 95:5287–5292
Salgado J, Zabalegui N, Garcia-Amigot F et al (2005) Structure-based assessment of BRCA1 and BRCA2 mutations in a small Spanish population. Oncol Rep 14:85–88
Csokay B, Udvarhelyi N, Sulyok Z et al (1999) High frequency of germ-line BRCA2 mutations among Hungarian male breast cancer patients without family history. Cancer Res 59:995–998
Wu K, Hinson SR, Ohashi A et al (2005) Functional evaluation and cancer risk assessment of BRCA2 unclassified variants. Cancer Res 65:417–426
Easton DF, Deffenbaugh AM, Pruss D et al (2007) A systematic genetic assessment of 1.433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet 81:873–883
Goldgar DE, Easton DF, Deffenbaugh AM et al (2004) Integrated evaluation of DNA sequence variants of unknown clinical significance: application to BRCA1 and BRCA2. Am J Hum Genet 75:535–544
Thompson D, Easton D (2001) Variation in cancer risk, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet 68:410–419
Wagner T, Stoppa-Lyonnet D, Fleischmann E et al (1999) Denaturing high-performance liquid chromatography detects reliably BRCA1 and BRCA2 mutations. Genomics 62:369–376
Notredame C, Higgins DG, Heringa J (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol 302:205–217
BIC (Breast Information Core) database. http://www.research.nhgri.nih.gov/bic/
Chodick G, Struewing JP, Ron E et al (2008) Similar prevalence of founder BRCA1 and BRCA2 mutations among Ashkenazi and non-Ashkenazi men with breast cancer: evidence from 261 cases in Israel, 1976–1999. Eur J Med Genet 51:141–170
Distelman-Menachem T, Shapira T, Laitman Y et al (2009) Analysis of BRCA1/BRCA2 genes’ contribution to breast cancer susceptibility in high risk Jewish Ashkenazi women. Fam Cancer 8:127–133
Haraldsson K, Loman N, Zhang Q et al (1998) BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res 58:1367–1371
Thomassen M, Hansen TVO, Borg A et al (2008) BRCA1 and BRCA2 mutations in Danish families with hereditary breast and/or ovarian cancer. Acta Oncol 47:772–777
Grzybowska E, Zientek H, Jasińska A et al (2000) High frequency of recurrent mutations in BRCA1 and BRCA2 genes in Polish families with breast and ovarian cancer. Hum Mutat 16:482–490
Bignell G, Micklem G, Stratton MR et al (1997) The BRC repeats are conserved in mammalian BRCA2 proteins. Hum Mol Genet 6:53–58
Kwiatkowska E, Teresiak M, Lamperska K et al (2001) BRCA2 germline mutations in male breast cancer patients in the Polish population. Hum Mutat 17:73
Davies AA, Masson J-Y, Mcllwraith MJ et al (2001) Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell 7:273–282
Górski B, Narod SA, Lubiński J (2005) A common missense variant in BRCA2 predisposes to early onset breast cancer. Breast Cancer Res 7:R1023–R1027
Spurdle AB, Hopper JL, Chen X et al (2002) The BRCA2 372 HH genotype is associated with risk of breast cancer in Australian women under age 60 years. Cancer Epidemiol Biomark Prev 11:413–416
Healey CS, Dunning AM, Teare MD et al (2000) A common variant in BRCA2 is associated with both breast cancer risk and prenatal viability. Nat Genet 26:362–364
Palli D, Falchetti M, Masala G et al (2007) Association between the BRCA2 N372H variant and male breast cancer risk: a population- based case-control study in Tuscany, Central Italy. BMC Cancer 7:170
Jakubowska A, Nej K, Huzarski T et al (2002) BRCA2 gene mutations in families with aggregations of breast and stomach cancers. Br J Cancer 87:888–891
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balabas, A., Skasko, E., Nowakowska, D. et al. Novel germline mutations in BRCA2 gene among breast and breast-ovarian cancer families from Poland. Familial Cancer 9, 267–274 (2010). https://doi.org/10.1007/s10689-010-9338-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-010-9338-5