Abstract
Eggs of several brood parasites have thicker and stronger shells than expected for their size. The present study evaluated the puncture resistance hypothesis for the occurrence of thick-shelled eggs in common cuckoos Cuculus canorus by investigating costs of cuckoo egg ejection in four Acrocephalus warblers—the great reed warbler A. arundinaceus, reed warbler A. scirpaceus, marsh warbler A. palustris and sedge warbler A. schoenobaenus. The three latter species all suffered ejection costs, while ejection was not costly in the larger great reed warbler. The occurrence of ejection costs was negatively related to host bill size. In the marsh warbler, we compared ejection costs in naturally parasitized nests and two experimental treatments, in which broods were parasitized artificially with great reed warbler and conspecific eggs. Hosts damaged their own eggs significantly more often when ejecting the thick-shelled cuckoo eggs than when ejecting the similarly sized but thinner-shelled great reed warbler eggs, providing some support for the puncture resistance hypothesis. Ejection of conspecific eggs did not involve any costs. Furthermore, contrary to predictions derived from the laying damage hypothesis, there was no evidence that egg damage was associated with cuckoo egg laying. Hosts damaging their own eggs during ejection were more likely to subsequently desert their clutches than those that did not. The frequency of clutches smeared with the contents of the ejected egg were positively related to the hypothesized difficulty of foreign egg puncturing. Potential advantages of thicker shells in common cuckoo eggs are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One peculiar trait of many brood-parasitic cuckoos and cowbirds is that their eggs are unusually thick-shelled and more resistant to breakage than expected for their size (e.g. Lack 1968; Rahn et al. 1988; Picman 1989; Brooker and Brooker 1991). Two main hypotheses have been proposed to explain this phenomenon. The laying damage hypothesis (Lack 1968) states that the greater structural strength of the parasitic egg represents an adaptation to protect it from damage during laying. Evidence that some cuckoos parasitize cavity-nesting hosts or hosts with domed nests where they cannot enter to lay provides some indirect support for this hypothesis (Wyllie 1981). Spaw and Rohwer (1987) tentatively rejected the laying damage hypothesis for the thick shells of cowbird eggs and proposed the puncture resistance hypothesis, which may also apply to cuckoos. Swynnerton (1918) was the first to suggest that the thick eggshells of the common cuckoo (hereafter cuckoo) Cuculus canorus egg may serve as a protection against puncturing attempts by hosts. The puncture resistance hypothesis states that brood parasites evolved thicker shells to resist puncture attempts by hosts and thereby avoid rejection. The most cost-efficient mode of rejection is ejection, i.e. selective removal of the parasitic egg from the nest (Rothstein 1975). However, many small host species are not able to grasp the parasitic egg in their mandibles due to their small bill size. Such hosts have first to puncture the egg in order to carry it in the bill and remove it from the nest (Rohwer and Spaw 1988; Moksnes et al. 1991). Puncture ejection may be costly because thick-shelled eggs often need considerable pecking effort to be punctured (Martin-Vivaldi et al. 2002), and there is a risk of accidental breakage or soiling of host eggs during the process, i.e. ejection costs. The puncture resistance hypothesis generates two main predictions: (1) ejection of the thick-shelled parasitic egg would be costly and (2) costs of ejection of the thick-shelled parasitic egg will be higher than for a similarly sized egg having normal eggshell thickness.
The existence of ejection costs has been well documented in a number of hosts of brown-headed cowbirds Molothrus ater (Rothstein 1975, 1976; Rohwer and Spaw 1988; Rohwer et al. 1989; Weatherhead 1991; Lorenzana and Sealy 2001) and cuckoos (Davies and Brooke 1988, 1989; Moksnes et al. 1991, 1994; Moskát et al. 2002; Stokke et al. 2002), which seems to provide support to the first prediction in both groups of brood parasites. However, most studies of rejection behaviour in hosts of the cuckoo involved the use of hard egg models, which are impossible for the hosts to puncture (e.g. Davies and Brooke 1989; Moksnes et al. 1991; Lindholm 2000, but see e.g. Procházka and Honza 2003, 2004; Honza et al. 2004 for the use of real eggs). Martin-Vivaldi et al. (2002) showed that the use of such egg models may lead to substantial overestimation of the frequency and magnitude of rejection costs. The results of their study together with the scarcity of data on costs associated with rejection of real cuckoo eggs made them suggest that there may be a misleading impression that rejection costs are common among cuckoo hosts. On the other hand, Martin-Vivaldi et al. (2002) compared costs suffered by potential host species when rejecting hard egg models to costs when rejecting real house sparrow Passer domesticus eggs. However, as Martin-Vivaldi et al. (2002) found, house sparrow eggs are significantly less resistant to puncturing than cuckoo eggs, which raises the possibility that their results, as well, may be biased towards an underestimation of the real costs that hosts suffer when confronted with real cuckoo eggs. Thus, at present there is a general lack of data to support the first prediction of the puncture resistance hypothesis for the eggs of the cuckoo.
The second prediction of the puncture resistance hypothesis was supported for at least one puncture ejector host of the brown-headed cowbird (Rohwer et al. 1989). In that study, northern orioles Icterus galbula ejecting real brown-headed cowbird eggs suffered significantly higher costs than when ejecting the thinner-shelled cliff swallow Hirundo pyrrhonota eggs. However, as far as we know, there are no comparable tests for any of the cuckoo hosts.
The aim of the present study was to evaluate the puncture resistance hypothesis for the greater structural strength of the cuckoo egg. To this end, we collected data on naturally parasitized nests of four species of Acrocephalus warblers in order to investigate if ejection of cuckoo eggs is costly to the host. Three of these species (great reed warbler A. arundinaceus, reed warbler A. scirpaceus and marsh warbler A. palustris) are among the most frequently parasitized hosts of the cuckoo in Europe, while the sedge warbler A. schoenobaenus is an occasional host (Wyllie 1981; Schulze-Hagen 1992; Moksnes and Røskaft 1995). Furthermore, by using observational and experimental data on one of these species, the marsh warbler, we tested the second prediction of the puncture resistance hypothesis, i.e. that ejection leads to more damage when the egg being removed is thick-shelled. We chose the marsh warbler because it is a small host and is known to reject cuckoo eggs mostly by puncture ejection (Gärtner 1981), making it a suitable study species for testing the occurrence of ejection costs. To this end, we monitored ejection costs in three groups of nests: (1) naturally parasitized by the cuckoo; 2) experimentally parasitized with great reed warbler eggs, which are known to be similar in size to cuckoo eggs but thinner-shelled and less resistant to puncture (Honza et al. 2001); (3) experimentally parasitized with foreign conspecific eggs. It was expected that marsh warblers would experience greater difficulties in ejecting the foreign egg as eggshell thickness and egg size increased. More specifically, we predicted (1) that marsh warblers ejecting cuckoo eggs would suffer significantly more rejection costs than those ejecting great reed warbler eggs and (2) ejection of foreign conspecific eggs would be less costly than ejection of great reed warbler eggs.
Methods
Bill size
Measurements of bill size were carried out at the Naturhistoriches Museum in Vienna, Austria in 1998. Mandibles of ten randomly selected specimens of each of the four Acrocephalus warblers were measured, and the mean length and width were used when calculating grasp index (see Rohwer and Spaw 1988; Moksnes et al. 1991). This procedure was justified by the fact that there are no significant sex differences in bill size in these species (Cramp 1992).
Study areas and natural parasitism
The occurrence of ejection costs when rejecting cuckoo eggs were studied in naturally parasitized nests of four Acrocephalus warblers—the great reed warbler, reed warbler, marsh warbler and sedge warbler in south-eastern Czech Republic during 1993–1998 (see e.g. Stokke et al. 1999 for a description of the study area) and for marsh warblers in north-western Bulgaria during 2002–2004. The study area in Bulgaria was situated between the villages Zlatia (43°46’N 23°30’E), Ignatovo (43°46’N 23°28’E) and Dolni Tsibar (43°48’N 23°31’E). The majority of nests were monitored from the nest-building stage and onwards, allowing us to know the nest history in detail both before and after cuckoo parasitism (see e.g. Moksnes et al. 1993a; Stokke et al. 1999).
Experimental procedure
In addition to monitoring ejection costs in naturally parasitized marsh warbler nests, we also carried out experiments with foreign eggs in our study area in Bulgaria. These experiments were performed in 2004.
Each experimental marsh warbler nest was artificially parasitized at the end of laying (four or five eggs laid), and only nests found during the nest-building or laying stage were used in this study. One randomly chosen host egg was taken in exchange for the foreign experimental egg to mimic the natural behaviour of the cuckoo (Wyllie 1981). Before introduction of the experimental egg, all host eggs were carefully examined for any kind of damage. No such damage was recorded. Two types of experimental eggs were used: (1) great reed warbler eggs taken from nests in the same general area, which had been used in other experiments involving egg exchange or had been deserted; (2) conspecific eggs—real marsh warbler eggs taken from other experimental nests in the study area. Each nest was subject to only one of these experimental egg-types.
Great reed warbler eggs are similar in size to cuckoo eggs (Honza et al. 2001; Antonov et al. in press). However, cuckoo eggs are known to be thicker-shelled and ≈2.2 times stronger against outside pressure than the eggs of the great reed warbler (Table 1). Foreign marsh warbler eggs used in the present study (1.76±0.12 ml, N=14, see Hoyt (1979) for calculation) were about 60% smaller than either cuckoo or great reed warbler eggs, and their eggshell thickness is known to be much less than in cuckoo or great reed warbler eggs (Table 1). As eggshell thickness and strength are positively related to egg size (Ar et al. 1979), it was predicted that conspecific eggs would be easiest to puncture eject or grasp eject.
Cuckoo eggs in our Bulgarian study area were a significantly better match of marsh warbler eggs than were great reed warbler eggs (Antonov et al. in press). There is thus a theoretical possibility that, in naturally parasitized nests, egg mimicry might have affected marsh warbler ejection behaviour. A mimetic egg may confuse the host, leading to accidental pecking of some of its own eggs, and in this way, a positive relationship between mimicry and ejection costs would be expected. Thus, higher costs may reflect both the thicker shell of the cuckoo egg as well as recognition difficulties. In order to investigate if this might affect our results, we compared the degree of mimicry of naturally parasitized marsh warbler clutches that suffered costs with those that did not.
Most naturally parasitized marsh warbler clutches in Bulgaria and Czech Republic were photographed on a Kodak Grey Card together with a Kodak Color Card in a standardized manner with a Canon EOS camera and macro lens using Kodak 100 or 200 ASA film for subsequent scoring of egg mimicry. The mimicry was assessed on a scale of one (perfect mimicry) to five (no mimicry) (Moksnes et al. 1993a). The mean of the assessments of three test persons was used as the measure of egg mimicry. This was justified by the high degree of concordance of the scores (repeatability=0.73, F=9.00, df=36, 110, P<0.001; Lessells and Boag 1987).
It is known that cuckoos sometimes partially depredate host clutches without parasitizing them (Wyllie 1981; Moksnes et al. 2000). If partial predation events are frequent, this could in theory lead to overestimation of the true costs of ejection if there are many cuckoo females in an area and all the missing eggs associated with ejection of the cuckoo eggs are interpreted as ejection costs. To control for such a possible bias, we designated 17 unparasitized marsh warbler nests in our Bulgarian study area as controls. These nests were visited for the same duration of time as experimental and naturally parasitized ones and were examined in the same way for missing or damaged eggs. Both parasitized and unparasitized nests were situated within the same general area, and if partial predation by cuckoos was common, it should have occurred at both parasitized and unparasitized nests. Regarding the Czech data, we do not possess data of egg disappearance from unparasitized nests on a daily basis, but regarding reed warblers and great reed warblers, the total egg loss in the period from start of egg laying until the sixth day of incubation have previously been published elsewhere (Røskaft et al. 2002). We discuss the results from that study with our results in the “Discussion” section.
General procedure
Each nest was visited daily after experimental egg introduction or natural parasitism for at least 6 days to examine if there were any signs of rejection. At each nest check, the foreign egg as well as host eggs were carefully inspected for damage, i.e. holes, slits, cracks and/or remnants of egg contents (see Moksnes et al. 1994), or missing eggs, i.e. evidence of ejection. Only nests in which the foreign egg was rejected by ejection were considered here as we were interested in the influence of the egg strength on the chance of incurring ejection costs. Ejection was a representative rejection response in the marsh warbler as it accounted for about 66% of all rejections of cuckoo eggs (Antonov et al. in press). Ejections were classified as “without costs” or “with costs”. An ejection was considered to be without costs when the foreign egg was removed from the nest and all host eggs remained unharmed. Cases where the foreign egg was either removed or left in the nest and at least one host egg was damaged, e.g. had puncture holes or cracks, or had disappeared were classified as ejections with costs, regardless of whether or not the nest was later deserted.
Statistical tests were performed in SPSS 11.0 and STATISTICA 6.1. Proportions were analysed using generalized linear models (GLMs) with binomial error distribution and logit link. In cases where the frequency for one of the categories was zero, we used Fisher’s exact test. All tests were two-tailed.
Results
Ejection costs in naturally parasitized nests
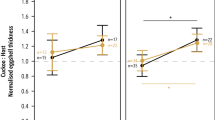
Ejection of the cuckoo egg was associated with costs in the three Acrocephalus warblers with the smallest bills (Table 2). In the marsh warbler, where data from two areas were available, no between-area differences in the occurrence of costs were detected (GLM; χ 2=0.8, df=1, P=0.36). Ejection costs were observed in 44.4% of cuckoo egg ejection events in the three smaller species. On the other hand, the great reed warbler, which has a much larger bill, ejected cuckoo eggs without any damage to its own eggs. The frequency of ejection costs, measured both as the mean number of host eggs lost and the frequency of nests with costs, was negatively related to the respective host species grasp indices (Table 2, r sp=−1.00, N=4).
Egg loss (either damage or disappearance) was not recorded at any of the 17 unparasitized marsh warbler control nests in Bulgaria. Both in Czech Republic and in Bulgaria, no naturally parasitized nests contained damaged host eggs when first discovered with a cuckoo egg. All cases of damaged eggs were recorded on subsequent visits after natural or experimental parasitism. The disappearance of host eggs, together with acceptance of the foreign egg, was not recorded in any nest. All egg damages were in the form of small puncture holes or slits. In four cases of marsh warbler egg ejection (three cuckoo eggs and one great reed warbler egg), the foreign egg was found in the nest with a large puncture hole and almost empty of contents prior to actual ejection, indicating puncture ejection.
Costs for marsh warblers in ejecting three types of foreign eggs
The frequencies of clutches suffering ejection costs in the three experimental groups of marsh warblers are presented in Table 1. Marsh warblers ejecting cuckoo eggs were significantly more likely to damage own eggs than those ejecting great reed warbler eggs (GLM; χ 2=5.7, df=1, P=0.017) or conspecific eggs (Fisher’s exact test P=0.006). Only two pairs (9.1%) that ejected great reed warbler eggs damaged their own eggs during the process of ejection (in each case, one host egg was punctured). No host eggs were damaged when a foreign conspecific egg was ejected. Ejection of great reed warbler eggs did not lead to significantly higher costs than ejection of conspecific eggs (Fisher’s exact test P=0.51).
The occurrence of costs in marsh warblers did not differ between the Czech and Bulgarian study areas (see above), and therefore the two data sets were pooled to test the effect of mimicry on the chance of making ejection errors. No significant relationship was evident (Mann–Whitney test, U=124.5, N 1=23, N 2=14, P=0.26).
Altogether, 26.1% (N=23) of marsh warblers attempting to eject the cuckoo egg subsequently deserted their nests. The probability of desertion was significantly higher among pairs that suffered ejection costs as compared to those that did not suffer such costs (5/10 vs 1/13 respectively, GLM; χ 2=4.2, df=1, P=0.041). All the pairs that suffered costs and subsequently deserted did not in fact eject the cuckoo egg. Only one pair that successfully ejected the cuckoo egg without suffering costs later deserted their nest. No desertions following ejection were recorded in the two experimental groups.
In 69.2% (N=13) of the marsh warbler nests in which the cuckoo egg was ejected without costs, the host eggs were smeared with egg contents, suggesting that the parasitic egg was first punctured in the nest (see also above). The corresponding figures for ejection of great reed warbler and conspecific eggs were 35.0% (N=20) and 21.4% (N=14), respectively. The proportion of clutches with smeared eggs differed significantly among the three treatments (GLM; χ 2=6.2, df=2, P=0.046). There was a higher occurrence of smeared eggs following ejections of cuckoo eggs as compared to ejection of conspecific eggs (GLM; χ 2=5.7, df=1, P=0.017). Ejection of great reed warbler eggs did not differ significantly in the proportion of smeared clutches compared to the other treatments (Ps>0.06).
Discussion
Is ejection of cuckoo eggs costly?
By recording the frequency of damaged eggs associated with ejection in naturally parasitized nests in four cuckoo host species, we showed that the ejection process may be costly. Furthermore, no eggs disappeared from or were damaged in unparasitized nests of Bulgarian marsh warblers. Therefore, all the cases of own egg damage were observed after the cuckoo eggs were laid. From Czech Republic, we have data on the disappearance of eggs from unparasitized nests of reed warblers and great reed warblers for the period from egg laying until the sixth day of incubation (see Røskaft et al. 2002). In unparasitized Czech reed warbler nests, the average egg loss was 0.4 (N=290), which is less than 50% of the average egg loss when ejecting cuckoo eggs (Table 2). Furthermore, the corresponding data for great reed warblers was 0.4 (N=23), which in fact is higher than when ejecting cuckoo eggs (0%; Table 2). These data taken together indicate that egg loss from unparasitized nests in Czech Republic can be explained, in part, by partial predation. Therefore, the loss of own eggs when ejecting cuckoo eggs found in the present study can, in the main, be attributed to the host activity while trying to eject the parasitic egg.
Moksnes et al. (1991) demonstrated that the mode of rejection of cuckoo hosts depended on the host’s bill size and more specifically its grasp index (sensu Rohwer and Spaw 1988). Hosts having large bills are able to grasp the parasitic egg, while smaller-billed hosts are forced to puncture the foreign egg before grasping and removing it (Moksnes et al. 1994). In the present study, the three smaller Acrocephalus species suffered considerable costs when ejecting cuckoo eggs, while no such costs were recorded in the great reed warbler. Furthermore, the frequency of ejection costs was negatively related to differences in bill size (Table 2). Due to its larger size and high grasp index, the great reed warbler seems to be able to grasp eject the parasitic egg without damaging its own eggs as was established by experiments with artificial cuckoo-sized hard model eggs, which were first pecked but finally grasp ejected with negligible costs (unpublished data). Despite the small sample, our study suggests that ejection of real cuckoo eggs is not costly to great reed warblers. In line with this, Lotem et al. (1995) reported only two cases out of 57 ejections (3.5%) in which a host egg disappeared along with the cuckoo egg or cuckoo egg model. On the other hand, Moskát and Honza (2002) noted that as many as 31.6% (6/19) of Hungarian great reed warblers ejecting cuckoo eggs lost one or more of their own eggs. Since the cuckoo density in this Hungarian population is extremely high (Moskát and Honza 2002), the elevated occurrence of ejection costs could partly be due to partial predation events by cuckoos (see also Wyllie 1981; Moksnes et al. 2000). Alternatively, the differences in the costs of ejection between Czech and Hungarian great reed warblers could be due to a generally better cuckoo egg mimicry in the latter area (Edvardsen et al. 2001; Moskát and Honza 2002), making errors in rejection more likely.
The present study provides evidence that the use of eggs of medium-sized passerines in egg experiments (e.g. Martin-Vivaldi et al. 2002) may underestimate the ejection costs of real cuckoo eggs. If we had based our estimates of rejection costs on great reed warbler eggs, marsh warblers would have appeared to suffer few costs (9%), while in fact as many as 43.5% of them faced with a real cuckoo egg suffered such costs. Thus, the only proper way to document true ejection costs in a host species is by the use of real parasitic eggs. Further experiments may reveal that ejection costs are important in other small cuckoo hosts, too.
Does the eggshell strength of cuckoo eggs render ejection by the host costly?
This critical prediction of the puncture resistance hypothesis was tested by the experiments performed on the marsh warbler. Observations of punctured cuckoo eggs and the fact that as many as 82.6% of attempted ejections of such eggs involved either damage of host eggs and/or smearing with yolk (see also Moksnes et al. 1994) provide evidence that the marsh warbler is a puncture ejector, confirming the results by Gärtner (1981).
Marsh warblers were presented with three types of foreign eggs differing in shell thickness and strength and thus in how easily they could be punctured. They incurred considerable costs when ejecting real cuckoo eggs because one or more of their own eggs were damaged in 43.5% of the cases. In addition, 50% of the nests where ejection with costs occurred were subsequently deserted. This could be due to inability to eject the cuckoo egg or just a response to clutch reduction as a result of egg damage (see e.g. Rothstein 1976; Øien et al. 1998). In accordance with prediction (1), great reed warbler eggs, which are considerably less resistant to puncture (Honza et al. 2001), were ejected with significantly lower costs than cuckoo eggs. Given that great reed warbler eggs were a significantly poorer match of marsh warbler eggs compared to cuckoo eggs, the effect of the strong eggshells of the cuckoo egg may have been confounded by the effect of mimicry. Better mimetic eggs may be associated with elevated ejection costs due to recognition problems, i.e. a host may peck occasionally at some of its own eggs. However, the occurrence of ejection costs in marsh warblers ejecting cuckoo eggs was unrelated to mimicry, and thus, the difference in ejection costs were due to eggshell thickness rather than mimicry. Therefore, we conclude that the increased eggshell thickness and structural strength of cuckoo eggs lead to increased ejection costs.
Ejection of conspecific eggs involved no egg damage. Contrary to prediction (2), great reed warbler eggs were not more costly to eject than foreign conspecific eggs. One possible explanation is that there may be an egg strength threshold that allows puncturing with no or negligible costs. Thus, great reed warbler eggs may fall below that threshold for the marsh warbler and were accordingly associated with minimal costs. Due to the small size, some conspecific eggs may have been grasp ejected, potentially lowering the costs of ejection, although some of them were surely puncture ejected, as was revealed by the presence of content spillage.
The frequency of clutches smeared with spilled egg content after ejection seems related to difficulties in puncturing. The harder the foreign egg to puncture, the more pecking and moving of the egg are needed in order to eject it. Increased pecking effort should thus lead to increased probability of smearing the host’s own eggs with contents from the punctured egg. In line with this, the proportion of clutches with remnants of yolk was smallest when ejecting conspecific eggs, which were supposedly the easiest to remove. Successful ejection of the larger cuckoo and great reed warbler eggs more often resulted in smearing of the remaining host eggs. It is not clear if smearing with egg content has any detrimental effect on the host eggs. It could cause sticking of eggs together or to the nest lining, leading to difficulties in egg turning during incubation and subsequent fracture or hatching failure (Rothstein 1975). Spilling the contents on the other eggs may also make the nest more vulnerable to ant infestation (Clark and Robertson 1981). We did not follow the hatching pattern closely enough to see if such smearing leads to increased hatching failure.
Potential advantages for the cuckoo of laying a thick-shelled egg
The laying damage hypothesis (Lack 1968) proposes that the thick eggshells of parasitic cuckoos protect them during laying when they fall down into the host nest. If the cuckoo evolved greater eggshell thickness to reduce the risk of damage during laying, we should expect damages also on host eggs caused by the collision with the cuckoo egg at laying to be common (Gaston 1976; Spaw and Rohwer 1987; Soler 1990; Soler et al. 1997). However, in the present study, no dents or other features indicating such damage were noted on host eggs when clutches were first recorded parasitized. Damage of host eggs was always recorded as puncture holes or slits, one or more days after the cuckoo egg was first observed, indicating that they were not caused by parasite egg-laying. These results taken together provide stronger support for the puncture resistance hypothesis than for the laying damage hypothesis, but do not allow us to definitively reject the latter hypothesis for the evolution of thick eggshells in cuckoos.
Intuitively, the puncture resistance hypothesis seems most plausible for the eggs of cowbirds (Spaw and Rohwer 1987; Rohwer and Spaw 1988; Rohwer et al. 1989). As the parasitic chick does not evict host eggs or nestlings in these species, the high risk of egg damage during rejection of the parasitic egg may outweigh the benefits of rejection at least in most small-billed hosts and thus favour acceptance (Spaw and Rohwer 1987). As an alternative to the evolutionary lag hypothesis (Rothstein 1975), the fact that many cowbird hosts accept non-mimetic eggs may actually represent “forced” acceptances resulting from the costs of ejection (Rohwer and Spaw 1988). The same argument could be used for non-evicting cuckoos, but previous studies on Clamator cuckoos have found convincing support in favour of the laying damage hypothesis (Gaston 1976; Soler et al. 1997; Soler and Martínez 2000). In addition, the thick-shelled eggs of cowbirds and non-evicting cuckoos would protect first-laid parasitic eggs from breakage by conspecific parasites, either during predation or during laying (see Brooker and Brooker 1991).
In evictor cuckoos like the common cuckoo, ejection costs are often considered to be of little significance as a constraint of egg rejection because rejection should always be a more advantageous strategy than acceptance due to the high costs of parasitism (Moksnes et al. 1991). In such parasites, egg mimicry is considered to be of primary importance as an adaptation enhancing the probability of acceptance (Spaw and Rohwer 1987). Nevertheless, thicker and stronger eggshells evolved in the cuckoo (Honza et al. 2001), and we have shown that this does render the ejection process costly in some of its main European hosts. Due to the high costs of accepting the parasitic egg, it is reasonable to assume that all hosts that recognize the parasitic egg will try to reject it. Furthermore, the great majority of cuckoo hosts are not able to employ grasp ejection due to bill size constraints and would rather accomplish ejection by puncturing (Moksnes et al. 1991). Thicker-shelled eggs, however, entail considerable pecking effort on the part of the host (Martin-Vivaldi et al. 2002). It is likely that individuals within a host population would differ in their ability to successfully puncture the thick-shelled egg and/or in the motivation and hence “perseverance” to actually make a puncture hole [see Kramer and Lemon (1985) and Weary et al. (1988) for a discussion of the role of motivation in bird song output]. Lindholm (2000) observed that in many cases, reed warblers pecked at artificial impenetrable model eggs but left them in the nest without deserting, thus providing supportive evidence that the motivation to reject may vary among individuals that are aware of the foreign egg. Similarly, some marsh warblers in our Bulgarian study area showed intensive pecking at model cuckoo eggs but finally left them in the nest without deserting (A. Antonov, unpublished data; see also Martin-Vivaldi et al. 2002). Lindholm (2000) raised the suggestion that reed warblers may peck at real cuckoo eggs as well without rejecting them. Furthermore, several studies have found an increase in host rejection rate of cuckoo eggs when presented with a stimulus in addition to the parasitic egg (e.g. a cuckoo dummy; Davies and Brooke 1988; Moksnes et al. 1993b; Lindholm 2000). This elevation in rejection rate could be due to a higher host motivation for rejecting the cuckoo egg by stimulus summation, leading to more intense pecking followed by ejection or a lower threshold of desertion. As it is possible that hosts would peck at the foreign egg but would differ in the motivation and hence effort at pecking, the likely selective advantage of the thicker shell is clear. The following reasoning would support this statement. A total of 50% (N=70) of cuckoo eggs laid in nests of Bulgarian marsh warblers were rejected, while 92% (N=24) of experimentally added great reed warbler eggs were rejected (Antonov et al. in press). Much of this difference in rejection rate of cuckoo and great reed warbler eggs can be attributed to differences in egg matching, but interestingly, the mode of rejection also differed. All great reed warbler eggs were ejected, while 10 out of 35 cuckoo eggs were deserted, and furthermore, 6 out of the 23 pairs that ejected cuckoo eggs also later deserted their nest. Hypothetically, the 45 cuckoo eggs that were accepted or deserted might have been pecked but then left unharmed. Theoretically, pecking would be less intense and last for shorter times (lower motivation) when mimicry is good, and the eggshell thickness would be important in determining the outcome of the given pecking effort (i.e. for a specific level of mimicry). In addition, there may also be some selection pressure against abandonment of the current breeding attempt because in many single-brooded passerines, clutch size and breeding performance generally decrease with season (Clark and Robertson 1981) and also a new breeding attempt may eventually be exploited by the parasite. Thus, for the parasite, egg ejection is a worse scenario than nest desertion. Furthermore, increasing the costs of the breeding in the current season may lead to reduced future reproductive effort (Nager et al. 2001; Visser and Lessells 2001). In line with this, clutch size in our Bulgarian marsh warbler population decreased significantly during the course of the season, while the probability of parasitism did not (A. Antonov, unpublished data). Furthermore, probably due to a short breeding season, marsh warblers often do not produce replacement clutches (1.2 breeding attempts per female; Schulze-Hagen et al. 1996), suggesting that there may indeed be a selection against desertion.
Further studies involving experimental introduction of real cuckoo eggs and video-recording host responses under different stimulus exposure (e.g. sighting of cuckoo or not) are needed to show if “forced” acceptances really exist among cuckoo hosts. This would help clarify the advantages of the thick-shelled cuckoo egg in the light of the puncture resistance hypothesis.
References
Antonov A, Stokke BG, Moksnes A, Røskaft E (in press) Egg rejection behavior in a marsh warbler Acrocephalus palustris population heavily parasitized by common cuckoos Cuculus canorus. Auk
Ar A, Rahn H, Paganelli CV (1979) The avian egg: mass and strength. Condor 81:331–337
Brooker MG, Brooker LC (1991) Eggshell strength in cuckoos and cowbirds. Ibis 133:406–413
Clark KL, Robertson RJ (1981) Cowbird parasitism and evolution of anti-parasite strategies in the yellow warbler. Wilson Bull 93:249–258
Cramp S (ed) (1992) The birds of the Western Palearctic. Warblers, vol VI. Oxford University Press, Oxford
Davies NB, Brooke M de L (1988) Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 36:262–284
Davies NB, Brooke M de L (1989) An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 58:207–224
Edvardsen E, Moksnes A, Røskaft E, Øien IJ, Honza M (2001) Egg mimicry in cuckoos parasitizing four sympatric species of Acrocephalus warblers. Condor 103:829–837
Gärtner K (1981) Das Wegnehmen von Wirtsvogeleiern durch den Kuckuck (Cuculus canorus). Ornithol Mitt 33:115–131
Gaston AJ (1976) Brood parasitism by the pied crested cuckoo Clamator jacobinus. J Anim Ecol 45:331–348
Honza M, Picman J, Grim T, Novák V, Čapek M Jr, Mrlík V (2001) How to hatch from an egg of great structural strength. A study of the common cuckoo. J Avian Biol 32:249–255
Honza M, Procházka P, Stokke BG, Moksnes A, Røskaft E, Čapek M Jr, Mrlík V (2004) Are blackcaps current winners in the evolutionary struggle against the common cuckoo? J Ethol 22:175–180
Hoyt OF (1979) Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77
Kramer GH, Lemon RE (1985) Song switching and agonistic stimulation in the song sparrow: five tests. Anim Behav 33:135–149
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lindholm AK (2000) Tests of phenotypic plasticity in reed warbler defences against cuckoo parasitism. Behaviour 137:43–60
Lorenzana JC, Sealy SG (2001) Fitness costs and benefits of cowbird egg ejection by gray catbirds. Behav Ecol 12:325–329
Lotem A, Nakamura H, Zahavi A (1995) Constraints on egg discrimination and cuckoo–host co-evolution. Anim Behav 49:1185–1209
Martin-Vivaldi M, Soler M, Møller AP (2002) Unrealistically high costs of rejecting artificial model eggs in cuckoo Cuculus canorus hosts. J Avian Biol 33:295–301
Moksnes A, Røskaft E (1995) Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J Zool (Lond) 236:625–648
Moksnes A, Røskaft E, Braa AT (1991) Rejection behaviour by common cuckoo hosts towards artificial brood parasite eggs. Auk 108:348–354
Moksnes A, Røskaft E, Bicík V, Honza M, Øien IJ (1993a) Cuckoo Cuculus canorus parasitism on Acrocephalus warblers in Southern Moravia in the Czech Republic. J Ornithol 134:425–434
Moksnes A, Røskaft E, Korsnes L (1993b) Rejection of cuckoo (Cuculus canorus) eggs by meadow pipits (Anthus pratensis). Behav Ecol 4:120–127
Moksnes A, Røskaft E, Solli MM (1994) Documenting puncture ejection of parasitic eggs by chaffinches Fringilla coelebs and blackcaps Sylvia atricapilla. Fauna Norv Ser C 17:115–118
Moksnes A, Røskaft E, Greger Hagen L, Honza M, Mørk C, Olsen PH (2000) Common cuckoo Cuculus canorus and host behaviour at reed warbler Acrocephalus scirpaceus nests. Ibis 142:247–258
Moskát C, Honza M (2002) European cuckoo Cuculus canorus parasitism and host’s rejection behaviour in a heavily parasitized great reed warbler Acrocephalus arundinaceus population. Ibis 144:614–622
Moskát C, Szentpéteri J, Barta Z (2002) Adaptations by great reed warblers to brood parasitism: a comparison of populations in sympatry and allopatry with the common cuckoo. Behaviour 139:1313–1329
Nager RG, Monaghan P, Houston DC (2001) The cost of egg production: increased egg production reduces future fitness in gulls. J Avian Biol 32:159–166
Øien IJ, Moksnes A, Røskaft E, Honza M (1998) Costs of cuckoo Cuculus canorus parasitism to reed warblers Acrocephalus scirpaceus. J Avian Biol 29:209–215
Picman J (1989) Mechanisms of increased puncture resistance of eggs of brown-headed cowbirds. Auk 106:577–583
Procházka P, Honza M (2003) Do common whitethroats (Sylvia communis) discriminate against alien eggs? J Ornithol 144:354–363
Procházka P, Honza M (2004) Egg discrimination in the yellowhammer. Condor 106:405–409
Rahn H, Curran-Everett L, Booth DT (1988) Eggshell differences between parasitic and non-parasitic Icteridae. Condor 90:962–964
Rohwer S, Spaw CD (1988) Evolutionary lag versus bill-size constraints: a comparative study of the acceptance of cowbird eggs by old hosts. Evol Ecol 2:27–36
Rohwer S, Spaw CD, Røskaft E (1989) Costs to northern orioles of puncture-ejecting parasitic cowbird eggs from their nests. Auk 106:734–738
Røskaft E, Moksnes A, Meilvang D, Bicík V, Jemelíková J, Honza M (2002) No evidence for recognition errors in Acrocephalus warblers. J Avian Biol 33:31–38
Rothstein SI (1975) An experimental and teleonomic investigation of avian brood parasitism. Condor 77:250–271
Rothstein SI (1976) Experiments on defenses cedar waxwings use against cowbird parasitism. Auk 93:675–691
Schönwetter M (1979) Handbuch der Oologie, Band II. Akademie Verlag, Berlin
Schulze-Hagen K (1992) Parasitierung und Brutverluste durch den Kuckuck (Cuculus canorus) bei Teich- und Sumpfrohrsänger (Acrocephalus scirpaceus, A. palustris) in Mittel- und Westeuropa. J Ornithol 133:237–249
Schulze-Hagen K, Leisler B, Winkler H (1996) Breeding success and reproductive strategies of two Acrocephalus warblers. J. Ornithol 137:181–192
Soler M (1990) Relationships between the great spotted cuckoo Clamator glandarius and its corvid hosts in a recently colonized area. Ornis Scand 21:212–223
Soler M, Martínez JG (2000) Is egg-damaging behavior by great spotted cuckoos an accident or an adaptation? Behav Ecol 11:495–501
Soler M, Soler JJ, Martínez JG (1997) Great spotted cuckoos improve their reproductive success by damaging magpie host eggs. Anim Behav 54:1227–1233
Spaw CD, Rohwer S (1987) A comparative study of eggshell thickness in cowbirds and other passerines. Condor 89:307–318
Stokke BG, Moksnes A, Røskaft E, Rudolfsen G, Honza M (1999) Rejection of artificial cuckoo (Cuculus canorus) eggs in relation to variation in egg appearance among reed warblers (Acrocephalus scirpaceus). Proc R Soc Lond B 266:1483–1488
Stokke BG, Honza M, Moksnes A, Røskaft E, Rudolfsen G (2002) Costs associated with recognition and rejection of parasitic eggs in two European passerines. Behaviour 139:629–644
Swynnerton CFM (1918) Rejections by birds of eggs unlike their own: with remarks on some of the cuckoo problems. Ibis 6:127–154 (Tenth Series)
Visser ME, Lessells CM (2001) The costs of egg production and incubation in great tits (Parus major). Proc R Soc Lond B 268:1271–1277
Weary DM, Krebs JR, Eddyshaw R, McGregor PK, Horn A (1988) Decline in song output by great tits: exhaustion or motivation? Anim Behav 36:1242–1244
Weatherhead PJ (1991) The adaptive value of thick-shelled eggs for brown-headed cowbirds. Auk 108:196–198
Wyllie I (1981) The cuckoo. Batsford, London
Acknowledgements
We want to thank the staff at the Naturhistoriches Museum in Vienna, Austria, for allowing us to make bill measurements of the study species. We would like to thank all students and cooperators working in our Czech study area for assistance in the field. We are very grateful to Manuel Soler, Lesley Brooker and one anonymous referee for comments that significantly improved a previous version of the manuscript. B.G.S. was funded by the Research Council of Norway, grant no. 151641/432. All experiments complied with the current laws of Bulgaria and Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Soler
Rights and permissions
About this article
Cite this article
Antonov, A., Stokke, B.G., Moksnes, A. et al. Eggshell strength of an obligate brood parasite: a test of the puncture resistance hypothesis. Behav Ecol Sociobiol 60, 11–18 (2006). https://doi.org/10.1007/s00265-005-0132-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0132-6