Abstract
Biogeographic studies in Amazonia typically describe biodiversity across interfluvia, rarely within them, where geographic variability in morphological traits might be observed. We tested for intraspecific phenotypic variation in three bird species within the Purus–Madeira interfluvium (Central Amazon) and whether phenotypes were correlated with environmental heterogeneity or geographic distance among sites. We compared coloration indexes derived from reflectance spectra and morphometrics of up to five adult individuals of each sex among 11 sites within the interfluvium and contrasted them with proxies for geographic distance and environmental variation (tree basal area and bird community). Environmental heterogeneity was minimally spatially autocorrelated, and there were no obvious geographical barriers to dispersal in the study region. The null hypothesis was that we would see either no phenotypic variation or random variation that was not explained by the tested variables. Half of the cases analyzed showed intraspecific morphological variation. Coloration varied more frequently than morphometrics, and color was better explained by environmental heterogeneity, particularly in males, whereas brightness also varied with geographic distance. Geographic distance explained the only case of variation in morphometrics. Our results indicate that coloration, particularly plumage brightness, is more labile than morphometric traits and that plumage color might be under stronger effects of local adaptation than brightness, which also seems to be under effects of neutral drift and gene flow among populations. Higher frequencies of association between male coloration and the environment suggest a role of non-arbitrary mechanisms of sexual selection on the expression of male phenotypes, whereas arbitrary intersexual selection might explain the randomly distributed variation that is not explained by environmental heterogeneity or geographic distance. We revealed intraspecific phenotypic variation in a spatial extent usually not considered in biogeographic studies in the Amazon and demonstrate that both local adaptation and neutral drift are important to explain intraspecific trait diversification at this geographical scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphological traits may vary among geographic localities within a species range as a result of adaptation to local environments (Mayr 1947; Cadena et al. 2011) or neutral drift (Lee et al. 2016), which are not mutually exclusive and may act together on phenotypic traits (Sun et al. 2013; Engen and Sæther 2016). Nevertheless, because genetic drift is usually considered a null hypothesis in evolutionary biology, its relative importance in modeling intraspecific phenotypic variation remains poorly understood. Thus, results from studies that have considered geographical and environmental variables encourage future studies in understanding the origins of biological diversity to consider the relative importance of these components, as this is still an intensive debate in evolutionary ecology (Wang and Summers 2010).
In adaptive evolution, the variation observed in phenotypic characters generally reflects the degree of environmental variation experienced by different populations of the same species at different sites (Amézquita et al. 2009). As postulated by the Hypothesis of Environmental Gradients (Endler 1977), biotic or abiotic environmental differences may be sufficient to lead to divergence among populations, and may lead subsequently to speciation by parapatry, in a process later described as ecological speciation among either sympatric, parapatric or allopatric populations (Schluter 2009). Under such circumstances, environmental heterogeneity may be an important factor in generating phenotypic diversity (Calsbeek et al. 2007; Price 2008).
In general, phenotypes reflect the ecology of the species bearing them in terms of the different habitats used and the different strategies deployed to exploit available resources (MacArthur and Pianka 1966; Levin 1992). The composition of predator species in local assemblages, for example, may exert selective pressures on populations of prey species and therefore their phenotypes (Hoagstrom and Berry 2008). For instance, changes in the local composition of predator species explain variation in aposematic coloration in frogs (Siddiqi et al. 2004; Hegna et al. 2012). Similarly, intraspecific phenotypic variation may occur because of local selection to changes in the degree of competition for resources (Goldberg and Lande 2006). Through competitive release, a species may extend its foraging area in the absence of competitors, leading to changes in phenotypic traits as the species adapts to newly available biotic environments (Endler 1977; Grant and Grant 1993, 2000; Hsu et al. 2014).
Sexual selection is a common cause of phenotypic variation among populations or sister taxa (Endler and Houde 1995; Safran and McGraw 2004; Uy et al. 2008). Some mechanisms of sexual selection by female choice thus predict a closer relationship between the variation in phenotypes and the environment (review in Prum 1997). Among those, the hypothesis of sensory drive postulates that the production and transmission of signals used in intersexual communication evolve in response to environmental conditions during signaling, reducing the cost to find mates (Endler 1992; Endler and Mclellan 1988; Endler and Basolo 1998). This process has been evoked to explain color evolution in birds (e.g., Endler 1993; Endler and Théry 1996; Gomez and Théry 2004; but see Anciães and Prum 2008; review in Hill and McGraw 2006). Hence, the emergence of new social characters with positive effects to the sensory receptors may accumulate in populations in a relatively short period of time (Endler and Théry 1996). If there is low gene flow or some variation in inter-sexual preferences among populations, this variation may lead to geographic variation in phenotypic traits and, ultimately, to speciation (Seddon et al. 2008; Seehausen et al. 2008; Maan and Seehausen 2011).
Under expectations of a pure neutral mechanism, on the other hand, populations may exhibit intraspecific variation even in the absence of selective forces due to the effects of random genetic drift alone. Thus, random neutral genetic recombination accumulates through time, and allelic frequencies will vary among localities; in addition, because of higher gene flow among geographically closer localities, more distant populations will be isolated by distance (Wright 1943). Consequently, there will be subtle changes among neighboring populations and increasing differentiation as they get farther apart. Finally, the evolution of phenotypic traits may obey arbitrary mating preferences, which become genetically correlated even in the absence of deterministic mechanisms of mate choice, such as honest signals or environmental drivers acting on sensorial systems (Fisher 1930; Kirkpatrick and Ryan 1991; Prum 2012). Therefore, in the absence of direct selection on female preferences, Fisherian selection should result in differentiation in secondary sexual traits and lead to polymorphisms among populations (review in Andersson 1994). Thus, the geographic distribution of such polymorphisms may be clinal or spatially random among populations.
In Amazonia, most studies focus on the distribution of biodiversity across extensive interfluvial areas. Classical biogeographic studies have addressed diversification processes acting on groups of closely related taxa among the so-called “areas of endemism” (Endler 1982; Haffer 1997; Patton and da Silva 1998; Ribas et al. 2012). However, studies have seldom evaluated patterns and mechanisms underlying taxonomic or phenotypic variation within interfluvia (e.g., Naka et al. 2012; Fernandes et al. 2013; Weir et al. 2015). Hence, much speculation still occurs regarding the determinants of intraspecific geographic variation (Wang and Summers 2010). Some of the hypotheses proposed to explain the origin of biodiversity in this region are based on adaptive mechanisms (Endler 1977), neutral mechanisms (Wallace 1858; Cracraft 1985; Ribas et al. 2012), or both (Haffer 1969; Bates 2001), and might be effective within interfluvia. Naka et al. (2012), for example, found phenotypic and phylogenetic breaks in the absence of current geographic barriers for sister taxa replacing each other along continuous forest within the Rio Branco and Rio Negro interfluvium, highlighting the value of studies focusing on the magnitude and the nature of variation in biological data among populations within interfluvia in this region. Another recent study found that acoustic signals of a nurse frog species (Allobates sp.) responded to a gradient of forest structure along a 600 km transect within the Purus–Madeira Interfluvium, highlighting that environmental heterogeneity over a subtle gradient can influence phenotypic variation in central Amazonia (Faria 2014).
The selected taxa are suboscines, which are good models for studies addressing questions in evolutionary biology and, specifically, the roles of ecology, sexual selection and neutral drift on their evolution. High diversity in suboscines in addition to details of their signaling behavior make this group ideal for field-based observational and experimental studies of signal evolution (Tobias et al. 2012). For our study, they are particularly well suited because they (1) occur throughout the study region; (2) are sexually dimorphic and therefore likely subjected to sexual selection; (3) are understory birds and likely not good dispersers; and (4) are subject of ongoing phylogeographic studies and, as such, are good candidates for future research relating to their genetic data. Furthermore, (5) variation in their breeding system and levels of sexual dichromatism among the three species (W. poecilinotus being the least and L. coronata the most dichromatic) suggest varying degrees of sexual selection, with greater variation in coloration in polygynous than monogamous taxa (Uy et al. 2008; Safran et al. 2012; Seddon et al. 2013). This implies different roles of the environment for phenotypic diversification, favoring signal conspicuousness through sexual selection or crypsis through natural selection (Endler 1991, 1993; Maan and Seehausen 2011). Finally, multiple characters are suitable for addressing the roles of alternative mechanisms of trait evolution (Dayrat 2005; Padial et al. 2010), owing to its differences in lability and because different traits are likely subjected to different evolutionary mechanisms. For instance, morphometric traits might be generally more constrained than coloration (Safran and McGraw 2004; Martin and Mendelson 2014), which might be better explained by environmental heterogeneity than morphometrics.
Here, we focus on three Amazonian suboscine passerines to address patterns of intraspecific phenotypic variation in coloration and morphometrics within the Purus–Madeira interfluvium by testing two non-exclusive alternative hypotheses addressing the roles of deterministic and stochastic mechanisms in phenotypic differentiation. Specifically, for each species, we test the hypotheses that (1) intraspecific phenotypic variation results from adaptation to local environments, and thus any observed phenotypic variation is explained by environmental heterogeneity among localities or that (2) phenotypic variation results from neutral genetic drift and isolation by distance and is explained by the geographical distance among localities. Thus, our null expectations were either of no phenotypic variation in the study region or of random variation not explained by the tested variables (Table 1). The high biological heterogeneity found in the Purus–Madeira interfluvium, combined with a low spatial autocorrelation of environmental data (Menger 2011), makes the region suitable for testing hypotheses regarding the relative importance of environmental and geographical factors in producing phenotypic diversity on fine geographical and taxonomic scales.
The species selected for the study were the white-throated antbird (Gymnopithys salvini; Thamnophilidae), the scale-backed antbird (Willisornis poecilinotus; Thamnophilidae), and the blue-crowned manakin (Lepidothrix coronata; Pipridae). G. salvini and W. poecilinotus are facultative ant-followers (WilIis 1968) and monogamic species. Both are sexually dichromatic (G. salvini males are mostly gray, white and black, and females are reddish brown and black; W. poecilinotus males are brownish black and white, and females are similar with more brown patches). Although previous studies showed intraspecific morphological variation in plumage coloration for these species (Hellmayr 1929; Isler and Whitney 2011; Kirwan and Green 2011), there have been no previous quantitative assessments of phenotypic variation in coloration in these species. G. salvini is restricted to the Inambari Area of Endemism (WilIis 1968; Cracraft 1985), whereas W. poecilinotus is found in the Amazon of Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname, and Venezuela (Sick 1997; Zimmer and Isler 2003). On the other hand, L. coronata is a frugivorous species with a polygynous lekking system, in which multiple males display in mating territories to attract females (Sick 1997; Durães 2009). It is a widespread Manakin species distributed throughout much of western Amazonia, the Andes foothills and Central America, exhibiting considerable geographic variation in male plumage coloration (from entirely black bodied to green and intermediate forms) across its range (Hellmayr 1929; Cheviron et al. 2005; Anciães et al. 2009).
Materials and methods
Study area
We conducted the study along the middle and lower regions of the interfluvium formed by the Purus and Madeira rivers, in the Central Brazilian Amazon (Fig. 1). The Purus–Madeira interfluvium is intersected by the BR-319 highway, which connects the Brazilian states of Amazonas and Rondônia and has been closed to regular vehicles since 1998 (Fearnside and Graça 2009). On a regional scale, lowland dense rain forest covers the northern Purus–Madeira interfluvium, while a transition towards open lowland rainforest dominated by palm trees occurs in the southern region, likely related to rainfall seasonality (IBGE 1997). Generally speaking, these physionomies are considered upland Terra Firme forests.
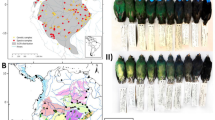
Map of the study area in the Purus–Madeira interfluvium with the 11 sampling localities (PPBio modules, red dots in the left panel) and schematic figure of the PPBio module (adapted from Marciente 2012) which shows uniform distribution of the plots along two parallels trails of 5 km of length and 1 km of width (lower right panel)
Geographic variations in forest structure have already been reported in previous studies for this region with a much lower basal area, lower canopy heights and lower mean wood densities in the seasonally flooded forests from the north compared to the central and southern sites (Cintra et al. 2013). Intensity of the rainy season varies strongly throughout the interfluvial area, declining from 2800 to 2100 mm annual precipitation with increasing rainfall seasonality. The soil texture of the Purus–Madeira interfluvium is mostly characterized by plinthosols with a predominance of silt and varying smaller percentages of sand and clay (Cintra et al. 2013). This environmental variation has been summarized into 13 environmental units (or ecoregions) based on 21 environmental variables including hydrology, landform, vegetation and soil (Ximenes 2008). Despite these ecoregions accurately represent the coarse environmental heterogeneity of the interfluvium, they do not characterize the finer-scale environmental variation within the studied transect (i.e., the variation within each of the aforementioned ecoregions), which has been conducted by other studies that evaluated local environmental and biological variation in the region, including in situ measures of the floristic composition of trees (Souza 2012), palms (Emilio et al. 2013) and herbs (Moulatlet et al. 2014); bird assemblages (Menger 2011); forest structure (Schietti et al. 2016) and soil chemical and physical properties (Martins et al. 2014).
Sampling design
We carried out fieldwork for the study in 11 localities (or study sites) in the Purus–Madeira interfluvium, which has established lodging infrastructure along and perpendicular to the BR-319 highway (Fig. 1, Table 2) belonging to the Brazilian Research Program on Biodiversity—PPBio (http://www.ppbio.inpa.gov.br) and created by the Brazilian Ministry of Science, Technology and Innovation (MCTI). The 11 study sites are distributed on average 60 km apart from each other along BR-319, totaling ca. 600 km of linear extension. Each site consists of two parallel trails 5 km in length, separated by 1 km and connected by perpendicular secondary trails located at 0 m, 3 km and 5 km (Fig. 1). Each 5 km-long trail contains six plots every 1 km consisting of a central line 250 m in length that follows the isocline to minimize environmental variation within the set of trails of the study site, according to RAPELD methods (Magnusson et al. 2005), where the environmental data described above were measured.
Data collection
Coloration and morphometric data were taken from adult specimens of each sex captured in the field between October and November 2012 and July and October 2013 (Table 2). At each RAPELD/PPBio site, 10 mist nets (12 × 2.5 m) were set up in four randomly selected plots (a total of 40 nets per site), open between 6 am and noon, for two consecutive days. For the three focal species, we collected up to five adult individuals of each sex at each site, totaling 195 individuals, which were prepared as voucher specimens (skins) and deposited at the bird collection of the Instituto Nacional de Pesquisas da Amazonia (INPA). Age and sex were first assessed through inspection of definitive plumage patterns (coloration for males and formative plumage for females, Ryder and Durães 2005; Johnson et al. 2011) and were confirmed during skin preparation through verification of gonads, skull ossification and bursa. When captures at a study site exceeded five adult individuals of each sex for a species, a screening procedure was performed to age and sex birds as described above, and feathers were removed from each body patch for which coloration would be measured (see details below). Birds were then banded with standard metallic bands from CEMAVE (National Research Center for Conservation of Wild Birds) and released. FHTA took all measurements in the field and prepared skins and feathers for reflectance analyses.
Color analysis
We obtained color spectra from all feathers collected during fieldwork (i.e., from collected specimens or from feathers of released individuals) and from specimens belonging to the bird collections at Instituto Nacional de Pesquisas da Amazônia (INPA) and Museu Paraense Emilio Goeldi (MPEG) collected at the study plots. We used an Ocean Optics USB-2000 UV–VIS spectrophotometer connected to a cosine corrected probe (R400-2 UV–VIS) and a PX2 pulsed Xenon light source to measure spectra on nine plumage patches (i.e., a plumage region irrespective to presenting distinct coloration patterns from adjacent regions): crown, neck, chest, belly, back, rump, remiges, greater wing coverts and rectrices. Individuals of G. salvini and W. poecilinotus have patches within male rectrices (white and brown bars in males and females, respectively, of G. salvini and white spots in W. poecilinotus), and thus measurements from these spots were also included for each species. As the primary objectives of this study do not include comparisons among species, such inclusions do not invalidate the results. Each measurement recorded was the average of ten individual readings of the same point in a plumage patch using an integration time of 20 ms and a zero boxcar correction. For G. salvini and W. poecilinotus, we analyzed spectra between 300 and 700 nm, corresponding to the visual range commonly associated with birds, including the ultra-violet region (Cuthill 2006; Mullen and Pohland 2007). For L. coronata, we analyzed the spectra between 400 and 700 nm corresponding to the range of maximum visual sensitivity associated with the family Pipridae, which are typically sensitive to the violet region (Ödeen and Håstad 2013). The same investigator (FHTA) measured all color spectra (figure S1).
We visualized and manipulated spectral data in Avicol (Gómez 2006) and derived color metrics using PAVO (Maia et al. 2013a) implemented in (R Development Core Team 2013). We estimated the coloration pattern of each bird species for each of the 11 study sites using 9 metrics to describe color parameters corresponding to hue (the peak location of the color spectrum), saturation (the color purity) and one metric that describes brightness (the intensity of a color spectra) (table S1), for all analyzed patches. We included the colorimetric variables of each plumage area into two PCAs for each species and sex separately. The first PCA was intended to reduce multidimensionality of the group of variables related to hue and saturation, whereas the second analysis included only the variable for absolute brilliance.
In the first PCA, we analyzed metrics for each of the color patches (9 or 10 patches), generating matrices with 81 (for L. coronata) or 90 colorimetric variables (for G. salvini and W. poecilinotus) in total. Because PCA analyses with numerous variables can generate many axes, each explaining only a small portion of the observed variation, we applied a scaling procedure to select variables, initially including 81 or 90 variables in the PCA, from which we selected the 10 variables most associated with the first two axes (at least 25% of the explained variation). Then, we inserted these ten variables into a second PCA and used the first two axes of greatest variation in color, which explained between 75 and 95% of the cumulative variation in the datasets for each species and sex as the dependent variable in the statistical analyses described below. Spots in the crown of G. salvini males were very dark with a percentage average brightness below 2%. These were therefore excluded from the analysis of hue and saturation and were used only in the analysis of brightness.
Because we are estimating variation in visual signals whose perception relies on specific properties of visual systems, it is important to consider thresholds of discrimination among signals produced at the compared sampling localities. Therefore, to account for discriminatory power of the coloration patterns quantified here, we calculated, for each patch, average pairwise perceptual distances (DS) in a tetrachromatic color space including all individuals per locality to estimate DS values among localities, according to the Vorobyev and Osorio (1998) receptor noise visual model. DS is expressed in terms of just noticeable differences (jnds), and values larger than 2 jnds are considered to represent significant color discrimination (see Vorobyev and Osorio 1998; Vorobyev et al. 2001). In addition to DS, we also estimated DL, which refers to differences in brightness among stimuli, calculated from the signal at receptors sensitive to large wavelengths (Siddiqi et al. 2004). Both DS and DL were estimated proportional to one jnd in PAVO (Maia et al. 2013a). For a given patch, we first estimated pairwise coloration distances (DS and DL) for each pair of individuals from two different localities (i.e., one individual from each locality) and then averaged this result to represent the average pairwise distance between any two localities, and repeated this for all locality pairs. Finally, we averaged DS and DL values among pairs for each pairwise comparison among localities. Thus, our DS and DL values represent distances in coloration averaged among patches.
Morphometric analyses
We took the following morphometric measurements from individuals of each focal species: bill length (from the bill tip to the nasal-frontal hinge), bill height (at the proximate edge of the nostrils), bill width (at the proximate edge of the nostrils), tarsus length (from the tibio-tarsus joint to the distal end of the tarso-metatarsus), wing length (from the carpal joint to the tip of the longest primary feather), and tail length (from the uropygium to the tip of the central rectrices). We measured beak length, height and width and tarsus length using a 0.1 mm-unit dial caliper and wing length and tail length using a 1 mm-unit flat ruler. All morphometrics measurements were taken by the same researcher (FHTA). We tested whether morphometric variation among individuals was significantly larger than the measurement error following recommendations in Harris and Smith (2009). For this, we conducted a one-way Analysis of Variance (ANOVA) for each morphometric character of each species to assess the significance of morphometric variation among individuals in relation to measurement error, for a total of 12 ANOVA tests (see Sokal and Rohlf 1995). We included individuals as a random factor and repeated measures of the morphometric character nested within individuals. The ANOVA tests were all significant, indicating that the variation within individuals was lower than that among individuals (L. coronata: Df = 34, F value = 6.3–6.7; P < 0.05; W. poecilinotus: Df = 64, F value = 3.8–9.8, P < 0.05; G. salvini: Df = 57, F value = 5.1–7.7, P < 0.05). Thus, any observed variation in further tests should not be attributed to measurement error.
We reduced the multidimensionality of the morphometric variables using PCA for all individuals within species and tested the significance of sexual dimorphism for each species with ANOVA tests. Because morphometrics were sexually monomorphic for all species, we analyzed the morphometric dataset with all individuals together and used the first two axes of greatest variation in variable response in the statistical analyses described below.
We also assessed the degree of correspondence between color, brightness and morphometrics through cross-correlations between the scores of the first two PCA axes of color, brightness and morphometrics variables for each species and sex separately. As the data were normally distributed, we applied a parametric correlation test. We used a Pearson Correlation and the Cross-correlation method (Ranta et al. 1998). Because the only significant cross-correlations were between color and morphometric datasets of females for both G. salvini and W. poecilinotus (r = 0.69, P = 0.026 and r = − 0.66, P = 0.05; respectively), we ran statistical analyses for all datasets separately.
Phenotypic variation
We ran ANOVA tests for color, brightness and morphometrics data for each species across study sites to evaluate the extent of phenotypic variation along the studied transect. We considered the significance of the test to determine whether there was significant geographic variation (i.e., whether inter-population variation was greater than intra-population variation).
We calculated means and standard deviations of color and morphometric data for each species in each site as well to evaluate the extent of phenotypic variation along the studied transect. We considered non-overlapping 95% confidence intervals as significant geographic variation.
Predictor variables
To characterize the environmental variation among the 11 study sites along the Purus–Madeira interfluvium, we used three environmental variables previously measured at the same transects where the birds were captured for the present study. The database used is available in the PPBio data repository (see metadata: http://ppbio.inpa.gov.br). The environmental variables tested replace a set of local environmental factors and represent differences present across the sampling localities (study sites). We used the following environmental variables: (1) density of the ten most abundant palm species in the Purus–Madeira interfluvium; (2) palm and tree basal area (m2 ha−1) and density (n ha−1) of plants with diameter above 10 cm and (3) species composition in the assemblage of avian species.
We used the latitudinal geographic coordinates for each site to represent geographic distances among study sites since the difference between latitudes of two locations results in the geographical distance between them. Next, we reduced the dimensionality of each of the compounded variables (1—density of palm trees and 3—bird community) separately and tested the cross-correlation among the four environmental variables described above. Thus, as basal area of trees was correlated with the variable density of palm trees (r = 0.83, p < 0.01), we chose to keep only the variables bird community and palm and tree basal area in subsequent analyses because we believe that these variables are more directly associated with the biology of the studied species. Bird community may indicate interactions between species and can promote divergence of eco-morphological traits and sexual signals such as coloration, besides indicating environmental differences, whereas tree basal area can represent obstruction to movement in the forest and may thus affect morphometric traits associated with flight performance and could affect visual communication and, as such, bird coloration.
Finally, we tested for the level of spatial autocorrelation in the study region. We used a correlation test between palm and tree basal area and latitude, and considered the findings from Menger (2011), which had already indicated low spatial autocorrelation between bird community and geographic distance along the Purus–Madeira interfluvium. We found a poor correlation between palm and tree basal area with latitude (r = − 0.18, p = 0.84). Such a result further strengthens the evidence for low spatial autocorrelation along the study region.
Relationship between phenotypes and the environment and geography
We built generalized linear models (GLM) to assess whether the phenotype of the bird species studied varies along the Purus–Madeira interfluvium and how this is related to environmental heterogeneity or geographic distance among the study sites. Using this, we related the first two PCA axes for phenotypic variables (color, brightness or morphometrics) with environmental variables and geographic distances among study sites. All variables were Z-transformed to avoid bias from the variable values themselves in the results. Altogether, we generated three groups of models: one for color metrics (hue and saturation), one for brightness and one for morphometrics, with models generated for each explanatory variable (environmental and geographical) and all possible combinations, totaling seven models in addition to the null model (a constant model). Subsequently, we used the Akaike Information Criterion (Akaike 1973), corrected for the effect of small sample sizes and number of parameters (AICc; Burnham and Anderson 2002), for choosing the most parsimonious models (i.e., those that “best” explain the phenotypic variation). We consider model sets with the lowest relative AICc value (i.e., DAICc = 0) the best, and models with a DAICc ≤ 2 equally plausible (Burnham and Anderson 2002). We also estimated Akaike weights (ωi), which correspond to the proportion of support received by a model relative to the total support of all models and the model evidence ratio (ER) to estimate how likely the best model is compared to the constant (no-effect or null) model. We performed all statistical analyses using R 3.0.3 (R Development Core Team 2013).
Results
Phenotypic variation among sites
Approximately half of the cases analyzed (each species for both sexes and all characters) showed intraspecific morphological variation (Table 3, p < 0.05, F > 2.62 for all significant comparisons). Considering that the percentage of significant comparisons approaches 50% (9/15 comparisons were significant, or 60%), we applied the BH method of correction (Benjamini–Hochberg, which is the default FDR in the statistical package R) to control for false positives (Benjamini and Hochberg 1995) in R. After applying this method, all our comparisons remained significant.
Coloration (color and brightness) varied relatively more among localities than morphometrics for the three studied species: males and females of G. salvini showed variation only in color and brightness, whereas in W. poecilinotus males presented variation in color and brightness but females presented variation only in brightness, and this was the only species presenting variation in morphometrics. Males of L. coronata, on the other hand, showed variation only in plumage color but not in brightness among localities (Figs. 2 and 3), whereas females did not show variation in coloration (color or brightness).
Geographic variation in saturation in a Gymnopithys salvini, b Willisornis poecilinotus and c Lepidothrix coronata among localities within the Purus–Madeira interfluvium (M1-11). Boxes around the median represent the standard deviation, and bars above boxes represent the 95% confidence intervals. See Appendix S1 in Supplementary Material and table S2 for interpretation of PCA scores
Geographic variation in brightness in a G. salvini, b W. poecilinotus and c L. coronata among localities. Boxes around the median represent the standard deviation, and bars above boxes represent the 95% confidence intervals. See Appendix S1 in Supplementary Material and table S2 for interpretation of PCA scores
In general, differences in coloration were more discernable for birds (taking into account the threshold of 2.0 jnds) as geographic distance among populations increased, with a few cases of variation in coloration not discernable for birds between neighboring populations. For Thamnophlids, most statistically significant differences in coloration between localities farther apart were above the threshold of bird visual discrimination (DS and DL values > 2.0 jnds for color and brightness, respectively), whereas some of the statistically significant comparisons between neighboring localities were below the threshold of bird visual discrimination. For males of L. coronata, most pairwise comparisons among localities were above 2.0 jnds for DS values, although differences in coloration were frequently small between nearby localities.
For all three species, the coloration metrics that varied the most among localities were those describing the saturation of a plumage patch (S1 variable, tables S1 and S2) at different wavelengths (UV, violet, green, blue, etc.). Morphometrics varied only in beak width and tail length in W. poecilinotus. Variation among localities was not significant for any other trait (Table 3 and Figs. 2, 3 and 4).
Geographic variation in morphometrics in a G. salvini, b W. poecilinotus and c L. coronata among localities. Boxes around the median represent the standard deviation, and bars above boxes represent the 95% confidence intervals. See Appendix S2 in Supplementary Material and table S2 for interpretation of PCA scores
Color, brightness and morphometrics varied independently among localities. For example, morphometric characters of G. salvini differed between sites 7 and 9, while brightness and saturation did not vary between them, though they varied among other study sites. Males of L. coronata in definitive plumage showed one phenotypic break in plumage color along the studied transect between study sites 3 and 7 (Fig. 2), so that males in the northern part of the interfluvium were mostly black (sites 1 and 2), while those in the extreme south were mostly green (sites 10 and 11).
Effects of environment and geographic location on coloration
We found effects of geographic distance and environment in explaining differences in coloration across populations (Tables 3, S3 and S4). Environmental variables explained the greatest variation in color (particularly in males) and geographic distance on brightness. In females, brightness was the only metric affected by the tested predictor variables and was explained only by geographic distance. We more frequently observed effects of tree basal area on color variation, whereas bird community explained variation in color and brightness. Lastly, environment and geographic distance did not explain two of the eight cases of variation in coloration among populations.
Patterns of variation in color and brightness were different among species, and between sexes. In males of G. salvini, variation in color (saturation metrics) was better explained by both bird community and palm and tree basal area (GLM, ωi = 0.35); the relationship was negative for bird community and positive for palm and tree basal area (Table 3 and S3; Fig. 5). However, variation in brightness among study sites in males of G. salvini was best explained by bird community and geographical distance among sites (GLM, ωi = 0.30) (Tables 3 and S4). Geographic variation in color in females of G. salvini (metrics of saturation) was explained neither by geographical distance nor by environmental variation, and the null model (i.e., no variation or random variation not explained by tested variables) explained the observed data (GLM, ωi = 0.40) (Tables 3 and S3). The variation in brightness in females of G. salvini was better explained by geographic distance, and this relationship was negative (GLM, ωi = 0.29) (Tables 3 and S4, Fig. 6). In male W. poecilinotus, the null model best explained color variation among sites (GLM, ωi = 0.30) (Tables 3 and S3). A model combining the effects of the bird community and geographic distance best explained the variation in brightness in this species (GLM, ωi = 0.43), and their effects were negative in both cases (Tables 3 and S4, Fig. 6). In female W. poecilinotus, the variation in brightness was most closely related to palm and tree basal area and geographical distance (GLM, ωi = 0.30), and the relationships were both positive (Tables 3 and S4, Fig. 6). Finally, variation in color (hue and saturation) in L. coronata males was best explained by a full additive model combining the effects of all explanatory variables (bird community, palm and tree basal area and latitude) (GLM, ωi = 0.73) (Tables 3 and S3; Fig. 5).
Effects of environment and geographic location on morphometrics
Because we found variation in morphometrics among locations only in W. poecilinotus, we tested the relationship between morphometrics and environmental heterogeneity and/or geographical distance (latitude) only for this species and found that morphometric variation in W. poecilinotus was best explained by geographical distance (GLM, ωi = 0.52) (Tables 3 and S5; Fig. 7).
Discussion
Our findings indicate that morphological traits of the three studied species vary along the Purus–Madeira. The relevance of the observed variation for the visual systems of the studied species indicates that most of the differences within variable plumage traits (color and brightness) would be perceived as different by birds from different localities, particularly more distant localities. Thus, our results show differences in plumage coloration that are not only statistically significant but also biologically meaningful. The variation in color that could be perceived by the human eye differed from the variation modeled considering the visual systems of the studied birds. L. coronata males in definitive plumage in the northern part of the interfluvium are predominantly black, those in the extreme south are green and central populations present a plumage color intermediate between black and green, which is suggestive of clinal variation for males of L. coronata along the study region, despite the more abrupt break observed in our analysis.
The lack of male samples in definitive plumage for sites 4–6 may have prevented us from obtaining a more gradual change between sites 3 and 7. Thus, our results indicate subtler changes between sites 1 and 3 and between sites 7 and 11 (Fig. 2). Although the geographic variation in male plumage of L. coronata across its range has been previously documented (Cheviron et al. 2005; Anciães et al. 2009), this is the first quantitative assessment of such variation. It is also the first study to suggest a phenotypic break in male plumage color among populations of this species within an interfluvium where there is no obvious influence of rivers or other barriers to gene flow, indicating the effect of other mechanisms in driving the observed colorations. The suggestive clinal variation pointed to by our data is nevertheless intriguing, given the relatively reduced geographic extant of our study, as it indicates that these populations are at least partially isolated by distance, suggesting that alternative mechanisms, such as secondary contact between populations that diverged outside the interfluvium should be considered.
According to our results, environmental heterogeneity and stochastic factors explain equally well the morphological variation among populations of the studied species within the Purus–Madeira interfluvium. While color was better explained by environmental heterogeneity, plumage brightness within thamnophilids also varied with geographic distance, which alone explained the only case of variation in morphometrics. These results indicate that coloration, particularly plumage brightness, is more labile than morphometric traits and that plumage color might be driven mostly by adaptation to local environments, whereas plumage brightness is also under the effects of neutral drift in the absence of strong barriers to gene flow among populations. In both cases, accumulated differences were expressed as phenotypic divergence (e.g., Wang and Shaffer 2008). The results by Naka et al. (2012) also implied roles of both natural selection and neutral drift in the phenotypic and phylogenetic diversification among Amazonian birds from the Rio Branco–Negro interfluvium. These results, along with ours, provide compelling evidence that environmental heterogeneity and geographical distances within an interfluvium can be enough to influence the diversification of phenotypes among Amazonian birds and may, therefore, have further implications for taxonomic diversification, affecting, e.g., speciation rates through the origin or reinforcement of reproductive isolation (Uy et al. 2008; Wang and Summers 2010; Maia et al. 2013b).
Higher frequencies of association between male coloration and the environment suggest roles of natural and sexual selection on the expression of these phenotypes. Tree basal area frequently affected color traits, suggesting its role in the transmission and perception of the studied visual signals. This finding further indicates that color variation among the studied populations might be mediated by natural selection for crypsis and sexual selection for conspicuity through the sensory drive mechanism, which has previously been invoked in the context of the evolution of plumage coloration in birds (Gomez and Théry 2004; Heindl and Winkler 2003a, b; Uy and Endler 2004; but see Anciães and Prum 2008). The variation in palm and tree basal area along the Purus–Madeira interfluvium may therefore have led to variation in light environments available to birds and, as such, affected the diversification of plumage coloration among the studied populations in order to favor detection or concealment in their habitats.
Body plumages of L. coronata males varied from greener to bluer-black as the scores of the first axis of the color PCA increased (see Appendix S1 in Supplementary Material). Because green plumages are brighter than blueish-black ones, variation in male body plumages among the studied populations might result from adaptation for brightness contrast to the darker and lighter environments, respectively, corroborating previous findings on the role of brightness contrast in the evolution of plumage coloration in birds (Marchetti 1993, but see McNaught and Owens 2002; Zahavi and Zahavi 1997). The typical male blue-crown, however, contributed little to the variation in male plumage color among populations of this species. While this might indicate strong stabilizing sexual selection and developmental constraints associated with structural colors (Andersson 1999, 2000), it is important to consider that males may maximize the conspicuousness of their blue-crowns behaviorally by selecting light environments or specific backgrounds to display, similar to other species of manakins (Heindl and Winkler 2003b; Uy and Endler 2004; Uy and Stein 2007; Anciães and Prum 2008). Therefore, the lack of variability among populations in this trait might be due to the gain in conspicuousness through individual microhabitat selection in local populations, thus reducing the advantages to adapt to different habitat conditions among localities.
The associations between coloration and basal area of palms and trees among thamnophilids are also suggestive of selective advantages for conspicuousness, as in G. salvini males, or crypsis, as in W. poecilinotus females. Plumages of G. salvini males from populations inhabiting more closed forests were more reflective in the ultraviolet. Light environments of closed forests are relatively poor in ultraviolet light (Endler 1990, 1993), suggesting that color contrast between male plumages and their background habitats were maximized among the studied populations in this species (e.g., Endler and Théry 1996; McNaught and Owens 2002; Uy and Stein 2007). Likewise, males of this species with darker plumages were sampled in more open habitats, and lighter plumages in darker habitats, suggesting a role of brightness contrast in the observed variation in plumage among the studied populations of this species (e.g., Marchetti 1993). In W. poecilinotus, female plumages were darker within more closed habitats, whereas lighter plumages were observed in more open forests, suggesting adaptation for crypsis or longer distance communication in more open vegetation (Zahavi and Zahavi 1997).
Our results showed that changes in the composition of local bird communities are also related to changes in coloration of male plumages in the three studied species. Bird communities might suggest that biotic and abiotic environments change among locations, which may result in different selective pressures acting on the conspicuity of visual signals. Although we do not have direct measures for interactions among species (i.e., competition or predation), species turnover might exert selective pressures acting on evolution of plumage coloration into signal space, particularly on brightness, to, e.g., maximize long distance detection (Marchetti 1993; Zahavi and Zahavi 1997) or enhance crypsis while individuals are in movement or displaying for conspecifics (Endler 1991; Gotmark 1993). Likewise, bird communities might reflect additional environmental variation not assessed by our direct measures of habitat, which, nevertheless, suggests habitat effects on the studied plumages.
A higher frequency of variation in coloration among populations of males than females, in addition to more frequent relationships between coloration of male populations and environmental heterogeneity, indicates that visual signals are more variable among males than females in dichromatic species and that males respond to a greater variety of ecological pressures (e.g., predator and competitor communities or adaptive mating preferences) than females. In dichromatic bird species, females are usually duller than males (Peterson 1996; Owens and Hartley 1998; Badyaev and Hill 2003) and engage in nesting activities and parental care either alone (in lek polygyny such as in L. coronata) or shared with males (in monogamy such as in thamnophilids) and, as such, the lack of variation in female coloration observed among populations in our study suggests that they are subjected to strong stabilizing selection for crypsis. Correlated variation between male plumages and the environment, on the other hand, suggests divergent selection driven by adaptive female preferences favoring conspicuity (Endler and Basolo 1998) in L. coronata and G. salvini, which might be counteracted by effects of biotic interactions, favoring crypsis or other changes to improve male fitness in local communities. In fact, natural and sexual selection interact and produce phenotypes of reduced adaptive value for males, such as conspicuous displays that attract predators (Fisher 1930; Prum 2010, 2012). These conclusions are supported by additional evidence that males are easier to detect from a distance than females, even individuals with black and white plumage, which might seem not to be sexually selected (Gotmark 1993; Lundberg and Alatalo 1992), as in W. poecilinotus from our study. In this species, variation in color was better explained by a null model predicting that variation would not be explained by any tested alternatives, thus corroborating predictions of the null model of sexual selection by arbitrary preferences (Prum 2010, 2012), while variation in brightness indicated partial effects of both isolation by distance (non-adaptive variation) and responses to local communities. Considering that this species is the least dichromatic in our study, such findings are consistent with expectations of weaker sexual selection acting on brightness in this species when compared to the other two from our study.
The low variability of morphometric traits observed in our study might be explained by the small number of species studied and by the intrinsic low variability of morphological traits, which are usually maintained by ecological selection, when compared to sexually selected traits (Seddon et al. 2013; Martin and Mendelson 2014). Nevertheless, it is interesting that drift alone may cause variation in body size along a latitudinal gradient within a species range (W. poecilinotus), which corroborates theoretical work suggesting that incipient divergence between populations might result from random accumulation of phenotypic differences and that adaptation to new ecological conditions (i.e., ecological specialization) may evolve later within populations (see Schluter 2009) and could lead to the split between ecologically divergent lineages (Schluter 1998, 2001; Price 2008). Thus, the observed patterns of ecomorphological variation among species (e.g., Schulenberg 1983, on Amazonian species of Thamnomanes, Thamnophilidae) and the diversifying force attributed to niche shifts acting on morphological divergence and reproductive isolation (e.g., Ribeiro et al. 2014, on the non-Amazonian bird species Cercotrichas signata) might be anticipated by pre-existing phenotypic variation arising by random drift if populations are interconnected by relatively low levels of gene flow (see Wright 1931), as in those isolated by distance. The absence of habitat effects on the morphometric variation observed in our study might be due to a lack of specificity of biotic variables that could account for the variation in morphometrics observed among populations of W. poecilinotus.
In conclusion, our results show intraspecific phenotypic variation for the three studied species within one interfluvium in the Amazon where, at least for birds, the presence of barriers to gene flow is limited when compared to multiple interfluvial areas. Geographical distances combined with the effects of environmental heterogeneity within the interfluvium were enough to explain divergence among populations in the studied traits. While coloration was more labile than morphometrics, it was subjected mostly to the forces of both ecological and sexual selection; yet, neutral drift partially explained variation in coloration, particularly in brightness, and was the only mechanism underlying the observed variation in morphometrics. However, our data do not support the notion that morphometric traits are adapted to local environments, at least not among populations within a species. Furthermore, different phenotypic traits within a given species and sex seem to respond independently to different evolutionary mechanisms, being more variable and correlated with environmental variables among males than females, suggesting stronger effects of sexual selection acting on males. Finally, the intraspecific variation and mechanisms discussed with respect to our data highlight possible evolutionary forces acting during speciation.
References
Akaike H (1973) Information theory and the maximum likelihood principle in 2nd International Symposium on Information Theory (B.N. Petrov and F. Cs ä ki, eds.). Akademiai Ki à do, Budapest
Amézquita A, Lima AP, Jehle R, Castellanos L, Ramos O, Crawford AJ, Gasser H, Hödl W (2009) Calls, colors, shape, and genes: a multi-trait approach to the study of geographic variation in the Amazonian frog Allobates femoralis. Biol J Linn Soc 98:826–838
Anciães M, Prum R (2008) Manakin display and visiting behavior: a comparative test of sensory drive. Anim Behav 75:783–790
Anciães M, Durães R, Cerqueira MC, Fortuna J, Sohn N, Cohn-Haft M, Farias IP (2009) Diversidade de piprídeos (Aves: pipridae) amazônicos: seleção sexual, ecologia e evolução. Oecol Bras 13:165–189
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andersson S (1999) Morphology of UV reflectance in a Whistling-Thrush: implications for the study of structural color signalling in birds. J Avian Biol 30:193–204
Andersson S (2000) Efficacy and content in avian color signals. In: Espmark Y, Amundsen T, Rosenqvist G (eds) Animal signals: signalling and signal design in animal communication. Tapir Academic, Trondheim, pp 47–60
Badyaev AV, Hill GE (2003) Avian sexual dichromatism in relation to phylogeny and ecology. Annu Rev Ecol Evol Syst 34:27–49
Bates JM (2001) Avian diversification in Amazonia: evidence for historical complexity and a vicariance model for a basic diversification pattern. In: Diversidade Biológica e Cultural da Amazônia (Vieira, I., M. A. D’Incao, J. M. C. da Silva and D. Oren), 119–138. Museu Paraense Emilio Goeldi, Belém, Pará, Brazil
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 57:289–300
Bueno AS, Bruno RS, Pimentel TP, Sanaiotti TM, Magnusson WE (2012) The width of riparian habitats for understory birds in an Amazonian forest. Ecol Appl 22:722–734
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cadena CD, Cheviron ZA, Funk WC (2011) Testing the molecular and evolutionary causes of a “leapfrog” pattern of geographical variation in coloration. Evol Biol 24:402–414
Calsbeek R, Smith TB, Bardeleben C (2007) Intraspecific variation in Anolis sagrei mirrors the adaptive radiation of Greater Antillean anoles. Biol J Linn Soc 90:189–199
Cheviron ZA, Hackett SJ, Capparella AP (2005) Complex evolutionary history of a Neotropical lowland forest bird (Lepidothrix coronata) and its implications for historical hypotheses of the origin of Neotropical avian diversity. Mol Phylogenet Evol 36:336–357
Cintra R, Cancelli J (2008) Effects of forest heterogeneity on occurrence and abundance of the scale-backed antbird, Hylophylax poecilinotus (Aves: Thamnophilidae), in the Amazon forest. Rev Bras Zool 25:630–639
Cintra BBL, Schietti J, Emillio T, Martins D, Moulatlet G, Souza P, Levis C, Quesada CA, Schöngart J (2013) Soil physical restrictions and hydrology regulate stand age and wood biomass turnover rates of Purus–Madeira interfluvial wetlands in Amazonia. Biogeosciences 10:7759–7774
Cohn-Haft M, Pacheco AMF, Bechtoldt CL, Torres MFNM, Fernandes AM, Sardelli CH, Macêdo IT (2007) Inventário ornitológico. p. 145-178. Em: Rapp Py-Daniel L, Deus CP, Henriques, A.L., Pimpão, D.M., Ribeiro, O.M. (orgs.). Biodiversidade do Médio Madeira: Bases científicas para propostas de conservação. INPA: Manaus
Cracraft J (1985) Historical biogeography and patterns of differentiation within the South American avifauna: areas of endemism. Ornithol Monogr 36:49–84
Creegan HP, Osborne PE (2005) Gap-crossing decisions of woodland songbirds in Scotland: an experimental approach. J Appl Ecol 42:678–687
Cuthill IC (2006) Color perception. In: Hill G, McGraw K (eds) Bird coloration. Harvard, Cambridge, pp 1–40
Dayrat B (2005) Towards integrative taxonomy. Biol J Linn Soc 85:407–415
Durães R (2009) Lek structure and male display repertoire of blue-crowned manakins in eastern ecuador. Condor 111:453–461
Emilio TLS (2007) Distribuição de Palmeiras (Areaceae) ao longo de gradientes ambientais no Baixo Interflúvio Purus–Madeira, Brasil. Dissertação de mestrado. Instituto Nacional de Pesquisas da Amazônia, Manaus
Emilio T, Quesada CA, Costa FRC et al (2013) Soil physical conditions limit palm and tree basal area in Amazonian forests. Plant Ecol Divers 7:215–229
Endler JA (1977) Geographic variation, speciation and clines. Princeton University Press, Princeton
Endler JA (1982) Pleistocene forest refuges: fact or fancy? In: Prance GT (ed) Biological diversification in the tropics. Columbia University Press, New York, pp 641–657
Endler JA (1990) On the measurement and classification of color in studies of animal color patterns. Biol J Linn Soc 41:15–352
Endler JA (1991) Interactions between predators and prey. In: Krebs JA, Davies NB (eds) Behavioural ecology: an evolutionary approach, 3rd edn. Blackwell Scientific, Oxford, pp 169–196
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat 139:125–153
Endler JA (1993) The color of light in forests and its implications. Ecol Monogr 63:1–27
Endler JA, Basolo AL (1998) Sensory ecology, receiver biases and sexual selection. Tree 13:415–420
Endler J, Houde AE (1995) Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution 49:456–468
Endler JA, Mclellan T (1988) The processes of evolution: toward a newer synthesis. Annu Rev Ecol Evol Syst 19:395–421
Endler JA, Théry M (1996) Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am Nat 148:421–452
Engen S, Sæther B (2016) Phenotypic evolution by distance in fluctuating environments: the contribution of dispersal, selection and random genetic drift. Theor Popul Biol 109:16–27
Faria ES (2014) Evolução da variação intraespecífica em cantos de anúncio de Allobates sp. Dissertação de mestrado. Instituto Nacional de Pesquisas da Amazônia, Manaus
Fearnside PM, Graça PMLA (2009) BR-319: a rodovia Manaus-Porto Velho e o impacto potencial de conectar o arco de desmatamento à Amazônia central. NCN 12:19–50
Fernandes AM (2013) Fine-scale endemism of Amazonian birds in a threatened landscape. Biodivers Conserv 22:2683–2694
Fisher RA (1930) The genetical theory of natural selection. Oxford University Press, Oxford
Goldberg EE, Lande R (2006) Ecological and reproductive character displacement on an environmental gradient. Evolution 60:1344–1357
Gomez D (2006) AVICOL, a program to analyse spectrometric data. Last update October 2011. http://sites.google.com/site/avicolprogram/or from the author at dodogomez@yahoo.fr
Gomez D, Théry M (2004) Influence of ambient light on the evolution of color signals: comparative analysis of a Neotropical rainforest bird community. Ecol Lett 7:279–284
Gotmark F (1993) Conspicuous coloration in male birds is favoured by predation in some species and disfavoured in others. Proc R Soc Lond B Biol Sci 253(143):146
Grant BR, Grant PR (1993) Evolution of Darwin’s finches caused by a rare climatic event. Proc R Soc Lond B Biol Sci 251:111–117
Grant PR, Grant BR (2000) Non-random fitness variation in two populations of Darwin’s finches. Proc R Soc Lond B Biol Sci 267:131–138
Haffer J (1969) Speciation in amazonian forest birds. Sci New Ser 165:3889
Haffer J (1997) Alternative models of vertebrate speciation in Amazonia: an overview. Biodivers Conserv 6:451–476
Harris EF, Smith RN (2009) Accounting for measurement error: a critical but often overlooked process. Arch Oral Biol 1:107–117
Hegna RH, Saporito RA, Donnelly MA (2012) Not all colors are equal: predation and color polytypism in the aposematic poison frog Oophaga pumilio. Evol Ecol 27:831–846
Heindl M, Winkler H (2003a) Interacting effects of ambient light and plumage color patterns in displaying Wire-tailed Manakins (Aves, Pipridae). Behav Ecol Sociobiol 53:153–162
Heindl M, Winkler H (2003b) Vertical lek placement of forest-dwelling manakin species (Aves, Pipridae) is associated with vertical gradients of ambient light. Biol J Linn Soc 80:647–658
Hellmayr CE (1929) Catalogue of the birds of the Americas and the adjacent Islands in field museum of natural history. Science 66:153–154
Hill EG, Mcgraw KJ (2006) Bird coloration: function and evolution, vol II. Harvard University Press, Cambridge
Hoagstrom CW, Berry CR (2008) Morphological diversity among fishes in a Great Plains river drainage. Hydrobiologia 596:367–386
Hsu YC, Shaner PJ, Chang CI, Ke L, Kao SJ (2014) Trophic niche width increases with bill-size variation in a generalist passerine: a test of niche variation hypothesis. J Anim Ecol 83:450–459
IBGE (1997) Recursos naturais e meio ambiente: uma visão do Brasil. Instituto Brasileiro de Geografia e Estatística. Rio de Janeiro 2:208
Isler ML, Whitney BM (2011) Species limits in antbirds (Thamnophilidae): the Scale-backed Antbird (Willisornis poecilinotus) complex. J Wilson Ornithol 123:1–14
Johnson EI, Wolfe JD, Ryder TB, Pyle P (2011) Modifications to a molt-based ageing system proposed by Wolfe et al. 2010. J Field Ornithol 82:421–423
Kirkpatrick M, Ryan MJ (1991) The evolution of mating preferences and the paradox of the lek. Nature 350:33–38
Kirwan GM, Green G (2011) Cotingas and manakins. Princeton University Press, Princeton
Lee K, Shaner PL, Lin Y, Lin S (2016) Geographic variation in advertisement calls of a Microhylid frog—testing the role of drift and ecology. Ecol Evol 6:3289–3298
Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967
Lundberg A, Alatalo RV (1992) The pied flycatcher. Poyser, London
Maan ME, Seehausen O (2011) Ecology, sexual selection and speciation. Ecol Lett 14:591–602
MacArthur RH, Pianka EE (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Magnusson WE, Lima AP, Luizão R, Luizão F, Costa FRC, Castilho CV, Kinupp V (2005) RAPELD: a modification of the Gentry method for biodiversity surveys in long-term ecological research sites. Biota Neotrop 5:19–24
Maia R, Eliason CM, Bitton PP, Doucet SM, Shawkey MD (2013a) Pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol Evol 4:906–913
Maia R, Dustin RR, Matthew DS (2013b) Key ornamental innovations facilitate diversification in an avian radiation. Proc Natl Acad Sci USA 110:10687–10692
Marchetti K (1993) Dark habitats and bright birds illustrate the role of the environment in species divergence. Nature 362:149–152
Marciente R (2012) Efeito da obstrução gerada pela densidade da vegetação sub-bosque sobre morcegos frugívoros e animalívoros catadores (Chiroptera: Phyllostomidae) na Amazônia Central. Dissertação de Mestrado. Instituto Nacional de Pesquisas da Amazônia. Manaus
Martin MD, Mendelson TC (2014) Changes in sexual signals are greater than changes in ecological traits in a dichromatic group of fishes. Evolution 68:3618–3628
Martins DL, Schietti J, Feldpausch TR, Luizão FJ, Phillips OL, Andrade A, Castilho CV, Laurance SG, Oliveira A, Amaral IL, Toledo JJ, Lugli LF, Pinto JLPV, Mendoz EMO, Quesada CA (2014) Soil-induced impacts on forest structure drive coarse woody debris stocks across central Amazonia. Plant Ecol Div 8:229–241
Mayr E (1947) Ecological factors in speciation. Evolution 1:263–288
Mcnaught MK, Owens IPF (2002) Interspecific variation in plumage color among birds: species recognition or light environment? J Evol Biol 15:505–514
Menger JS (2011) Fatores Determinantes Da Distribuição De Aves No Interflúvio Purus-Madeira. Dissertação de Mestrado. Instituto Nacional de Pesquisas da Amazônia. Manaus
Moulatlet GM, Costa FRC, Rennó CD, Emilio T, Schietti J (2014) Local hydrological conditions explain floristic composition in lowland Amazonian forests. Biotropica 46:395–403
Mullen P, Pohland G (2007) Studies on UV reflection in feathers of some 1000 bird species: are UV-peaks in feathers correlated with VS/UVS cones? Ibis 150:59–68
Naka LN, Bechtoldt CL, Henriques LMP, Brumfield RT (2012) The role of physical barriers in the location of avian suture zones in the guiana shield, northern amazonia. Am Nat 179:115–132
Nokelainen O, Valkonen J, Lindstedt C, Mappes J (2014) Changes in predator community structure shifts the efficacy of two warning signals in Arctiid moths. J Anim Ecol 83:598–605
Ödeen A, Håstad O (2013) The phylogenetic distribution of ultraviolet sensitivity in birds. BMC Evol Biol 13:36
Owens IPF, Hartley IR (1998) Sexual dimorphism in birds: why are there so many diferent forms of dimorphism? Proc R Soc Lond B Biol Sci 265:397–407
Padial JM, Miralles A, De la Riva I, Vences M (2010) The integrative future of taxonomy. Front Zool 7:16
Patton JL, da Silva MNF (1998) Rivers, refuges, and ridges: the geography of speciation of Amazonian mammals. In: Haward DJ, Berlocher ST (eds) Endless forms: species and speciation. Oxford University Press, New York, pp 202–213
Peterson AT (1996) Geographic variation in sexual dichromatism in birds. Bull Br Orn Club 116:156–172
Price T (2008) Speciation in Birds. Roberts and Company Publishers, Greenwood Village
Prum RO (1997) Phylogenetic tests of alternative intersexual selection mechanisms: trait macroevolution in a polygynous clade (Aves: Pipridae). Am Nat 149:668–692
Prum RO (2010) The Lande–Kirpatrick mechanism is the null model of intersexual selection: implications for meaning, honesty, and design of intersexual signals. Evolution 64:3085–3100
Prum RO (2012) Aesthetic evolution by mate choice: Darwin’s really dangerous idea. Philos Trans R Soc Lond B Biol Sci 367:2253–2265
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ranta E, Kaitala V, Lundberg P (1998) Population variability in space and time: the dynamics of synchronous population fluctuations. Oikos 83:376–382
Ribas CC, Aleixo A, Nogueira ACR, Miyaki CY, Cracraf J (2012) A palaeobiogeographic model for biotic diversification within Amazonia over the past three million years. Proc R Soc Lond Ser B Biol sci 279:681–689
Ribeiro AM, Lloyd P, Dean JRJ, Brow M, Bowie RCK (2014) The ecological and geographic context of morphological and genetic divergence in an understory-dwelling bird. PLoS ONE 9:85903
Ryder TB, Durães R (2005) It’s not easy being Green: using molt and morphological criteria to age and sex Green-plumage manakin (Aves: Pipridae). Ornitología Neotropical 16:481–491
Safran RJ, McGraw KJ (2004) Plumage coloration, not length or symmetry of tail-streamers, is a sexually selected trait in North American barn swallows. Behav Ecol 15:455–461
Safran RJ, Flaxman SM, Kopp M, Irwin DE, Briggs D, Evans MR, Funk WC, Gray DA, Hebets EA, Seddon N, Scordato E, Symes LB, Tobias JA, Toews DPL, Uy JAC (2012) A robust new metric of phenotypic distance to estimate and compare multiple trait differences among populations. Curr Zool 58:426–439
Schietti J, Martins D, Emilio T, Souza PF, Levis C, Baccaro FB, Pinto JLPDV, Moulatlet GM, Stark SK, Sarmento K, de Araújo RNO, Costa FRC, Schöngart J, Quesada CA, Saleska SR, Tomasella J, Magnusson WE (2016) Forest structure along a 600 km transect of natural disturbances and seasonality gradients in central-southern Amazonia. J Ecol 104:1335–1346
Schluter D (1998) Ecological causes of speciation. In: Howard D, Berlocher S (eds) Endless forms: species and speciation. Oxford University Press, Oxford, pp 114–129
Schluter D (2001) Ecology and the origin of species. Trends Ecol Evol 16:372–380
Schluter D (2009) Evidence for ecological speciation and its alternative. Science 323:737–741
Schulenberg TS (1983) Foraging behaviour, ecomorphology and systematic of some antshrikes (Formicariidae: Thamnomanes). Wilson Bull 95:505–521
Seddon N, Merrill RM, Tobias JA (2008) Sexually selected traits predict patterns of species richness in a diverse clade of suboscine birds. Am Nat 171:620–631
Seddon N, Botero CA, Tobias JA, Dunn PO, Macgregor HE, Rubenstein DR, Uy JA, Weir JT, Whittingham LA, Safran RJ (2013) Sexual selection accelerates signal evolution during speciation in birds. Proc R Soc Lond B Biol Sci 280:20131065
Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HDJ, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H (2008) Speciation through sensory drive in cichlid fish. Nature 455:620–627
Sick H (1997) Ornitologia brasileira. Editora Nova Fronteira, Rio de Janeiro
Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K (2004) Interspecific and intraspecific views of color signals in the strawberry poison dart frog Dendrobates pumilio. J Exp Biol 207:2471–2485
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research. W. H. Freeman, New York
Souza PF (2012) Florística, distribuição e diversidade beta da Comunidade arbórea ao longo do gradiente ambiental hídrico em uma floresta de terra firme no interflúvio Purus-Madeira, Amazônia Central. Dissertação de mestrado. Instituto Nacional de Pesquisas da Amazônia, Manaus
Sun K, Luo L, Kimball RT, Wei X, Jin L, Jiang T, Li G, Feng J (2013) Geographic variation in the acoustic traits of greater horseshoe bats: testing the importance of drift and ecological selection in evolutionary processes. PLoS ONE 8:1–11
Tobias JA, Aben J, Brumfield RT (2010) Song divergence by sensory drive in amazonian birds. Evolution 64:2820–2839
Tobias JA, Brawn JD, Brumfield RT, Derryberry EP, Kirschel ANG, Seddon N (2012) The importance of Neotropical Suboscine birds as study systems in ecology and evolution. Ornitol Neotrop 23:259–272
Uy JAC, Endler JA (2004) Modification of the visual background increases the conspicuousness of golden-collared manakin displays. Behav Ecol 15:1003–1010
Uy JA, Stein AC (2007) Variable visual habitats may influence the spread of colorful. J Evol Biol 20:1847–1858
Uy JAC, Moyle RG, Filardi CE (2008) Plumage color and song differences mediate species recognition between incipient flycatcher species of the solomon islands. Evolution 63:153–164
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of color thresholds. Proc R Soc Lond Ser B Biol Sci 265:351–358
Vorobyev M, Brandt R, Peitsch D, Laughlin SB, Menzel R (2001) Color thresholds and receptor noise: behavior and physiology compared. Vis Res 41:639–653
Wallace AR (1858) On the tendency of species to form varieties; and on the perpetuation of the varieties and species by natural means of selection. J Proc Linn Soc Lond 2:53–62
Wang IJ, Shaffer HB (2008) Rapid color evolution in an aposematic species: a phylogenetic analysis of color variation in the strikingly polymorphic strawberry poison-dart frog. Evolution 62:2742–2759
Wang IJ, Summers K (2010) Genetic structure is correlated with phenotypic divergence rather than geographic isolation in the highly polymorphic strawberry poison-dart frog. Mol Ecol 19:447–458
Weir JT, Faccio MS, Pulido-Santacruz P, Barrera-Guzmán AO, Aleixo A (2015) Hybridization in headwater regions, and the role of rivers as drivers of speciation in Amazonian birds. Evolution 69:1823–1834
WilIis EO (1968) Taxonomy and behavior of Pale-faced Antbirds. Auk 85:253–264
Wright S (1931) Evolution in Mendelian populations. Genetics 16:97–159
Wright S (1943) Isolation by distance. Genetics 28:114–138
Ximenes AC (2008) Mapas auto-organizáveis para a identificação de ecorregiões no interflúvio Purus-Madeira: uma abordagem da biogeografia ecológica. Dissertação. Instituto Nacional de Pesquisas Espaciais: São José dos Campos
Zahavi A, Zahavi A (1997) The handcap principle. Oxford University Press, Oxford
Zimmer KJ, Isler ML (2003) Family Thamnophilidae (Typical Antbirds). In: Del Hoyo J, Elliott A, Christie DA (eds) Handbook of the birds of the world, vol 8. Lynx Edicions, Barcelona, pp 448–681
Acknowledgements
We are grateful to I. Kaefer, P. Simões, S. Borges, R. Cintra, F. Werneck and two anonymous referees for helpful comments on the manuscript and to A. Lima, W. Magnuson, M. J. Fortin and A. Stow for helpful sampling design suggestions. Centro Nacional de Pesquisa para Conservação das Aves Silvestres—CEMAVE (Number: 3245/3) and Instituto Chico Mendes de Conservação da Biodiversidade—ICMBio (Number: 5873-1) granted permits, and Fundação de Amparo à Pesquisa do Estado do Amazonas (PRONEX/FAPEAM/CNPQ 003/2009 to MA); Conselho Nacional de Desenvolvimento Científico e Tecnológico (UNIVERSAL—MCTI/CNPq 471092/2012-6 to MA); PRONEX/FAPEAM (1600/2006 to W. Magnusson), Hidroveg Universal CNPq (473308/2009-6), Centre for Amazonian Biodiversity Studies (INCT CENBAM), The Large Scale Biosphere–Atmosphere Experiment (LBA) and CAPES (fellowship to FHTA) funded the research. We thank the curators and collection managers at Instituto Nacional de Pesquisas da Amazônia (INPA) and the Museu Paraense Emilio Goeldi (MPEG) for access to specimens. The Brazilian Biodiversity Research Programme (PPBio) offered logistical support, and T. Emilio shared data on palm plant species and soil. We thank F. Assunção, A. Sousa, M. Bosholn, N. Soto, H. Santana, E. Dantas, S. Pereira, R. Dário, J. Capurucho and G. Leite for field assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Abreu, F.H.T., Schietti, J. & Anciães, M. Spatial and environmental correlates of intraspecific morphological variation in three species of passerine birds from the Purus–Madeira interfluvium, Central Amazonia. Evol Ecol 32, 191–214 (2018). https://doi.org/10.1007/s10682-018-9929-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-018-9929-4