Abstract
Daphnia is a widespread freshwater zooplankton species, which is both a classic and emerging new model for research in ecological physiology, ecotoxicology and evolutionary biology of adaptation to novel environments. Heat tolerance in Daphnia is known to depend both upon evolutionary history of a genotype and on individuals’ acclimation to elevated temperature and to correlate with the level of haemoglobin expression. We demonstrate the existence of north–south gradient of heat tolerance in North American D. pulex, which is not associated with any parallel changes in haemoglobin expression. Geographically distinct clones differ in the way their haemoglobin expression changes due to acclimation to a sub-stressful (28°C) temperature, but these changes are not correlated with the latitude of clones’ origin. Likewise, the effect of acclimation to sub-stressful temperature is independent from, and cannot be fully explained by, haemoglobin expression changes during acclimation. The degree of oxidative damage to haemoglobin, measured as the ratio of absorbance at 540:576 nm at the acclimation temperature, is a strong predictor of 28°C-acclimated Daphnia survival during an acute heat exposure. The comparison of haemoglobin expression in resistant and tolerant clones acclimated to different temperatures indicates that tolerant clones exhibit canalization of haemoglobin expression, possessing a high level of haemoglobin even at non-stressful temperatures. We discuss the evolutionary biology of adaptation and acclimation to elevated temperatures in an ecologically important component of freshwater ecosystems in the context of global climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organisms differ in their ability to tolerate stress for two fundamentally different reasons: (1) genetic differences at structural or regulatory genes relevant to stress tolerance (adaptation), and (2) individual, non-heritable, physiological changes occurring during the exposure to the stressful environment (acclimation). These two mechanisms shape the adaptive landscape and set limits to the evolution of stress tolerance. On the one hand, adaptive ability of organisms to adjust phenotypes as the response to changes in environment is expected to evolve if benefits of being plastic overweighs the costs of plasticity in a variable environment (Scheiner 1993; Scheiner 2006; Snell-Rood et al. 2010). On the other hand, acclimation to stress, just as any other kind of plasticity, impedes evolutionary adaptation to stress, hiding adaptive variations from positive selection (Whitman and Agrawal 2009; Pfennig et al. 2010; Snell-Rood et al. 2010). Finally, the plasticity mechanisms themselves may be a target for selection, and therefore may be capable of evolving (King and Wilson 1975; Schulte 2004; Pfennig et al. 2010).
Increased temperature, among many other environmental stressors, achieves a particular level of interest in the face of global climate changes. While even the most pessimistic scenarios of global warming project relatively modest increase of average temperatures over the next several decades (thus keeping the global averages well within tolerance limits of the majority of organisms), the frequency of extreme temperatures experienced by ecosystems is expected to increase dramatically (Meehl et al. 2007; Hofmann and Todgham 2010). Although data on population and evolutionary consequences of climate change have just recently started to accumulate (see Reusch and Wood 2007; Pörtner and Knust 2007, Pörtner and Farrell 2008; Hoffmann and Willi 2008 and Bradshaw and Holzapfel 2010 for recent reviews), it is already obvious that, in many cases, populations’ response to elevated temperatures is determined by the interplay between novel selection pressures and existing phenotypic plasticity (Hoffmann and Willi 2008). It is therefore important to be able to make predictions about the direction, rate and limits of evolutionary changes, which are likely to occur due to increased exposure to temperature extremes, particularly in species playing pivotal or keystone roles in ecosystems.
Ectothermic animals constantly experience metabolic changes due to changes in ambient temperature. Physical constraints (i.e. Q10 temperature coefficient) dictate increased metabolic rates at higher temperatures and the limits of heat tolerance are often set by the ability of organisms to maintain very high metabolic rates without sustaining damage to cells or running out of resources. Aquatic animals, in particular, often face heat-induced hypoxia because oxygen solubility in water decreases with temperature, which creates a mismatch between the demand for oxygen and the capacity of oxygen supply to tissues at extreme temperatures (Hochachka 1997; Pörtner 2002; Pörtner and Knust 2007). Facing such physical and physiological constraints, organisms can be selected for phenotypic plasticity, allowing them to adjust metabolism in order to prevent damage or to minimize its consequences. For example, up-regulation of heat shock proteins (chaperones preventing heat damage to proteins) is a ubiquitous reaction to elevated temperatures (as well as to a variety of other stressors) in a broad range of organisms (Schlesinger 1990; Horowitz 2007; Richter et al. 2010). Alternatively, metabolic rates may be adjusted in order to offset the direct effect of temperatures, a process known as temperature compensation of metabolism (Hochachka and Somero 2002). Such plasticity is not exhibited instantly and requires a more or less prolonged period of acclimation, i.e. physiological adjustment to elevated temperature.
Daphnia (Crustacea: Cladocera) has been a classic model organism for the study of ecological physiology (in general and in relation to temperature in particular) ever since the beginning of experimental zoology (Woltereck 1909; McArthur and Baillie 1929; Brown 1929; Stier and Wolf 1932; Banta 1939; Fox et al. 1953) and remains one during the genomic era (Colbourne et al. 2011). D. pulex is the first non-insect arthropod with a completely sequenced genome and has recently become an officially recognized model organism for biomedical research by the US National Institutes of Health (Colbourne and Pfrender 2009; Colbourne et al. 2011). In particular, the possibility to maintain genetically identical genotypes sampled from outbred populations in a variety of environments makes Daphnia an ideal model system for the studies of phenotypic plasticity and genotype-by-environment interactions. Since the classic Daphnia study in which the term “reaction norm” was first formulated (Woltereck 1909), a plethora of Daphnia reaction norm data has been obtained, including those on plasticity of morphological characters (e.g. Jacobs 1987; Spitze and Sadler 1996; Boersma et al. 1998; Tollrian and Heibl 2004; Spanier et al. 2010; Miyakawa et al. 2010), life-history traits (Lynch 1980; Spitze 1992; Ebert et al. 1993; Yampolsky and Ebert 1994; Spitze and Sadler 1996; Boersma et al. 1998; Weetman and Atkinson 2004; Latta et al. 2007; Chopelet et al. 2008; Beckerman et al. 2010) and tolerance to pollutants (Coors et al. 2004; Messiaen et al. 2010), which Daphnia exhibit in response to a wide variety of environmental factors, from temperature and food availability to the presence of predators, parasites or pollutants.
Here we will focus on the role of adaptations and plasticity in Daphnia tolerance to elevated temperatures. Heat tolerance in Daphnia has long been known to be strongly dependent on acclimation to elevated temperatures (MacIsaac et al. 1985; Paul et al. 2004a) and to depend on haemoglobin expression in tissues (Seidl et al. 2005). Haemoglobin expression is higher in heat-acclimated Daphnia (Fox et al. 1953; Kobayashi and Hoshi 1984; Kobayashi and Nezu 1986; Paul et al. 2004a, b) and haemoglobin-rich Daphnia are more heat- and hypoxia tolerant (Paul et al. 2004a, b) and have a higher thermopreference (Wiggins and Frappell 2000).
Clonal genotypes in Daphnia are often found to have significantly different performance at high temperatures (e.g. Palaima and Spitze 2004) and artificial selection in different thermal regimes can quickly shift clonal compositions of experimental populations (Scheiner and Yampolsky 1998; Van Doorslaer et al. 2009). There is little evidence, however, that heat tolerance in Daphnia shows a strong latitudinal differentiation (Mitchell and Lampert 2000; Chopelet et al. 2008) or differentiation among genotypes present in ponds during different seasons (Mitchell, Halves and Lampert 2004). The lack of latitudinal differentiation in heat tolerance in Daphnia can be explained by the ability of genotypes to avoid temperature extremes in resting stages (Mitchell and Lampert 2000; Mitchell et al. 2004) or by high migration rates (cf. Allen et al. 2010). One may also hypothesize, based on the existence of genotype-by-environment interactions, that clinal differentiation in heat tolerance may be observable in organisms acclimated to some, but not other temperatures (see below). On the other hand, there is evidence of a latitude-by-current temperature interaction in oxygen consumption (Chopelet et al. 2008), indicating that genotypes with different thermal adaptation have the highest metabolic rates at temperatures similar to those experienced in the evolutionary past.
Thus, heat tolerance in Daphnia is determined by three inter-correlated factors: acclimation to current temperature, haemoglobin concentration in haemolymph and, possibly, genetic tolerance factors caused by evolutionary history of the genotype. The interplay of these factors predicts possible response to selection acting on thermotolerance. How can we characterize this interplay? For example, can the acclimation effect be fully explained by the increase of haemoglobin concentration at higher acclimation temperatures? Is there any genotype-by-environment interaction, i.e. do genotypes of different geographic origin possess different amount of plasticity for heat tolerance (different strength of acclimation effect) or plasticity for haemoglobin expression? If differences in plasticity exist, what is the evolutionary direction of such differences, i.e. do more tolerant genotypes possess a higher or a lower amount of plasticity (indicative of adaptive evolution or canalization of phenotypic plasticity, respectively)? In this study we attempt to answer some of these questions using a set of clones of D. pulex originating at different latitude, whose heat tolerance during an acute exposure to 35°C and haemoglobin expression are measured after acclimation to either 18, 24, or 28°C.
Materials and methods
Origin of clones
Heat tolerance was studied in 14 clones of Daphnia pulex Leydig obtained from the Daphnia Genome Center stock collection at Indiana University, Bloomington. Geographic locations of clones’ origin are shown on Fig. 1, mean and maximal July temperatures at the location of origins and other details of clones’ origin are given in Supplementary Table 1. The four southernmost locations (two in Illinois and two in Indiana) are located in the prairie zone, while the remaining 10 clones originate from forested locations. Therefore, the latitudinal gradient, in addition to its correlation with mean summer temperatures, also reflects exposure of typical Daphnia habitats to solar radiation. The clones have been maintained in the stock collection for 5–7 years prior to this study, with the exception of clones WTM3 and CR344, which have been collected from nature less than a year before the start of the experiment.
Heat-shock experiment
Four replicates of each of the 14 D. pulex clones were maintained separately for two generations (each generation being neonate to a second-clutch neonate) prior to the experiment in 150 ml bottles containing COMBO water medium (Kilham et al. 1998) and stored at either 18°C, 24°C or 28°C under 12:12 photoperiod. Scenedesmus acutus culture was added daily to the final concentration of 200,000 cells/ml. The medium was replaced and neonates removed twice weekly. 20–30 s or third clutch offspring of the second-generation females were collected within 48 h from birth and placed in bottles containing 150 ml of COMBO medium. After reaching the age of 12–15 days they were transferred into plastic 200 ml culture flasks with fresh medium (no algae added), which were placed into a 35°C water bath with slow agitation, resulting in a 30 min temperature ramp from the initial temperature to 35°C. Individuals were checked every 30 min during the first 2 h of the exposure and every 10-15 min during the next 2 h. Individuals were scored as “immobilized” when observed motionless on the bottom of the bottle, no antennae motion visible (but filtering apparatus still moving). In addition, at 2, 3 and 4 h of the experiment, the individuals were removed from the water bath briefly and examined under dissecting microscope to detect heartbeat. Individuals were scored as dead when neither filtering apparatus motion nor heartbeat was detected; live individuals were returned to the culture flasks.
Haemoglobin measurement
Simultaneously with the heatshock experiment, in three out of the four acclimation replicates available, 3–4 individuals from the cohorts acclimated to either 18 or 28°C were used for haemoglobin concentration measurements. These individuals were harvested either prior to the onset of heatshock experiment (“unexposed”) or after 2 h of exposure to 35°C (“exposed”). Very little mortality occurs in the heatshock experiment during the first 2 h of exposure to 35°C. Only “unexposed” data are available for Daphnia acclimated at the intermediate 24°C acclimation treatment, so all analysis involving haemoglobin data for exposed and unexposed individuals was limited to acclimation temperatures 18 and 28°C.
Individual Daphnia were frozen in liquid nitrogen for storage, and later homogenized in 10 ul of 0.1 M phosphate buffer (pH 7.2) and the homogenate centrifuged at 14,000 rpm for 6 min. Absorption spectra of the supernatant was measured immediately by Nanodrop spectrophotometer ND-1000 at 230–700 nm. Haemoglobin-specific absorbance at each of the absorption peaks at 414, 540 and 576 nm (Pirow et al. 2001) was normalized by the following procedure. The slope of the absorption spectrum in the 350–450 and 520–600 nm ranges was determined by linear regression, from which the data points corresponding to the three haemoglobin absorption peaks (410–420, 530–545 and 570–580 nm) were excluded. The expected values determined by these linear regressions at each absorption peak were subtracted from the observed absorbance. The rationale for this background subtraction is that crude extracts of Daphnia tissues contain various non-haemoglobin compounds absorbing light in the 400–600 nm range, causing the slope in the spectrum (Pirow et al. 2001). The resulting difference was then normalized by dividing it by the absorbance at 260 nm (corresponding to the absorption peak of nucleic acid used as a proxy for the amount of tissues in the crude extract). The ratio of absorbance less background at 540:576 nm was used as an estimate of the portion of haemoglobin present in its oxidized (F3+) state (methaemoglobin). Methaemoglobin has a broad absorption peak at 500–550 nm but an absorption minimum at 570–590 nm, so increased concentration of methaemoglobin affects absorbance at 540 more than at 576 nm and therefore the ratio of the two absorbances can be used as an approximate measure of oxidative damage to haemoglobin, the ratio of >1 indicating an elevated oxidative damage.
Data analysis
Data were analyzed using general linear models and survival and reliability methods of JMP statistical software (SAS Institute 2001). For the purpose of analysis of variance, the acclimation temperature and the categorical geographic origin of clones (S vs. N clones) were treated as fixed effects, while clones (nested within the geographic region), as well as the continuous latitude, were treated as random effects. The data (replicate means) are available in Supplementary Table 2.
Results
Latitude, acclimation and heat tolerance
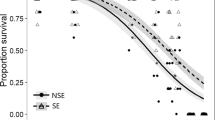
As expected, both the geographic location of the clone’s origin and acclimation to higher temperature had a significant effect on heat tolerance (Fig. 2; Table 1). Southern clones show higher heat tolerance than the northern ones, particularly when acclimated to lower temperatures. The latitude of clone’s origin location had a significant effect on mean 3-h mortality at 35°C in Daphnia acclimated to 18°C and 24°C (P < 0.03 and P < 0.05, respectively), and on mean 4-h mortality at 35°C in Daphnia acclimated to 24°C (P < 0.01; Fig. 2a, b).
Correlation between the geographic latitude of clones’ origin and mean mortality after 3 (a) and 4 (b) hours of exposure to 35°C and median time until immobilization during exposure (c) in Daphnia acclimated at either 18°C (open triangles), 24°C (filled circles) or 28°C (open squares). Linear regressions with regression coefficient significant at least P < 0.05 are shown as solid lines, non-significant ones as dotted lines
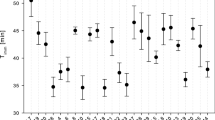
Median time until immobilization, while significantly effected by latitude in Daphnia acclimated to 18°C and 24°C (P < 0.05 and P < 0.005, Fig. 2c) show only a marginally significant N versus S effect in a two-way ANOVA (Table 1), possibly due to the large among-clone variance in animals acclimated to 28°C (Fig. 2c; see also Fig. 5 below). Together, the origin latitude and acclimation temperature explain (\( {\text{R}}_{\text{adj}}^{2} \)) 50% of variation in mortality and 55% of the variation of median time until immobilization. Despite much greater range of values than in latitude, longitude of clones’ geographic origin location was not a significant effect in any of the tests, either when Oregon clones were compared to Great Lakes region clones, or within the Great Lakes region. Mean mortality after 3 and 4 h of exposure to 35°C and median time until immobilization are shown in Fig. 3. Although there was no evidence of differences among clones (nested within the geographic region) in median time until immobilization, fitting a parametric survival model to immobilization data revealed a significant difference among clones nested within southern and northern groups of locations (Table 2).
Effects of origin latitude, acclimation and acute exposure on haemoglobin absorbance
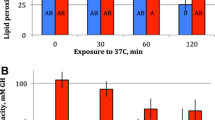
Genotypes, geographic origin, acclimation to 28°C and acute exposure had no effect on the concentration of haemoglobin measured as absorbance at 414 nm (Table 3; Fig. 4a). The geographic region (N vs. S) showed a significant difference (F = 19.2; P < 0.005; data not shown) when clones nested within geographic region were removed from the model, reflecting the fact that there were more “red” clones in the sample of northern clones (7 vs. 3) than in the sample of southern clones (2 vs. 2). Groups of clones visually identified as “red” and “pale” differed from each other significantly with respect to clones nested within such groups, indicating that visual evaluation of Daphnia colour provides an adequate estimate of haemoglobin concentration in haemolymph (data not reported). On the other hand, acclimation temperature had a significant effect at absorbance at both 540 and 576 nm (Table 3), possibly indicating changes in expression of different haemoglobin subunits (see Paul et al. 2004a; Lamkemeyer et al. 2005). Importantly, although clones as the main effect showed no significant effect on any absorbance values, there was a significant clone-by-acclimation temperature interaction for absorbance at 414 nm (nested within geographic region), as well as a geographic region-by-acclimation temperature interaction for absorbance at 576 nm (Table 3). This indicates that clones of different genotypes and/or latitudinal adaptation differ in their haemoglobin response to long-term acclimation to 18° versus 28°C. Specifically, southern clones showed an increased absorbance at 576 nm at 28°C, while northern clones showed no change. Absorbance at 540 nm, on the other hand, showed no difference between northern and southern clones; instead, there was a significant effect of acute exposure to 35°C, as well as an acclimation temperature-by-exposure interaction for absorbance at 540 nm. This effect can be summarized by the changes in 540:576 absorbance ratio (Fig. 4b): although all clones in both temperatures showed an increase of the ratio after a 2-h exposure, the highest values were observed in northern clones acclimated to 18°C, consistent with the expectation of the least-adapted and un-acclimated animals to show the highest oxidative damage.
Haemoglobin concentration measured as relative absorbance at 414 nm and the ratio of absorbance at 540 nm: 576 nm (b) in Daphnia from southern (S) and northern (N) clones, acclimated at either 18 or 28°C. Open bars: measurements at acclimation temperature. Grey bars: measurements after 2 h of exposure to 35°C. Paired t-test: ** P < 0.015; * P < 0.05
Effects of haemoglobin concentration on heat tolerance
Haemoglobin concentration measured as relative absorbance at 414 nm had only a marginally significant effect on mortality after 3 h, no effect on mortality after 4 h and a significant (P < 0.013) effect on median time until immobilization (Table 4), with median time being nearly twice as high in the clones with the highest haemoglobin level than in those with the lowest. On the other hand, the ratio between absorbance at 540 and 576 nm has a significant effect on mortality after 4 h (but not after 3) as well as on time until immobilization (Table 4; Fig. 4), higher ratios resulting in lower time until immobilization and higher mortality. There was also a significant acclimation temperature-by-absorbance ratio for both mortality after 4 h and time until immobilization (Table 4): high ratio correlated with high mortality and low time until immobilization in Daphnia acclimated to 28°C, but not to 18°C (Fig. 5). Interestingly, both the main effect and the interaction were significant only for the 540:576 absorbance ratio measured at the acclimation temperature, but not in animals exposed to 35°C (data not shown).
The effect of oxidative damage to haemoglobin (measured at acclimation temperature as the ratio of absorbance at 540:576 nm) on mortality after 4 h (a) and time until immobilization (b). Open circles—acclimation at 18°C, closed circles—acclimation at 28°C. Regressions and R2 values for 28°C are y = 0.18x + 0.26; R2 = 0.14 (a) and y = −1.038x + 4.88; R2 = 0.26 (b)
Discussion
Caveats
We report several patterns describing a complex interplay between genetic factors (adaptation possibly associated with latitudinal differentiation) and physiological changes (acclimation, haemoglobin concentration in haemolymph, resistance to acute heat exposure) affecting heat tolerance in Daphnia. This interplay is rich in significant interactions. The latitudinal cline is only visible in Daphnia acclimated to low or intermediate temperatures, but not to high temperatures. Acclimation, while significant as the main effect, also demonstrates a significant interaction with the 540:576 nm absorbance ratio, a likely measure of the degree of oxidative damage to haemoglobin. Haemoglobin concentration itself is not a uniform variable, but rather demonstrates different trends when measured as absorbance at different absorption peaks and different degree of reaction to acclimation to a substressful temperature in Daphnia of different latitudinal origin. Before attempting to interpret these results in terms of their significance for the adaptive landscape of heat tolerance in Daphnia, it is important to discuss several caveats. First, the data reported have been obtained on the material of only 14 clones, each population represented by just one clone (i.e. interpopulational variance pooled with the variance among clones within a population). Further, the latitudinal range sampled represents only a fraction of the species’ range, notably leaving out any Arctic and sub-Arctic populations, undoubtedly different in their heat tolerance. Finally, most clones have been maintained in constant condition of the stock collection for several years prior to the experiments, possibly losing, due to mutation accumulation or laboratory selection, some of the latitudinal differentiation they represented. Thus, the data reported are quite preliminary with respect to their ability to characterize geographic differentiation in heat tolerance.
In addition, some parts of the statistical analysis are suspect due to heteroscedasticity in the data. As indicated in the Results (see Fig. 2), the variance within the acclimation to 28°C treatment is much higher than that in 18 and 24°C treatments, rendering P values in Tables 1, 2, 3 and 4 approximate. The problem is partially ameliorated by the use of log-transformed data, which yields similar results. These caveats notwithstanding, examination of Figs. 2, 3, 4 suggests that the interactions we report here are real, at least qualitatively.
Latitudinal cline is more pronounced when individuals are acclimated to lower temperatures
The differences in slopes of the linear regression of clonal mean heat tolerance on the latitude of clone’s origin (Fig. 2) indicates that whatever the genetic factors of heat tolerance and geographic adaptation are, they play a role only in Daphnia not acclimated to high temperatures and are swamped by plastic effects when the organism has time to acclimate. In terms of evolutionary response to increased temperatures in nature this means that phenotypic plasticity will predominate in the phenotype in the situations when genotypes find themselves in a novel environment either due to migration or to climate change, i.e. in cases in which temperature change occurs slowly.
Changes in haemoglobin concentration
We observed that acclimation to a substressful (28°C) and acute exposure to stressful (35°C) temperatures have different effects on absorbance at different haemoglobin absorption peaks. For the effects of acclimation, one may hypothesize that this indicates differential expression of different subunits of Daphnia haemoglobin (Paul et al. 2004a; Zeis et al. 2004; Lamkemeyer et al. 2005), an effect also corroborated by microarray expression data (data in preparation). However, such a hypothesis is not feasible for an acute (2 h) exposure, because the changes in haemoglobin subunit expression take several days to occur (Paul et al. 2004a; Lamkemeyer et al. 2005; B. Zeis, personal communication). Rather, the apparent changes in absorbance at 540 and 576 nm is likely to be due to oxidative damage to haemoglobin, the absorbance at 540 nm being affected greater than that at 576 nm due to the shape of absorption spectrum of the main product of haemoglobin oxidation, methaemoglobin (which has a higher absorbance at 540 than at 576 nm; Zijistra et al. 1991). This interpretation is corroborated by two observations. First, the 540:576 ratio is uniformly higher in Daphnia exposed to 35°C than in unexposed ones. Second, it is the highest in northern Daphnia clones acclimated to 18°C and then exposed to 35°C (i.e. in the animals least prepared for the acute heat exposure both genetically and physiologically). It is, therefore, not surprising that the 540:576 ratio has a significant effect on survival during the heat exposure. What is less expected is that this ratio measured after 2 h of exposure has no effect on survival, while the one measured prior to exposure at the acclimation temperatures does (Table 4). This can be explained in two different ways. Either we are not adequately measuring the relative abundance of haemoglobin and methaemoglobin in heat-exposed animals, for example because other absorbing compounds are produced during heat exposure, or the amount of oxidative damage to haemoglobin only matters during the initial phases of heatshock, while after 2 h of exposure it does not matter any more. The latter explanation is partially corroborated by the fact that the increase in overall haemoglobin concentration measured at 414 nm tends to reduce mortality after 3 h of exposure, but not after 4 h (Table 4). It should be noted that it remains unknown whether any of the 540:576 ratio effects observed are caused by the actual damage to haemoglobin, or merely reflect the overall degree of oxidative damage in tissues. Either way, the joint effect of acclimation and the 540:576 ratio on mortality and immobilization of Daphnia during an acute heat exposure indicates that the acclimation to sub-stressfully high temperature may occur, in part, by activating antioxidant mechanisms.
Evidence for canalization of haemoglobin expression in heat-tolerant clones
In partial contradiction with previous results (Seidl et al. 2005), the effect of acclimation to elevated temperature in our experiments appears to be independent from the effect of haemoglobin concentration changes (Table 4). In general, a plastic response of a physiological or biochemical trait to an unfavorable environmental factor (for example an increase of haemoglobin concentration in response to a temperature change) does not, of course, prove that this plasticity is adaptive, i.e. that the plastic change increases the organism’s tolerance to the environmental stress. This plasticity may be a downstream change directly caused by the environmental factor due to a physical or developmental constraint. Short of manipulative experiments, one reasonable way to distinguish between adaptive and constrained plasticity is to compare its magnitude in tolerant versus sensitive genotypes. A constrained downstream response should manifest equally in both, while the magnitude of adaptive plasticity contributing to tolerance may be either higher in tolerant genotypes, indicating recent expansion of plasticity, or lower in tolerant genotypes, indicating canalization. Table 5 summarizes the situations in which sensitive and tolerant genotypes differ with respect of plastic (inducible) and non-plastic (constitutive) expression of a particular trait and possible interpretations of each. These interpretations are graphically represented in Fig. 6a–c (with the assumption that naïve, sensitive genotype is the ancestral state, while the tolerant genotype is the evolved, derived state). Adaptive evolution occurring through the expansion of plasticity is known as Baldwin effect (Baldwin 1896; Badyaev 2005; Crispo 2007; Lande 2009), while the opposite phenomenon, adaptive evolution by reduction of plasticity, is called canalization (Waddington 1942; Crispo 2007). Some Daphnia traits, such as melanistic colouration, have been demonstrated to evolve rapidly through the Baldwin mechanism (Latta et al. 2007; Scoville and Pfrender 2010) with the molecular mechanism of plasticity providing a target for selection. Does plasticity of haemoglobin expression follow any of the three patterns—Baldwin effect (Fig. 6a), canalization (Fig. 6b) or elimination of previously existing direct harmful effect of the environment (Fig. 6c)? In order to answer this question, we compared plasticity of haemoglobin expression (measured as 540 nm absorbance, as the value reflecting both haemoglobin concentration and the degree of possible oxidative damage) in clones with different mean mortality, representing the range of “genetic” heat tolerance. The results of this comparison (Fig. 6d) indicate that, assuming tolerant genotypes being the derived state, the evolution of heat tolerance was accompanied by canalization of haemoglobin expression. Indeed, the difference between haemoglobin concentrations in Daphnia acclimated to 18° and 28°C is the highest in the least tolerant clones and the lowest in the most tolerant. Thus, the plasticity observable during acclimation to a sub-stressful temperature is canalized in the tolerant genotypes. Data based on the absorbance at 576 nm demonstrates a similar pattern (not shown). Interestingly, this is in opposition to a recent experimental study of thermal evolution in Daphnia (Van Doorslaer et al. 2009), demonstrating the increase in thermal plasticity of fitness (but decrease in plasticity of clutch size).
Three possible situations with genotypeXenvironment interactions in a trait related to stress tolerance. a: Adaptive enhancement of plasticity (Baldwin effect), b: canalization, c: escaping a direct harmful effect of the environment present in sensitive genotypes. Vertical dashed arrows represent the amount of plasticity present in a sensitive or tolerant genotype. d: Evidence of canalization of haemoglobin expression during acute exposure to lethal temperature and 2-generations acclimation to sub-lethal temperature. Open circles, solid line—clonal means for Daphnia acclimated at 18°C; closed circles, dashed line: clonal means for Daphnia acclimated at 28°C. The horizontal axis (1 − Mortality) represents the mean portion of Daphnia in the same clone surviving 3 h of exposure to 35°C
What if the tolerant genotypes represent the ancestral state and the evolution proceeded towards heat-sensitive ones? Then the observed pattern will have to be interpreted as neutral expansion of plasticity upon relaxation of stabilizing selection. A much broader phylogeographic survey of heat tolerance in Daphnia would be necessary to distinguish between the two scenarios.
Cost and limitations of haemoglobin expression plasticity
Phenotypic plasticity places limits on the rate of adaptive evolution by masking genetic variations from natural selection. However, it can be an evolving trait itself, allowing a change in phenotypic means across environments due to either expansion or canalization. It appears that haemoglobin expression in Daphnia is canalized in heat-tolerant clones. This can be interpreted (Table 5) as either evolution to reduce costs of plasticity (Scheiner 1993; DeWitt et al. 1998; Ernande and Dieckmann 2004), as the result of selection for faster response (i.e. for reducing the lag-time limitation of plasticity; DeWitt et al. 1998), or as neutral loss of the un-induced phenotype (due to relaxation of stabilizing selection in populations never experiencing the non-inducing environment). The latter explanation is hardly applicable to Daphnia, which inhabits highly seasonal environments and in which, therefore, even the most heat-adapted phenotypes regularly experience low temperatures. It is therefore interesting to distinguish between selection to reduce the cost of plasticity and selection for fast response. Cost of plasticity is hard to demonstrate in Daphnia (Scheiner and Berrigan 1998), but in the case of haemoglobin expression at least some hypotheses are possible. On the one hand, there are data indicating that in Daphnia (at least in lake species), haemoglobin, in addition to oxygen transporting role, also plays a role of protein storage (Schwerin et al. 2010). Turning off haemoglobin production when not necessary for the reasons of thermal adaptation may not be possible due to correlated detrimental effects on protein storage. On the other hand, constant expression of haemoglobin (i.e. lack of plasticity) may be costly, because high-haemoglobin Daphnia are visibly darker and therefore more vulnerable to fish predators. In this study, we find at least some evidence favoring the hypothesis of selection for a fast response, as the baseline (unexposed) haemoglobin concentration correlates with performance during heatshock exposure, while the exposed levels do not.
References
Allen MR, Thum RA, Cáceres CE (2010) Does local adaptation to resources explain genetic differentiation among Daphnia populations? Mol Ecol 19:3076–3087
Badyaev AV (2005) Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc R Soc B Biol Sci 272:877–886
Baldwin JM (1896) A new factor in evolution. Am Nat 30:441–451
Banta AM (1939) Studies on the physiology, genetics, and evolution of some cladocera. Carnegie Inst. Wash. Publ. No. 513, 285 p
Beckerman AP, Rodgers GM, Dennis SR (2010) The reaction norm of size and age at maturity under multiple predator risk. J Anim Ecol 79:1069–1076
Boersma M, Spaak P, De Meester L (1998) Predator-mediated plasticity in morphology, life history, and behavior of Daphnia: the uncoupling of responses. Am Nat 152:237–248
Bradshaw WE, Holzapfel CM (2010) Light, time, and the physiology of biotic response to rapid climate change in animals. Ann Rev Physiol 72:147–166
Brown LA (1929) The natural history of cladocerans in relation to temperature. II. Temperature coefficients for development. Am Nat 63:346–352
Chopelet J, Blier PU, Dufresne F (2008) Plasticity of growth rate and metabolism in Daphnia magna populations from different thermal habitats. J Exp Zool Part A Ecol Genet Physiol 309:553–562
Colbourne JK, Pfrender ME (2009) Daphnia. NIH model organisms for biomedical research 2009. Available from http:\\www.nih.gov/science/models/Daphnia/
Colbourne JK, Pfrender ME, Gilbert D et al (2011) The ecoresponsive genome of Daphnia pulex. Science 331:555–561
Coors A, Hammers-Wirtz M, Ratte HT (2004) Adaptation to environmental stress in Daphnia magna simultaneously exposed to a xenobiotic. Chemosphere 56:395–404
Crispo E (2007) The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61:2469–2479
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Ebert D, Yampolsky L, Stearns SC (1993) Genetics of life-history in Daphnia magna. 1. Heritability in two food levels. Heredity 70:335–343
Ernande B, Dieckmann U (2004) The evolution of phenotypic plasticity in spatially structured environments: implications of intraspecific competition, plasticity costs and environmental characteristics. J Evol Biol 17:613–628
Fox HM, Phear EA, Gilchrist BM (1953) Influence of temperature and iron on haemoglobin synthesis by Daphnia. Nature 171:347
Hochachka PW (1997) Oxygen—a key regulatory metabolite in metabolic defense against hypoxia. Am Zool 37:595–603
Hochachka PW, Somero GN (2002) Biochemical Adaptation. Oxford University Press, Oxford
Hoffmann A, Willi Y (2008) Detecting genetic responses to environmental change. Nat Rev Genet 9:421–432
Hofmann GE, Todgham AE (2010) Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Ann Rev Physiol 72:127–145
Horowitz M (2007) Heat acclimation and cross-tolerance against novel stressors: genomic-physiological linkage. Progr Brain Res 162:373–392
Jacobs J (1987) Cyclomorphosis in Daphnia. Mem Ist Ital Idrobiol 45:325–352
Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L (1998) COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147–159
King MC, Wilson AC (1975) Evolution at two levels in humans and chimpanzees. Science 188(4184):107–116
Kobayashi M, Hoshi T (1984) Analysis of respiratory role of haemoglobin in Daphnia magna. Zool Sci 1:523–532
Kobayashi M, Nezu T (1986) Variation of haemoglobin content in Daphnia magna. Physiol Zool 59:35–42
Lamkemeyer T, Paul RJ, Stöcker W et al (2005) Macromolecular isoforms of Daphnia magna haemoglobin. Biol Chem 386:1087–1096
Lande R (2009) Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J Evol Biol 22:1435–1446
Latta LC IV, Bakelar JW, Knapp RA, Pfrender ME (2007) Rapid evolution in response to introduced predators. II: the contribution of adaptive plasticity. BMC Evol Biol 7:21
Lynch M (1980) The evolution of cladoceran life histories. Quart Rev Biol 55:23–42
MacIsaac HJ, Hebert PDN, Schwartz SS (1985) Inter- and intraspecific variation in acute thermal tolerance of Daphnia. Physiol Zool 58:350–355
McArthur JW, Baillie WHT (1929) Metabolic activity and duration of life. I. Influence of temperature on longevity in Daphnia magna. J Exp Zool 53:221–242
Meehl GA, Stocker TF, Collins WD et al. (2007) Global climate projections. In: Solomon S, Qin D, Manning M et al (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge.
Messiaen M, De Schamphelaere KAC, Muyssen BTA, Janssen CR (2010) The micro-evolutionary potential of Daphnia magna population exposed to temperature and cadmium stress. Ecotoxicol Environ Saf 73:1114–1122
Mitchell SE, Lampert W (2000) Temperature adaptation in a geographically widespread zooplankter, Daphnia magna. J Evol Biol 13:371–382
Mitchell SE, Halves J, Lampert W (2004) Coexistence of similar genotypes of Daphnia magna in intermittent populations: response to thermal stress. Oikos 106:469–478
Miyakawa H, Imai M, Sugimoto N et al (2010) Gene up-regulation in response to predator kairomones in the water flea, Daphnia pulex. BMC Dev Biol 10:45
Palaima A, Spitze K (2004) Is a jack-of-all-temperatures a master of none? An experimental test with Daphnia pulicaria (Crustacea: Cladocera). Evol Ecol Res 6:215–225
Paul RJ, Lamkemeyer T, Maurer J, Pinkhaus O, Pirow R, Seidl M, Zeis B (2004a) Thermal acclimation in the microcrustacean Daphnia: a survey of behavioural, physiological and biochemical mechanisms. J Therm Biol 29:655–662
Paul RJ, Zeis B, Lamkemeyer T, Seidl M, Pirow R (2004b) Control of oxygen transport in the microcrustacean Daphnia: regulation of haemoglobin expression as central mechanism of adaptation to different oxygen and temperature conditions. Acta Physiol Scand 182:259–275
Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP (2010) Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol 25:459–467
Pirow R, Bäumer C, Paul RJ (2001) Benefits of haemoglobin in the cladoceran crustacean Daphnia magna. J Exp Biol 204:3425–3441
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol 132A:739–761
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Reusch TBH, Wood TE (2007) Molecular ecology of global change. Mol Ecol 16:3973–3992
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40:253–266
SAS Institute (2001) JMP 5.0 user manual. Cary, NC, USA
Scheiner SM (1993) Genetics and evolution of phenotypic plasticity. Ann Rev Ecol Syst 24:35–68
Scheiner SM (2006) Genotype-environment interactions and evolution. In: Fox CW, Wolf JB (eds) Evolutionary genetics. Concepts and case studies. Oxford University Press, Oxford
Scheiner SM, Berrigan D (1998) The genetics of phenotypic plasticity. VIII. The cost of plasticity in Daphnia pulex. Evolution 52:368–378
Scheiner SM, Yampolsky LY (1998) The evolution of Daphnia pulex in a temporally varying environment. Genet Res 72:25–37
Schlesinger MJ (1990) Heat shock proteins. J Biol Chem 265:12111–12114
Schulte PM (2004) Changes in gene expression as biochemical adaptations to environmental change: a tribute to Peter Hochachka. Comp Biochem Physiol Part B Biochem Mol Biol 139:519–529
Schwerin S, Zeis B, Horn W, Horn H, Paul RJ (2010) Hemoglobin concentration in Daphnia (D. galeata-hyalina) from the epilimnion is related to the state of nutrition and the degree of protein homeostasis. Limnol Oceanogr 55:639–652
Scoville AG, Pfrender ME (2010) Phenotypic plasticity facilitates rapid parallel adaptation to an introduced predator. Proc Nat Acad Sci USA 107:4260–4263
Seidl MD, Pirow R, Paul RJ (2005) Acclimation of the microcrustacean Daphnia magna to warm temperatures is dependent on haemoglobin expression. J Therm Biol 30:532–544
Snell-Rood EC, Van Dyken JD, Cruickshank T, Wade MJ, Moczek AP (2010) Toward a population genetic framework of developmental evolution: the costs, limits, and consequences of phenotypic plasticity. Bioessays 32:71–81
Spanier KI, Leese F, Mayer C et al (2010) Predator-induced defenses in Daphnia pulex: Selection and evaluation of internal reference genes for gene expression studies with real-time PCR. BMC Mol Biol 11:50
Spitze K (1992) Predator-mediated plasticity of prey life history and morphology: Chaoborus americanus predation on Daphnia pulex. Am Nat 139:229–247
Spitze K, Sadler TD (1996) Evolution of a generalist genotype: multivariate analysis of the adaptiveness of phenotypic plasticity. Am Nat 148:S108–S123
Stier JB, Wolf E (1932) On temperature characteristics for different processes in the same organism. J Gen Physiol 16:367–374
Tollrian R, Heibl C (2004) Phenotypic plasticity in pigmentation in Daphnia induced by UV radiation and fish kairomones. Funct Ecol 18:497–502
Van Doorslaer W, Stoks R, Duvivier C et al (2009) Population dynamics determine genetic adaptation to temperature in Daphnia. Evolution 63:1867–1878
Waddington CH (1942) Canalization of development and the inheritance of acquired characters. Nature 150:563–565
Weetman D, Atkinson D (2004) Evaluation of alternative hypotheses to explain temperature-induced life history shifts in Daphnia. J Plankton Res 26:107–116
Whitman DW, Agrawal AA (2009) What is phenotypic plasticity and why is it important? In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity of insects. Mechanisms and consequences. Science Publishers, USA, pp 1–63
Wiggins PR, Frappell PB (2000) The influence of haemoglobin on behavioural thermoregulation and oxygen consumption in Daphnia carinata. Physiol Biochem Zool 73:153–160
Woltereck R (1909) Weitere experimentelle Untersuchungen über Artverinderung, speziell über das Wesen quantitativer Artunterschiede bei Daphniden. Verhand Deut Zool Ges 1909:110–172
Yampolsky LY, Ebert D (1994) Variation and plasticity of biomass allocation ratio in Daphnia. Funct Ecol 8:435–440
Zeis B, Lamkemeyer T, Paul RJ (2004) Molecular adaptation of Daphnia magna hemoglobin. Micron 35:47–49
Zijistra WG, Buursma A, Meeuwsen-van der Roest WP (1991) Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin Chem 37:1633–1638
Acknowledgments
We are grateful to Matthias Westphal for providing D. pulex clones, to Fred Hooker and Chelsea Ross for laboratory assistance, to Tom Jones, Karl Joplin, John Colbourne, Michael Pfrender and two anonymous reviewers for suggestions on improving the manuscript and to Bettina Zeis for extremely valuable advise on the analysis and interpretation of haemoglobin data. This work was partially supported by NSF grants DUE-0525447 and DGE-0742364.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Williams, P.J., Dick, K.B. & Yampolsky, L.Y. Heat tolerance, temperature acclimation, acute oxidative damage and canalization of haemoglobin expression in Daphnia . Evol Ecol 26, 591–609 (2012). https://doi.org/10.1007/s10682-011-9506-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9506-6