Abstract
Host specialization evolved in many parasite-host systems. Evolution and maintenance of host specificity may be influenced by host life-history traits, active host selection by the parasite, and host anti-parasite strategies. The relative importance of these factors is poorly understood in situations that offer parasites a choice between hosts with similar habitat requirements. The common cuckoo Cuculus canorus is a generalist parasite on the species level, but individual females prefer particular host species. In reed beds of the Yellow River Delta, China, two potential hosts with similar nest characteristics, Oriental reed warblers Acrocephalus orientalis and reed parrotbills Paradoxornis heudei, breed in sympatry. We found that warblers were parasitized at much higher rates than parrotbills. Both hosts recognized and rejected non-mimetic model eggs well, indicating that they have been involved in an arms-race with cuckoos. Cuckoo eggs closely resembled warbler eggs, and such eggs were mostly accepted by warblers but rejected by parrotbills. Only warblers recognized adult cuckoos as a specific threat. Both hosts were equally good at raising cuckoo chicks. Low nest density, partial isolation by breeding time, small scale differences in nest and nest site characteristics, and high rejection rates of natural cuckoo eggs are likely cumulatively responsible for the low current parasitism rate in parrotbills. This study emphasizes the importance of integrating the study of general host life-history characteristics and specific anti-parasitism strategies of hosts across all breeding stages to understand the evolution of host specificity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generalist parasites often form specialized races, each adapted to a particular host via highly polygenic morphological and physiological traits (Futuyma and Moreno 1988), yet still belonging to a single species (i.e., host races may be connected by gene flow). For example, the common cuckoo, Cuculus canorus (hereafter cuckoo), exploits the parental care of many small passerines across Eurasia (Moksnes and Røskaft 1995). The cuckoo is a generalist at the species level, but individual cuckoo females prefer one or a few host species (Gibbs et al. 2000; Fossøy et al. 2011). Cuckoos have evolved distinct egg morphs that mimic particular host species in colour, spottiness, and size (Moksnes and Røskaft 1995) to reduce the risk of host egg rejection (Davies and Brooke 1989).
Despite intense research, especially during recent decades, the mechanisms underlying host selection in cuckoos remain poorly known. This is because there are many factors that may contribute to host selection (Soler et al. 1999), making it difficult to investigate their relative importance. Some, like foreign egg rejection, may represent specific anti-parasite adaptations (Davies and Brooke 1989; Moksnes et al. 1991a) while others, like nest design (Grim et al. 2009) or chick diet (Yang et al. 2013), probably did not evolve in response to parasitism. A strong test to understand causes of host selection is to analyse parasitism in sympatrically occurring potential hosts with similar nest characteristics, food and habitat requirements. This has rarely been done so far (Ortega and Cruz 1991; Peer and Bollinger 1997; Mermoz and Fernández 1999; Edvardsen et al. 2001; Grim et al. 2011).
In the reed beds of the Yellow River Delta, China (YRD), two potential cuckoo hosts breed in sympatry: the Oriental reed warbler, Acrocephalus orientalis, and the reed parrotbill, Paradoxornis heudei (hereafter, warbler and parrotbill, respectively). The two species are phylogenetically distant, but they construct nests that are surprisingly similar in appearance and position (Fig. 1). Previous work, however, showed that warblers are commonly victimized by cuckoos in YRD and elsewhere in Eastern China, whereas parrotbills are almost never parasitized (Yang et al. 2014). These differences are striking considering the extremely homogeneous habitat (reed beds) that both passerines share. Disentangling reasons for such patterns requires a comprehensive approach that considers all potential factors, including nest site locations (Moskát and Honza 2002) and host responses to parasites at all stages of development, i.e. adults (Sealy et al. 1998), eggs (Davies and Brooke 1989), and chicks (Grim 2006a). Therefore we combined these approaches and studied both general life-history traits and potentially specific anti-parasite host responses at adult, egg, and chick stages in order to comprehensively understand host selection in a homogeneous ecological setting (see Grim et al. 2011).
Materials and methods
We carried out the fieldwork from May to August in 2008 (parrotbills only) and in 2010–2012 (warblers and parrotbills) in the YRD National Nature Reserve (37°35′ − 38°12′ N, 118°33′ − 119°20′ E), Shandong, Eastern China. The study area is located in the second largest estuarine wetland area of China and contains extensive reed habitats, interspersed with stretches of black locust Robinia pseudoacacia forests and farmlands (see Li et al. 2015a for more details). Warblers are recognized as the main hosts of the cuckoo in lowland reed habitats of Eastern Asia (Yang et al. 2012, 2014). Parrotbills are potential cuckoo hosts, but reports of parasitism are very rare (Lin et al. 2008; Yang et al. 2012, 2014). Although a larger area was searched for nests (see below), most nests were found in three specific reed bed plots (Fig. S1). We systematically searched for nests of the two passerines throughout the breeding season. Most nests (80.0 %, n = 526) were found when they were at the nest building or egg stage and we randomly selected nests for experiments. All nests, experimental or not, were monitored every 1–4 days until the final nest fate was determined.
Our focal host species (one warbler and one parrotbill) is an ideal study system—simple and tractable (cf. multiple hosts in other habitats making such systems less tractable, see also discussion in Grim et al. 2011). Cuckoo parasitism rate was defined as a proportion of active nests that we found parasitized. Parasitism rates in any hosts may potentially be underestimated due to the high egg rejection rates by hosts (Davies 2000). However, we find this possibility unlikely for the following reasons. Cuckoos typically remove one or several eggs from each host nest during the act of parasitism (Moksnes et al. 2000). However, first, among parrotbill nests that were visited daily during the egg-laying stage, we only found one case of a missing host egg (n = 32). Second, non-parasitized parrotbills usually have a clutch size of 5 eggs (83.1 %, n = 83), and there was no significant difference in the percentage of nests with less than 5 eggs before (17.9 %, n = 46) and after (23.3 %, n = 37) cuckoo parasitism commenced in the area (Chi square test: χ2 = 0.02, df = 1, p = 0.88). This suggests that at most only a few parasitized parrotbill nests escaped our attention.
The local abundance and distribution of each species was comprehensively surveyed along the roads or paths that pass through almost all reed habitats at the study site. One survey was conducted every year when most territories were established, in the first week of June. All three study species were easily visible due to their conspicuous behaviour. Territories were marked using a GPS (Garmin 60 s), from which local population sizes were estimated. The spatial breeding distribution of each bird species was measured on GoodyGIS (Version. 3.21; http://www.goodygis.com/) based on the GPS data. The nest density of each host species was calculated as the total number of nests divided by the breeding area per year (see, e.g., Samaš et al. 2013, see also Jelínek et al. 2014). This estimate constitutes a suitable index for comparing the relative breeding population for each host species because both parrotbill and warbler nests were searched with similar search effort. Importantly, the method directly takes into account all simultaneously active nests that are available to cuckoos in the peak part of the season.
We investigated differences in characteristics of nests and nest sites between parrotbills and warblers, following the protocol of Moskát and Honza (2000): (1) distance from nest to the closest potential cuckoo perch site, i.e. tree or electric wire (to the nearest m); (2) distance to the closest reed edge (m); (3) nest visibility to a human observer (DL), on ordinal scale of five degrees from bad to good visibility; (4) the height of the reeds above the nest (m); (5) the number of green reed stems in a 0.5 × 0.5 m quadrat at breast height (1.5 m) around the nest; (6) the mean height of the five highest reed stems in a 0.5 × 0.5 m quadrat of the nest site (m); not used by Moskát and Honza (2000); (7) the nest height above the water or ground (m); (8) the nest volume, an index expressing the size of the nest, which was calculated by multiplying three values: (a) outer nest height from the bottom of the nest to the rim; (b) maximum outer nest width; and (c), minimum outer nest width (all to the nearest cm).
In order to investigate specific host responses to adult parasites, we presented stuffed dummies near host nests, following the protocol of Sealy et al. (1998) and Campobello and Sealy (2010). Dummies of three species were used (two specimens per each to minimize the risk that differences between treatments would be a by-product of a particular specimen, see Trnka and Grim 2013), representing a brood parasite (common cuckoo, gray morph), a predator of adults (sparrowhawk Accipiter nisus), and non-threatening species as a control (spotted dove Spilopelia chinensis). A randomly chosen specimen was placed on bamboo sticks postured in typical life-like position about 0.5 m from the focal nest and at the same height as the nest rim with the head facing the rim.
The responses of nest owners were observed from a distance of about 10–20 m from a hide. Behaviour was also video-recorded by a camera placed 1–2 m from the nest. Generally, the nest owners would return within 2–3 min, and the responses were recorded for 5 min from the return of the first bird. Each nest was tested with all three dummies presented in a random order and separated by 30 min. Nests were randomly selected during the egg-laying or early incubation stage (≤6 incubation days). Host responses were rated on a four-step scale following Moksnes et al. (1991b): (1) No reaction, in which the hosts paid no attention to the dummy, and in some cases, the host even sat on the nest directly; (2) Distress calling, in which the host uttered distress and alarm calls but was not willing to approach the dummy; (3) Mobbing, in which the host approached the dummy with aggressive postures and alarm calls, but never made physical contact; (4) Attacks, in which the host vigorously attacked (i.e., contacted) the dummy. The aggressive response was combined into two categories: no aggression (1 and 2) and aggression (3 and 4) for statistical analysis (Røskaft et al. 2002; Grim et al. 2011). In cases when most individuals respond aggressively but some respond with few attacks whereas others respond with many attacks, ordinal or nominal behavioural scales may lack power to detect existing and biologically relevant effects (see analyses in Trnka et al. 2012). Therefore, we additionally recorded the exact number of contact body attacks during 5 min based on the video recordings (for a separate analysis, see below).
Variation in appearance between the eggs of cuckoos and those of the two potential host species was measured by spectrophotometry. Egg reflectance spectra (300–700 nm; 0.597 nm intervals) of three randomly selected portions of the background, a light spot, and a dark spot on each egg were measured using a miniature fibre optic spectrometer (AVANTES) connected to a portable computer. All measurements were taken at a 45° angle and cover an area of approximately 1 mm2. In total, 792 spectra readings were collected from 55, 19, and 14 eggs of warblers, cuckoos, and parrotbills, respectively.
We artificially parasitized nests with one of three egg types: real cuckoo eggs, blue model eggs, and conspecific eggs. We used natural cuckoo eggs to directly quantify selection pressure by hosts to natural parasitism; some naturally laid (i.e. non-experimental) parasite eggs may be rejected by hosts before researchers notice them (but see above), therefore an experimental approach is needed to avoid biases in estimates of egg rejection rates (Moksnes and Røskaft 1992, Samas et al. 2014). We employed non-mimetic blue models as a standardized stimulus, which was identical for both study host species (see also Grim et al. 2011). Conspecific eggs were used because rejection of both real cuckoo eggs and non-mimetic models may represent a by-product of host egg rejection abilities evolved due to conspecific parasitism (Samas et al. 2014, Liang et al. 2016).
Real cuckoo eggs were transferred from parasitized warbler nests into non-parasitized warbler and parrotbill nests. In six cases, warbler eggs were used instead of cuckoo eggs as the experimental eggs in the experiments with reed parrotbills, because of scarcity of cuckoo eggs and due to that warbler eggs are very similar to cuckoo eggs in appearance (see results). Indeed, there was no difference in parrotbill rejection of cuckoo (76.9 %, n = 12) and warbler eggs (83.3 %, n = 6; Fisher Exact Test: p = 1.00). The data from the two groups were therefore merged.
The blue model eggs were made of synthetic clay. The mass of these eggs (3.14 ± 0.04 g; n = 46) were similar to those of real cuckoo eggs (3.13 ± 0.20 g, n = 38; t = 0.40, df = 82, p = 0.69), but the egg sizes (egg length: 19.44 ± 0.76 mm; egg breadth: 15.42 ± 0.41 mm; n = 46) were slightly smaller than real cuckoo eggs (egg length: 22.02 ± 0.90 mm; egg breadth: 16.26 ± 0.42 mm; n = 38; all p < 0.0001). Finally, we included a control group, i.e. nests whose content was not manipulated but was monitored to estimate the baseline nest desertion rates (Samas et al. 2014).
Host nests were monitored daily or every second day for six consecutive days after the initial placement of the eggs, following the standard criteria for rejection (Moksnes et al. 1991a, b). Responses were defined as (1) ejection: the parasitic egg disappeared or were still incubated but heavily pecked (model eggs); (2) desertion: the nest was abandoned with or without any damage to either the parasitic egg or the hosts’ own eggs; and (3) acceptance: the clutch with the parasitic egg was still warm without peck-broken egg(s) and was incubated at the end of the monitoring period. Nests that were depredated within the 6-day period were excluded from analyses. Desertions were included as a rejection response because no control nests were deserted (Results).
We carried out cross-fostering experiments in order to investigate whether warblers and parrotbills differed in their ability to raise a cuckoo chick. We exchanged 14 cuckoo chicks (aged 3–5 days) from naturally parasitized warbler nests with host chicks in non-parasitized parrotbill (n = 6) or warbler (n = 8) nests. Another 18 cuckoo chicks in naturally parasitized nests served as the control group. We cross-fostered cuckoos between naturally parasitized and non-parasitized nests to check whether cross-fostering of cuckoos from warbler to parrotbill nests itself did not affect their growth and survival in parrotbill nests (see also Grim 2007). All the cuckoo chicks were monitored every other day until fledging or death. Body weight (0.01 g) and tarsus length (mm) were measured daily or every other day until fledging.
Statistical analyses
The date of the first egg laid in each focal nest was either recorded directly (for nests found in nest building and egg-laying stage) or inferred (nests found with completed clutches or chicks) using incubation time of 14 and 12 days for two hosts and cuckoo respectively, and fledging time of 14 and 20 days for two hosts and cuckoo, respectively (Li et al. unpublished data). We used the first egg-laying date (or egg laying for the cuckoo) for each nest to compare breeding time synchronization for the cuckoo and two hosts. Independent sample t-tests were used to compare the breeding time among groups because all data fitted normal distribution.
All egg and nest characteristics were assessed for normality using the Shapiro–Wilk tests. When necessary, data were ln transformed to achieve normality. In cases where there was still a lack of normality after transformation, we used Mann–Whitney U tests to compare the differences between groups, otherwise an independent sample t-tests were used. The sample size for nest sites and nest characteristics varied for different comparisons because weather or predators destroyed some nests. For analyses of differences in parasitism rates, egg rejection rates and fledgling success rates, Pearson’s χ2 tests were used with Yates’ continuity correction, except when 20 % of the expected values in the contingency table were <5, in which case we used Fisher exact tests.
We used the generalized linear mixed model (GLMM) to test the host responses to dummies with response variable as nominal (no aggression or aggression) or continuous (number of contact attacks per 5 min). All GLMMs included two main fixed factors: host species (nominal) and dummy species (nominal) and their interaction. Other potential confounding factors, namely, year and nest stage (two categories: egg-laying vs. early incubation, i.e. <6 days of incubation) were also statistically controlled for. Specimen id was modelled as a fixed (rather than a random) effect because the number of levels was lower than six (see Fox et al. 2015). Nest identity was entered as a random factor to control for non-independence among trials at the same nest. The first GLMM used a binomial distribution with a logit link, as the host aggression is a binary response (no aggression or aggression). The second GLMM used a Poisson distribution with a log link, because the response variable “number of attacks” is a count of occurrences during a fixed period of time. We followed backward elimination of non-significant terms. We checked the final (minimum adequate) model by adding the previously removed terms (one at a time) and found that none explained any significant variation. Test statistics and p values reported are from a sequential backward elimination procedure just before the particular term (being the least significant) and was removed from the model. The minimum adequate model contained only significant predictors.
Three separate principal component analyses were used to explore the colour variations in the background and the two types of spots for the three species. This type of analysis is useful for evaluating variation in spectral data because it reduce the correlated variables of reflectance spectra into a few orthogonal variables that describe achromatic (brightness) and chromatic (colour) variation (Cuthill et al. 1999; Cherry and Bennett 2001). The first principal component (PC1) represents variation in mean reflectance, or brightness, whereas the subsequent principal components represent variation in colour (Endler and Thery 1996). PC1 explained 83.6, 85.5, and 71.0 % of the overall colour variations of background, light spots, and dark spots, respectively, whereas the PC2 and PC3 explained the remaining variation among 8.4–13.9 % and 3.3–6.2 % respectively. The coefficients were plotted against wavelength to depict the variation in colour that was explained by each principal component (Fig. S2), and the differences in principal component scores between cuckoo eggs and those of the two hosts were compared to reflect the egg mimicry.
We used logistic regression to estimate standard growth parameters (K = growth rate, A = asymptotic mass) of cuckoo chicks (for details see Grim 2006b). Only chicks with growth data across the majority of nestling period could be used to estimate standard growth parameters (Starck and Ricklefs 1998; Grim 2006b). The benefit of this approach is to estimate K and A parameters at individual chick level that can be included in future meta-analyses. The disadvantage is that, chicks that died, due to predation or inclement weather, before reaching the asymptotic phase of growth had to be excluded. Therefore, we run a separate analysis based on all growth data, with chick id as a random effect; the single population estimates of K and A were very similar to those based on individual chicks (Table 3).
We used one-way ANOVAs to compare the estimated growth parameters (K, A) of young cuckoo raised in three groups (Kruskal–Wallis tests on the same data lead to same conclusions, results not shown). The differences in fledging age and body measures (body mass and tarsus length) were only compared between host species (naturally parasitized warblers and cross-fostered warblers were pooled because of the relatively small sample sizes) using independent sample t-tests.
All statistical analyses were carried out using IBM SPSS 20.0. The statistical tests were two-tailed, and estimates are reported as mean ± SE, except when stated otherwise. Significance levels were set at p < 0.05.
In the present study, blinded methods were not used. It was not possible to record data blindly because our study involved focal animals in the field.
Results
Parasitism rate
The overall parasitism rate in warblers (24.9 %; n = 362) was significantly higher than in parrotbills (0.8 %; n = 132; Chi square test: χ2 = 35.81, df = 1, p < 0.001; Table S1). There was no significant annual variation in the parasitism rate in the two hosts (warbler: χ2 = 0.44, df = 2, p = 0.80; parrotbill: χ2 = 2.32, df = 3, p = 0.51). Approximately 2.5 % of the warbler nests (n = 362) were multiply parasitized (contained two cuckoo eggs), and there was no annual variation in multiple parasitism (χ2 = 0.02, df = 2, p = 0.99, Table S1).
Spatial distribution, nest density, nest and nest site characteristics
Our survey disclosed that parrotbills have a considerably larger spatial breeding distribution (ca. 103 km2) than warblers (ca. 3.67 km2) in the Yellow River delta, but the estimated average nest density (11.0 nests/km2) of parrotbills was only about a quarter of that of warblers (40.8 nests/km2) from the data collected in three main study sites (Fig. S1 and Table S2). Warblers were concentrated in three main reed plots (>90 % of the population in the study area), where they bred in the densest and tallest reeds. The breeding populations showed dramatic annual fluctuations in each reed plots (Table S2) depending on the local hydrological conditions for reed growth. Parrotbills bred in a broader range of reeds, but the nest density was not uniform: parrotbill nest density in site A was much higher than in site B and C (Table S2). Within the three study plots, the nest density of parrotbills was much lower than that of warblers in site B and C, but was about half (0.58) of that of warblers in site A (Table S2).
Both parrotbills and warblers built cup nests of remarkably similar appearance sewn around 3–7 reed stems (Fig. 1). There were no significant differences between the distances of parrotbill and warbler nests to cuckoo perches, but parasitized warbler nests were closer to perches than non-parasitized ones (Table 1). There were no other nest or nest-site characteristics showing any statistically significant difference between parasitized and non-parasitized warbler nests. However, compared to parrotbills, warblers generally placed their nests further from the nearest reed edge (from water or dry land, but significantly so only for warblers that were not parasitized), in larger and denser reeds, nested in less dry reeds, had larger and more visible nests, and placed their nests higher in the reeds (Table 1).
Timing of breeding season
Parrotbills were local residents and started to breed earlier (first egg-laying date for the first nest in each season: 2008: April 28; 2010: May 15; 2011: May 8; 2012: April 25) than migratory warblers (2010: May 25; 2011: May 25; 2012: May 18) or migratory cuckoos (2010: June 1; 2011: June 6; 2012: May 30). There were no significant differences between the average first egg-laying dates of cuckoos and warblers during 2010–2011 (2010: t = 1.13, df = 176, p = 0.26; 2011: t = 0.27, df = 138, p = 0.79), but in 2012, cuckoos initiated their egg-laying season somewhat later than warblers (t = 2.57, df = 150, p = 0.01). The average first egg-laying dates of parrotbills were much earlier than those of cuckoos (for all years, p ≤ 0.005) and warblers (for all years, p ≤ 0.005) during the three years of study. In fact, on average, 55.3 % (2010: 38.5 %, n = 26; 2011: 69.4 %, n = 36; 2012: 53.7 %, n = 41) parrotbill clutches were completed and the eggs were nearly ready to hatch before the onset of cuckoo parasitism. However, still a considerable number of parrotbill nests (n = 46) were available to cuckoos, but very few actually were used (Table S1). Considering the time when only the parrotbill nests were available to cuckoos during 2010–2012, parasitism rate in parrotbills (0 %, n = 46) was also significantly lower than in warblers (25.9 %; n = 348; χ2 = 13.99, df = 1, p < 0.001).
Response to dummies
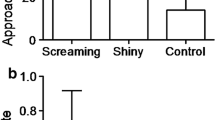
Ordinal scoring showed that warblers were more aggressive (68 % of trials) than parrotbills (19 % of trials; F1, 209 = 24.18, p < 0.0001; Fig. 2a). Both specimen id (F2, 204 = 0.15, p = 0.86), specimen-host species interaction (F4, 205 = 1.18, p = 0.32) and breeding stage (F1, 204 = 0.35, p = 0.56) were non-significant and removed from the final model. The final model controlled for significant differences among years (F2, 209 = 3.78, p = 0.024) and nests (Z = 3.09; p = 0.002).
Warblers launched a larger number of direct attacks (estimate ± SE: 6.83 ± 2.12) to all dummies than parrotbills (0.08 ± 0.04, F2,207 = 45.59, p < 0.0001), and were especially aggressive against the cuckoo when compared to the aggression of the parrotbill (14.61 ± 4.53 vs 0.07 ± 0.05; t = 5.32, p < 0.0001). Both the year (F2, 205 = 2.62, p = 0.08) and breeding stage (F1, 204 = 0.11, p = 0.75) were non-significant and removed from the final model. The final model controlled for significant differences among nests (Z = 4.60, p < 0.0001), specimens (F2,207 = 11.06, p < 0.0001), and the interaction between host species and specimens (F2,207 = 14.20, p < 0.0001). The warblers showed more aggressive attacks towards mounted cuckoos than hawks and doves (F2, 207 = 5.14, p = 0.007; cuckoo versus hawk: t = 3.21, df = 207, p = 0.002; cuckoo versus dove: t = 3.20, df = 207, p = 0.002; Fig. 2b), whereas parrotbills showed no significant variation in the number of attacks they made towards cuckoos versus the other two stuffed dummy types (F2, 207 = 1.12, p = 0.33; cuckoo versus hawk: t = 1.00, df = 207, p = 0.32; cuckoo versus dove: t = −1.24, df = 207, p = 0.22; Fig. 2b).
Egg mimicry
Cuckoo egg background colour was pale green–blue, speckled and blotched with pale grey, light and olive green, and dark brown spots (Fig. 3b), displaying almost perfect mimicry of warbler eggs to the human eye and according to the objective spectral analysis. However, both the background colour and the light spots of the cuckoo eggs were brighter than those of the warbler eggs (Fig. 3a). Parrotbill eggs were greenish-white with dark brown spots and blotches or clouds of underlying pale sienna spots (Figs. 1d, 3b), and differed distinctly from cuckoo eggs (Fig. 3b).
Background color and spots of eggs of the common cuckoo (n = 19) and its two hosts (warblers: n = 55; parrotbills: n = 14): a reflectance spectra (300–700 nm; 0.597-nm intervals) of background color and spots, b the difference between light spots (LS), corresponding to the square frame in red), and dark spots (DS), corresponding to the square frame in yellow
There were significant differences in three scores of PC1–3 between cuckoo and the two hosts (all p < 0.05), except for the PC1 of the dark spots between cuckoo and warbler (t = 1.85, df = 214, p = 0.07) and the PC2 of the background between cuckoo and warbler (t = 0.41, df = 223, p = 0.68), and the PC3 of the dark spots between cuckoo and both hosts (cuckoo vs. warbler: t = 0.95, df = 214, p = 0.34; cuckoo vs. parrotbill: t = −1.13, df = 98, p = 0.26; Fig. S3).
Egg recognition experiments
Neither parrotbills (n = 20) nor warblers (n = 32) in the control groups showed any egg rejection (rejection errors) or nest desertion (Fig. 4). Therefore, we included desertion as a specific response to parasitism.
Parrotbills (77.8 %, n = 18) displayed a higher rejection rate of real cuckoo eggs than did warblers (10.5 %, n = 19; χ2 = 14.40, df = 1, p < 0.001; Fig. 4). Both hosts used ejection of the eggs from the nest as their only egg rejection strategy, with no loss sustained due to erroneous or accidental ejection of own eggs.
Parrotbills (78.9 %, n = 19) and warblers (75.8 %, n = 33) showed a similar high rate of egg rejection for non-mimetic blue model eggs (χ2 = 1.97, df = 1, p = 0.16; Fig. 4). Ejection (both successful and attempted but unsuccessful) was the most common method of rejection of the foreign eggs in both parrotbills (93.3 %, n = 15) and warblers (92.0 %, n = 25; Fisher exact test: p = 1.00). However, while warblers successfully removed model eggs in most cases (91.3 %, n = 23), parrotbills only successfully ejected 7.1 % (n = 14) such eggs (χ2 = 22.20, df = 1, p < 0.001). The inability of the parrotbills to eject model eggs always ended in either the forced acceptance of the parasite eggs (35.8 %, n = 14) or complete desertion (57.1 %; n = 14) of the nest and breakage of almost all host own eggs (2–4 eggs). Two unsuccessful attempts to eject that finally led to nest desertion also contained two or three broken host eggs, but the rate of loss associated with damage to the host’s own eggs was significantly lower for warblers (8.7 %; n = 23) than parrotbills (71.4 %, n = 14; Fisher exact test: p < 0.001). Oriental reed warblers and reed parrotbills have distinctly different bill morphology (Xiong and Lu 2013). Due to their short and blunt bills, parrotbills are most probably not able to grasp eject eggs and therefore have to rely on puncture ejection (or desertion). This seemed to work without problems when ejecting real cuckoo eggs, but they obviously faced problems when trying to get rid of the thicker-shelled model eggs. Warblers, on the other hand, should be able to grasp eject eggs (Antonov et al. 2006), or puncture and remove even hard-shelled model eggs without any substantial costs (Honza and Moskát 2008). These results taken together strongly suggest that the “forced acceptance” of model eggs by parrotbills was simply due to their inability to remove such eggs (even though they were pecked, i.e. we observed pecking marks on the surface of the eggs).
Both parrotbills (12.0 %, n = 25) and warblers (11.1 %, n = 18) showed a low rejection rate of conspecific eggs which did not differ between the two hosts (Fisher Exact Test: p = 1.00). The rejection by both species was by both ejection and desertion.
Cuckoo chick cross-fostering experiments
There were no significant differences in the fledging success of cuckoo chicks among the three treatment groups (Table 2; χ2 = 2.57, df = 2, p = 0.28). The parrotbill hosts did not discriminate against cuckoo chicks—there were no differences in the growth parameters of the cuckoo chicks raised in the original warbler nests, cross-fostered to different warbler nests and cross-fostered to parrotbill nests (Fig. 5; Fig. S4; Table 3). Furthermore, there was no significant variation in the fledging age (warbler: 18.3 ± 0.3 days, parrotbill: 17.5 ± 0.5 days; t = −0.95, df = 8, p = 0.37), fledging body mass (warbler: 58.7 ± 0.9 g, parrotbill: 56.4 ± 2.6 g; t = −1.04, df = 8, p = 0.33) and fledging tarsus length (warbler: 25.4 ± 0.3 mm, parrotbill: 25.0 ± 0.9 mm; t = −0.51, df = 8, p = 0.63) of cuckoo chicks fostered by the two hosts.
The growth (body mass, g) of common cuckoo chicks raised by two hosts: a the filled circles and black solid line represent cuckoo chicks in the original (i.e., naturally parasitized) warbler nests (n = 15); the hollow circles and grey solid line represent cuckoo chicks cross-fostered between warbler nests (n = 5); b the triangle and the dotted line represent cuckoo chicks cross-fostered from warbler to parrotbill nests (n = 6)
Discussion
Despite the very homogeneous habitat, cuckoos in the Yellow River Delta parasitized warblers at much higher rates than parrotbills, and cuckoo eggs showed exquisite mimicry to warbler eggs, but not to parrotbill eggs. Hence, cuckoo parasitism was highly host-specific among these two sympatric reed bed breeding passerines, in line with the host preference hypothesis (Moksnes and Røskaft 1995; Skjelseth et al. 2004). This finding is intriguing, since our cross-fostering experiments showed that both hosts are equally good at raising cuckoo chicks.
Furthermore, egg recognition experiments disclosed that there were no significant differences in the egg recognition ability by warblers and parrotbills, as both show a high rejection rate of non-mimetic eggs. The highly developed ability to reject foreign eggs suggests that both warblers and parrotbills have been utilized by cuckoos in the past. However, cuckoos have only evolved mimetic eggs towards warbler eggs.
The question then is why a cuckoo gens specializing on warblers has evolved, while at the same time there seems to be no gens utilizing parrotbills. Obviously, the fact that the parrotbill show high rejection rate of natural “warbler” cuckoo eggs appears to be an important factor in explaining the apparent low parasitism rate on parrotbills in our study area and elsewhere (Yang et al. 2014). The rejection of cuckoo eggs by this species has most likely evolved due to past parasitism by cuckoos and was not explained as a “collateral damage” (Samas et al. 2014) from adaptations against conspecific parasitism because (1) parrotbills rejected conspecific eggs rarely and at much lower rates than cuckoo eggs, and (2) we detected no cases of conspecific parasitism. However, cuckoos have not responded by evolving a mimetic egg. Hence, parrotbills are now difficult for cuckoos to successfully parasitize since their eggs are so different from the parrotbill eggs, even though we cannot rule out that they still are hosts in other parts of their breeding range. Could there be other characteristics of parrotbills that make them less likely to be favoured by cuckoos?
There were no significant differences in the general habitat patterns of the reed bed between the nest patches of the two hosts. In fact, the parrotbill territories often overlapped with the warbler territories. However, parrotbills preferred to nest in patches of reeds that were lower in height and with a greater percentage of dry reed stems than warblers (see also Li et al. 2015b). Hence, we cannot rule out host selection based on small scale differences between parrotbill and warbler nest and nest site characteristics (Moskát and Honza 2000; Antonov et al. 2007, i.e. the habitat imprinting hypothesis: Teuschl et al. 1998).
Host behaviour can also be influential for explaining variation in cuckoo parasitism (Gill et al. 1997; Davies 2000). The dummy experiments disclosed that warblers, but not parrotbills responded aggressively to cuckoos. Further, parrotbills, in contrast to warblers, did not recognize cuckoos as a specific threat. The response of warblers to the intruders was consistent with previous work on closely related great reed warblers Acrocephalus arundinaceus in Europe (Honza et al. 2006; Trnka and Grim 2013). Since warblers are noisy, aggressive and large birds, they are easy to detect. Parrotbills on the other hand are smaller and less conspicuous, and since they do not behave aggressively towards cuckoos, they are more difficult to detect (i.e., lower opportunity for eavesdropping on these hosts by cuckoos).
Life-history features and adaptations by the hosts may contribute to host-specific parasitism (Antonov et al. 2010; Grim et al. 2011; Møller et al. 2011; Grim et al. 2014). The parrotbill is a resident of the area and starts to breed much earlier than the warbler and cuckoo, both of which are migratory, summer visitors. In fact, over half (52.7 %) the nests of parrotbills complete egg-laying before the cuckoo breeding season begins, thereby avoiding being parasitized by cuckoos, a fact that may likely contribute to the lower parasitism rate in reed parrotbills and lower selection on specific anti-cuckoo adaptations in this host (for similar cases see Peer and Bollinger 1997; Gill 1998). However, in other areas, many resident birds are common hosts for cuckoos even though their breeding period is poorly synchronized with that of the cuckoo (Kim 1996; Medina and Langmore 2016). Furthermore, although half of the nests of parrotbills in our study were available for parasitism, they still experienced a significantly lower parasitism rate than synchronously breeding warblers. Therefore, our results suggest that being a resident and having a partially separated breeding season cannot solely explain the lower parasitism rate on parrotbills by cuckoos. Still, we cannot rule out that parrotbills have adopted an earlier initiation of breeding in order to decrease the risk of parasitism by cuckoos.
Warblers had a higher local density and aggregated breeding than parrotbills, which may make them more available and suitable as cuckoo hosts than parrotbills (Stokke et al. 2007; Soler et al. 2009; Jelínek et al. 2014). Hence, significantly less search effort is most likely required from cuckoos utilizing warblers than parrotbills. However, the apparent low parasitism rate of the parrotbills cannot be completely explained by differences in density, because it appears that the density of warblers and parrotbills were nearly similar in some areas (e.g. Area A, Fig S1), but still parasitism rates were strikingly different.
The mismatch in breeding synchrony between cuckoos and parrotbills (Møller et al. 2011) and the differences in aggression and nest/nest site characteristics may boost a possible “density” effect and lead to a low availability of parrotbills for parasitism. Hence, it seems likely that not only one but rather several mechanisms may additively and interactively render parrotbills less suitable to cuckoos than warblers. It is important to acknowledge that our study is both restricted in time and space, making inference about the past and other sites impossible. Egg rejection in parrotbills may have evolved due to high parasitism in the past, perhaps in areas or times with higher density and a better breeding synchrony with cuckoos. Alternatively, rejection behaviour may be an ancestral trait that has been retained in the absence of parasitism even through evolutionary events on the species level (e.g. Rothstein 2001; Peer and Sealy 2004; but see Grim and Stokke 2016), or have evolved due to other reasons than brood parasitism (see Stokke et al. 2016 for a discussion of such events).
Conclusion
The host-specific parasitism in which the common cuckoo favoured Oriental reed warbler over reed parrotbill may be attributed to factors associated with both sides of the brood parasitism system (i.e., the initial selection by the cuckoo and constraints imposed by the hosts). In this study, we used a comprehensive approach to test the host responses to cuckoo parasitism at all developmental stages (e.g., adults, eggs, nestlings) and examined the influence of life-history traits that are not directly involved in parasite-host arms-races but still may affect their outcome. We showed that the main constraint preventing cuckoos from using parrotbills as hosts was the high frequency of egg rejection of non-mimetic cuckoo eggs. During the egg-laying stage specifically, the egg rejection rate reached ~90 %, which may be sufficient to make this host effectively secondarily unsuitable (see Grim et al. 2011) for cuckoos. The egg rejection was the only successful anti-parasitism strategy as there were no effective adaptations against cuckoo adults and chicks to counteract cuckoo parasitism in parrotbills. However, we did find an obvious partial separation in the breeding time between the cuckoo and the parrotbill, which would decrease the overall parasitism rate at the population level independently of other factors. Whether parrotbills have adopted an earlier breeding time in order to decrease the possibility of parasitism by cuckoos (Møller et al. 2011) is impossible to test directly but cannot be excluded. We suggest that future studies of host selection by parasitic birds will benefit from a comprehensive approach we used here, i.e. combination of observations and experiments across all developmental stages of parasite-host interactions.
References
Antonov A, Stokke BG, Moksnes A, Kleven O, Honza M, Røskaft E (2006) Eggshell strength of an obligate brood parasite: a test of the puncture resistance hypothesis. Behav Ecol Sociobiol 60:11–18
Antonov A, Stokke BG, Moksnes A, Røskaft E (2007) Factors influencing the risk of common cuckoo Cuculus canorus parasitism on marsh warblers Acrocephalus palustris. J Avian Biol 38:390–393
Antonov A, Stokke BG, Ranke PS, Fossøy F, Moksnes A, Røskaft E (2010) Absence of egg discrimination in a suitable cuckoo Cuculus canorus host breeding away from trees. J Avian Biol 41:501–504
Campobello D, Sealy SG (2010) Enemy recognition of reed warblers (Acrocephalus scirpaceus): threats and reproductive value act independently in nest defence modulation. Ethology 116:498–508
Cherry MI, Bennett TD (2001) Egg colour matching in an African cuckoo, as revealed by ultraviolet-visible reflectance spectrophotometry. Proc R Soc Lond B Biol Sci 268:565–571
Cuthill IC, Bennett ATD, Partridge JC, Maier EH (1999) Plumage reflectance and the objective assessment of avian sexual dichromatism. Am Nat 160:183–200
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & A. D Poyser, London
Davies NB, Brooke MdeL (1989) An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 58:207–224
Edvardsen E, Moksnes A, Røskaft E, Øien IJ, Honza M (2001) Egg mimicry in cuckoos parasitizing four sympatric species of Acrocephalus warblers. Condor 103:829–837
Endler JA, Thery M (1996) Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am Nat 148:421–452
Fossøy F, Antonov A, Moksnes A, Røskaft E, Vikan JR, Møller AP, Shykoff JA, Stokke BG (2011) Genetic differentiation among sympatric cuckoo host races: males matter. Proc R Soc Lond B Biol Sci 278:1639–1645
Fox GA, Negrete-Yankelevich S, Sosa VJ (2015) Ecological statistics: contemporary theory and application. Oxford University Press, Oxford
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Gibbs HL, Sorenson MD, Marchetti K, Brooke MdeL, Davies NB, Nakamura H (2000) Genetic evidence for female host-specific races of the common cuckoo. Nature 407:183–186
Gill BJ (1998) Behavior and ecology of the shining cuckoo Chrysococcyx lucidus. In: Rothstein SI, Robinson SK (eds) Parasitic birds and their hosts: studies in coevolution. Oxford University Press, Oxford, pp 143–151
Gill SA, Grieef PM, Staib LM, Sealy SG (1997) Does nest defence deter or facilitate cowbird parasitism? A test of the nesting-cue hypothesis. Ethology 103:56–71
Grim T (2006a) The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol Ecol Res 8:785–802
Grim T (2006b) Cuckoo growth performance in parasitized and unused hosts: not only host size matters. Behav Ecol Sociobiol 60:716–723
Grim T (2007) Experimental evidence for chick discrimination without recognition in a brood parasite host. Proc R Soc Lond B Biol Sci 274:373–381
Grim T, Stokke BG (2016) In the light of introduction: importance of introduced populations for the study of brood parasite-host coevolution. In: Weis JS, Sol D (eds) Biological invasions and animal behaviour. Cambridge University Press, Cambridge, pp 133–157
Grim T, Rutila J, Cassey P, Hauber ME (2009) Experimentally constrained virulence is costly for common cuckoo chicks. Ethology 115:14–22
Grim T, Samaš P, Moskát C, Kleven O, Honza M, Moksnes A, Røskaft E, Stokke BG (2011) Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts? J Anim Ecol 80:508–518
Grim T, Samaš P, Procházka P, Rutila J (2014) Are tits really unsuitable hosts for the common cuckoo? Ornis Fennica 91:166–177
Honza M, Moskát C (2008) Egg rejection behaviour in the great reed warbler (Acrocephalus arundinaceus): the effect of egg type. J Ethol 26:389–395
Honza M, Šicha V, Procházka P, Ležalová R (2006) Host nest defense against a color-dimorphic brood parasite: great reed warblers (Acrocephalus arundinaceus) versus common cuckoos (Cuculus canorus). J Ornithol 147:629–637
Jelínek V, Procházka P, Požgayová M, Honza M (2014) Common cuckoos Cuculus canorus change their nest-searching strategy according to the number of available host nests. Ibis 156:189–197
Kim CH (1996) Behavioral characteristics between the parasite and host: crow tits Paradoxonis webbiana and common cuckoo Cuculus canorus. Korean J Ornithol 3:831–839
Li D, Sun X, Lloyd H, Que P, Liu Y, Wan D, Zhang Z (2015a) Reed parrotbill nest predation by tidal mudflat crabs: evidence for an ecological trap? Ecosphere 6:art20
Li D, Wei H, Sun X, Zhang Z (2015b) Nest-site selection of reed parrotbills in the patchy reed habitats at post-harvest. Acta Ecol Sin 35:5009–5017
Liang W, Møller AP, Stokke BG, Yang C, Kovařík P, Wang H, Yao CT, Ding P, Lu X, Moksnes A, Røskaft E, Grim T (2016) Geographic variation in egg ejection rate by great tits across 2 continents. Behav Ecol. doi:10.1093/beheco/arw061
Lin J, Li Y, Zhang E, Wu XS (2008) Reed parrotbill. China Nat 2:22–25
Medina I, Langmore NE (2016) Batten down the thatches: front-line defences in an apparently defenceless cuckoo host. Anim Behav 112:195–201
Mermoz ME, Fernández GJ (1999) Low frequency of shiny cowbird parasitism on scarlet-headed blackbirds: anti-parasite adaptations or nonspecific host life-history traits? J Avian Biol 30:15–22
Moksnes A, Røskaft E (1992) Responses of some rare cuckoo hosts to mimetic model cuckoo eggs and to foreign conspecific eggs. Ornis Scand 23:17–23
Moksnes A, Røskaft E (1995) Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J Zool 236:625–648
Moksnes A, Røskaft E, Braa AT (1991a) Rejection behavior by common cuckoo hosts towards artificial brood parasite eggs. Auk 108:348–354
Moksnes A, Røskaft E, Braa AT, Korsnes L, Lampe HM, Pedersen HC (1991b) Behavioural responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour 116:64–89
Moksnes A, Røskaft E, Hagen L, Honza M, Mørk C, Olsen P (2000) Common cuckoo Cuculus canorus and host behaviour at reed warbler Acrocephalus scirpaceus nests. Ibis 142:247–258
Møller AP, Antonov A, Stokke BG, Fossøy F, Moksnes A, Røskaft E, Takasu F (2011) Isolation by time and habitat and coexistence of distinct host races of the common cuckoo. J Evol Biol 24:676–684
Moskát C, Honza M (2000) Effect of nest and nest site characteristics on the risk of cuckoo Cuculus canorus parasitism in the great reed warbler Acrocephalus arundinaceus. Ecography 23:335–341
Moskát C, Honza M (2002) European cuckoo Cuculus canorus parasitism and host’s rejection behaviour in a heavily parasitized great reed warbler Acrocephalus arundinaceus population. Ibis 144:614–622
Ortega CP, Cruz A (1991) A comparative study of cowbird parasitism in yellow-headed blackbirds and red-winged blackbirds. Auk 108:16–24
Peer BD, Bollinger EK (1997) Explanations for the infrequent cowbird parasitism on common grackles. Condor 99:151–161
Peer BD, Sealy SG (2004) Correlates of egg rejection in hosts of the brown-headed cowbird. Condor 106:580–599
Røskaft E, Moksnes A, Stokke BG, Bicík V, Moskát C (2002) Aggression to dummy cuckoos by potential European cuckoo hosts. Behaviour 139:613–628
Rothstein SI (2001) Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 61:95–107
Samas P, Hauber ME, Cassey P, Grim T (2014) Host responses to interspecific brood parasitism: a by-product of adaptations to conspecific parasitism? Front Zool 11:34
Samaš P, Grim T, Hauber ME, Cassey P, Weidinger K, Evans KL (2013) Ecological predictors of reduced avian reproductive investment in the southern hemisphere. Ecography 36:809–818
Sealy SG, Neudorf DI, Hobson KA, Gill SA (1998) Nest defense by potential hosts of the brown-headed cowbird. In: Rothstein SI, Robinson SK (eds) Parasitic birds and their hosts: studies in coevolution. Oxford University Press, New York, pp 194–211
Skjelseth S, Moksnes A, Røskaft E, Lisle Gibbs H, Taborsky M, Taborsky B, Honza M, Kleven O (2004) Parentage and host preference in the common cuckoo Cuculus canorus. J Avian Biol 35:21–24
Soler JJ, Møller AP, Soler M (1999) A comparative study of host selection in the European cuckoo Cuculus canorus. Oecologia 118:265–276
Soler J, Vivaldi M, Møller A (2009) Geographic distribution of suitable hosts explains the evolution of specialized gentes in the European cuckoo Cuculus canorus. BMC Evol Biol 9:88
Starck JM, Ricklefs RE (1998) Avian growth and development: evolution within the altricial-precocial spectrum. Oxford University Press, New York
Stokke BG, Hafstad I, Rudolfsen G, Bargain B, Beier J, Campàs DB, Dyrcz A, Honza M, Leisler B, Pap PL, Patapavičius R, Procházka P, Schulze-Hagen K, Thomas R, Moksnes A, Møller AP, Røskaft E, Soler M (2007) Host density predicts presence of cuckoo parasitism in reed warblers. Oikos 116:913–922
Stokke BG, Røskaft E, Moksnes A, Møller AP, Antonov A, Fossøy F, Liang W, López-Iborra G, Moskát C, Shykoff JA, Soler M, Vikan JR, Yang C, Takasu F (2016) Disappearance of eggs from nonparasitized nests of brood parasite hosts: the evolutionary equilibrium hypothesis revisited. Biol J Linn Soc 118:215–225
Teuschl Y, Taborsky B, Taborsky M (1998) How do cuckoos find their hosts? The role of habitat imprinting. Anim Behav 56:1425–1433
Trnka A, Grim T (2013) Color plumage polymorphism and predator mimicry in brood parasites. Front Zool 10:597–600
Trnka A, Prokop P, Grim T (2012) Uncovering dangerous cheats: how do avian hosts recognize adult brood parasites? PLoS One 7:e37445
Xiong L, Lu J (2013) Exploitation of reed beds by specialist passerines: reed parrotbill and Oriental reed warbler. Wilson J Ornithol 125:165–173
Yang C, Liang W, Antonov A, Cai Y, Stokke BG, Fossøy F, Moksnes A, Røskaft E (2012) Diversity of parasitic cuckoos and their hosts in China. Chin Birds 3:9–32
Yang C, Stokke BG, Antonov A, Cai Y, Shi S, Moksnes A, Røskaft E, Møller AP, Liang W, Grim T (2013) Host selection in parasitic birds: are open-cup nesting insectivorous passerines always suitable cuckoo hosts? J Avian Biol 44:216–220
Yang C, Li D, Wang L, Liang G, Zhang Z, Liang W (2014) Geographic variation in parasitism rates of two sympatric cuckoo hosts in China. Zool Res 35:67–71
Acknowledgments
We are grateful to John A. Endler, Sara Helms Cahan and two anonymous referees for constructive comments that significantly improved the manuscript. We thank the Yellow River Delta Management Bureau for permission to undertake this study including all experimental procedures. The experiments comply with the current laws of China. We are particularly grateful to two French volunteers (Andrieu Julie and Marion Soresina) for their help with data collection in the field; Yueliang Liu, Shuyu Zhu, Kai San, Xianghai Du, Shuyi Zhang, and Pengfei Guo for their kind support, and Baoshan Cui for sharing the laboratory in the field station. We would also like to thank Chao Li (Gudong Oil Production Plant, Sinopec Shengli Oilfield), Junlin Chen (Anhui University), Xianxian Liu, Xiaomei Shen, and Qiao Wu for their kind help during this study. This work was supported by the National Natural Science Foundation of China (Nos. 31301888 to DL; 31272328 and 31472013 to WL), Open Fund of Ministry of Education Key Laboratory for Biodiversity Sciences and Ecological Engineering, Beijing Normal University (K1401 to DL), General scientific research project of Education Department of Liaoning Province (L2015196 to DL), National Basic Research Program of China (2006CB403305 to ZZ) and United Foundation for Natural Science of National Natural Science Foundation of China and People’s Government of Guangdong Province (U0833005 to ZZ). BGS was funded by the Research Council of Norway (218144). TG acknowledges the support from Human Frontier Science Program (awards RGY69/2007 and RGY83/2012) and the Czech Science Foundation (Grant No. P506/12/2404). We declare that all authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, D., Zhang, Z., Grim, T. et al. Explaining variation in brood parasitism rates between potential host species with similar habitat requirements. Evol Ecol 30, 905–923 (2016). https://doi.org/10.1007/s10682-016-9850-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-016-9850-7