Abstract
Examples of phenotypic plasticity—the ability of organisms of identical genotypes to produce different phenotypes in response to the environment—are abundant, but often lack data on the causative physiology and biochemistry. Phenotypes associated with increased protection against or reduced damage from harmful environments may, in fact, be downstream effects of hidden adaptive responses that remain elusive to experimental measurement or be obscured by homeostatic or over-compensatory effects. The freshwater zooplankton crustacean Daphnia drastically increases its heat tolerance as the result of acclimation to high temperatures, an effect often assumed to be based on plastic responses allowing better protection against oxidative stress. Using several geographically distant Daphnia magna genotypes, we demonstrate that the more heat tolerant individuals have a higher total antioxidant capacity (TAC) both in the comparison of heat-acclimated vs. non heat-acclimated females and in the comparison of females to age- and body size-matched males, which show lower heat tolerance than females. However, experimental manipulations of hypothesized antioxidant pathways by either glutathione addition or glutathione synthesis inhibition had no effect on heat tolerance. Lipid peroxidation (LPO), contrary to expectations, did not appear to be a predictive measure of susceptibility to thermal damage: LPO was higher, not lower, in more heat tolerant heat-acclimated individuals after exposure to a lethally high temperature. We hypothesize that LPO may be maintained in Daphnia at a constant level in the absence of acute exposure to elevated temperature and increase as a by-product of a possible protective antioxidant mechanism during such exposure. This conclusion is corroborated by the observed short-term and long-term changes in phospholipid composition that included an increase in fatty acid saturation at 28 °C and up-regulation of certain long-chain polyunsaturated fatty acids. Phospholipid composition was more strongly affected by recently experienced temperature (4-day transfer) than by long-term (2 generations) temperature acclimation. This is consistent with partial loss of thermal tolerance after a short-term switch to a reciprocal temperature. As predicted under the homeoviscous adaptation hypothesis, the more heat tolerant Daphnia showed lower membrane fluidity than their less heat tolerant counterparts, in comparison both between acclimation temperatures and among different genotypes. We conclude that thermal tolerance in Daphnia is influenced by total antioxidant capacity and membrane fluidity at high temperatures, with both effects possibly reflecting changes in phospholipid composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organisms’ adaptive responses to stressful or damaging environmental factors have always generated a significant research interest, and identification of causative genetic determinants of adaptive plastic response to environmental stress is often seen as one of the central goals of modern ecological physiology and ecological genomics (Hoffmann and Willi 2008; Morris and Rogers 2014). There is a growing body of evidence that adaptive plasticity plays an important role in evolutionary response to environmental challenges, contributes to the evolution of novel phenotypes and species (Pfennig et al. 2010; Oomen and Hutchings 2015) and determines populations’ and species’ ability to survive climate changes (Hoffmann and Willi 2008; Canale and Henry 2010; Sgro et al. 2016). Yet, unequivocal demonstration that observed plastic changes are, indeed, causative to increased survival or reduced damage in the inducing environments has been difficult. In addition to the universal “correlation is not causation” problem, this approach suffers from a lack of unequivocal expectations. Why are we certain that the measured difference in presumed environmental response is a good measure of tolerance? There are several reasons why this might not be the case.
Genetic or phenotypic correlations between the amount of observed phenotypic change and tolerance to the environment that induces such change may be misleading in terms of the adaptive value of the measured plastic response. Such a correlative approach suffers from both false positives and false negatives. The experimentally measured plastic response may be passive (van Kleunen and Fischer 2005; Morris and Rogers 2014), i.e., constrained by the physical environment or resource availability and thus result in no adaptive outcome. Alternatively, acclimation to a stressful environment may be protective, but through a plastic response that eludes experimental measurement. Both scenarios result in an erroneous attribution of the measured response to the measured adaptive benefit such as reduced stress damage.
On the other hand, the stress-induced changes assumed to be a measure of environmental damage, such as lipid peroxidation (LPO), may be under homeostatic control, with a constant acceptable level maintained by organisms regardless of the environmental stress level (Rodriguez-Martinez and Ruiz-Torres 1992). This will result in a lack of any apparent plasticity, i.e., in a false negative. Furthermore, measurable environmental damage may, in fact, function as a signal of a stressful environment eliciting protective plastic response that we fail to measure or detect. Again, LPO, often assumed to be a measure of environmental stress, plays a role as functional component of a stress-detecting signaling pathway (Suzuki and Mittler 2006; Cohen et al. 2013; Farmer and Mueller 2013). This means that certain elevated level of LPO may result in increased, not decreased stress tolerance, i.e., have a hormetic effect (Maulucci et al. 2016). Heat- or oxidative stress-acclimated organisms may show a higher level of lipid peroxidation, resulting in a seemingly paradoxical result that higher levels of damage correlate with higher stress tolerance. Finally, in a comparison of stress-tolerant vs. stress-sensitive individuals, stress-sensitive ones may unexpectedly show a lower, not higher signal of environmental damage due to overcompensation, again, resulting in an inverse relationship between the hypothesized adaptive plasticity and differences in tolerance to the inducing environment. For example, ethanol-resistant Drosophila may experience lower internal ethanol concentrations due to more effective detoxification making potentially important plastic responses weaker, not stronger (“Fry conjecture”; Yampolsky et al. 2012). Given these confounding scenarios, it becomes obvious that there is a strong need for manipulative experiments or at least for correlative studies that include a number of alternative responses.
Approaches to the identification of adaptive plasticity that can partially ameliorate these methodological problems include comparison of plastic responses in evolved, stress-tolerant genotypes vs. naïve/ancestral stress-sensitive ones (Yampolsky et al. 2012) as well as comparison of long-term vs. acute plastic changes. While not entirely free from false positives and false negatives described above, such approaches at least offer an additional dimension that allows more specific predictions: stress-tolerant genotypes should show a stronger response than stress-sensitive ones and short-term vs. long-term responses, if adaptive, should mirror similar patterns of stress tolerance.
In this study, we apply these approaches, along with some manipulative experiments, to elucidate the role of plastic changes in antioxidant capacity, lipid peroxidation, and lipid composition in acclimation to high temperature, using a model organism Daphnia magna, a freshwater zooplankton crustacean. Specifically, we expect both antioxidant properties and heat tolerance to be higher in heat-acclimated Daphnia and intermediate in Daphnia acclimated to either high or low temperatures and then switched to the opposite temperature, thus partially eliminating the effects of long-term acclimation. If the observed changes in antioxidant capacity are causative to heat tolerance, we expect that direct addition of glutathione, a major antioxidant, and inhibition of glutathione antioxidant pathway, should result, respectively, in increased and decreased heat tolerance. Likewise, we expect temperature tolerance to increase after a short-term exposure to hypoxia, which is known to up-regulate antioxidant pathways in Daphnia (Becker et al. 2011; Klumpen et al. 2016). We also expect that the same acclimation and temperature switch treatments should result in mirroring changes in membrane fatty acid composition.
Materials and methods
Daphnia maintenance, temperature acclimation, and short-term switches
Daphnia magna clones (see Supplementary Table 1 and Yampolsky et al. 2014 for clones’ provenance) were maintained at 18 °C and under 12:12 photoperiod in 200 mL plastic culture flasks filled with COMBO water medium (Kilham et al. 1998) and fed every other day with Scenedesmus obliquus culture to the final concentration of 50,000 cells/ml. Four replicate flasks per clone were established. Each flask was started with 10 individuals less than 48 h of age; at Daphnia maturity each flask was split into two, with 5 adults in each. Every 4 days, the medium was changed and juveniles were removed; juveniles from 2nd or 3rd clutch were used to establish the next generation.
After 1 generation at 18 °C, each replicate in each clone was split into two temperature treatment groups, one remaining at 18 °C and the other moved into a 28 °C incubator with identical light and feeding condition. Temperature tolerance, hypoxia, short-term temperature switch, and antioxidants/LPO measurements were conducted after the 28 °C-acclimated individuals spent at least two generations at 28 °C, with the un-acclimated individuals remaining at 18 °C serving as controls.
In the third generation of acclimation to 28°/18 °C, half of the flasks containing adult Daphnia from a subset of three clones (Supplementary Table 1) were transferred to the reciprocal temperature 4 days (i.e., 1 moult cycle at 18 °C, less than 2 cycles at 28 °C) prior to the thermal tolerance experiment in which 3 to 4 replicates per clone per treatment were used. The same “stay” and “switch” replicates from one of the clones were used for the lipidomics experiment (see below).
To compare LPO and TAC in females and males (the latter known to have lower thermal tolerance than either age- or size-matched females; Yampolsky and Schaer, unpublished results), we acclimated males and females from two of the six clones that produce males regularly, from collection that occurred within 48 h of birth to the age of 3 weeks; at week 2, we added additional female neonates to the experiment, thus achieving matching the males to same age females (who were much larger) and same size females (who were much younger). Thus, the three classes of animals compared in this experiment were 3-week-old females (average body length ± SD: 2.59 ± 0.26 mm), 3-week-old males (1.97 ± 0.08 mm), and 1-week-old females (1.95 ± 0.11 mm).
Thermal tolerance experiments
As an estimate of temperature tolerance, we estimated time until immobilization at 37 °C (Williams et al. 2011; Yampolsky et al. 2014). For this, Daphnia were placed individually in 50 mL plastic culture flasks containing COMBO water without algae. For the experiments in which antioxidants and LPO were measured in Daphnia exposed to 37 °C and unexposed ones, half of these flasks remained at the corresponding acclimation temperature (18 or 28 °C) to serve as the unexposed controls. The rest of the flasks were transferred immediately into a 37 °C water bath, resulting in a 12- to 15-min temperature ramp inside the flasks. In experiments with different exposure times, Daphnia were placed into the water bath at staggered times, so that 30, 60, and 120-min exposures to 37 °C ended simultaneously. The flasks were monitored continuously (each one at least every 2 min) for Daphnia activity and time at immobilization was recorded when Daphnia could no longer lift themselves off the bottom of the flask.
Hypoxia exposure
A mildly hypoxic environment (approximately 5 mg O2/L, which corresponds to ~53, 65, and 75% of saturation at 18, 28, and 37 °C) was created by filling 50-ml plastic culture flasks with COMBO medium that was boiled for 10 min and allowed to cool down to approximately 80 °C, with air bubbles removed from flasks through a cap equipped with a pluggable vent. The flasks were then cooled down to room temperature and used for thermal tolerance experiments as described above. After Daphnia were placed into hypoxic vials through the vents, the flasks were topped with hypoxic COMBO water if needed to remove air bubbles. Flasks filled with COMBO water saturated with oxygen by intense ambient air bubbling for at least 24 h served as normoxic controls. Daphnia were placed into hypoxic or control flasks equilibrated to room temperature and then either used in the thermal tolerance experiments immediately, or remained in the experimental flasks for 90 min (at their respective acclimation temperatures) prior to the start of the thermal tolerance measurement. This was done to distinguish between direct effects of hypoxia and any physiological response to hypoxia (known to be most pronounced after 90 min of exposure, Becker et al. 2011).
Glutathione manipulations
To either augment or disrupt antioxidant properties in living Daphnia, individuals were exposed to various concentrations of either glutathione or the inhibitor of glutathione synthesis pathway buthionine sulfoximine (BSO). Despite low efficiency of cross-membrane transport of glutathione, supplementing Daphnia media with 0.1–0.2 mM reduced glutathione (GH) can modify oxidative stress tolerance (Podosinovikova et al. 2005). BSO efficiently inhibits gamma-glutamylcysteine synthetase, the rate-limiting enzyme in glutathione synthesis pathway (Anderson 1998), and is broadly used in oxidative stress studies in animal tissues. Because we are testing the hypothesis that enhancement of glutathione pathway can mimic the thermal tolerance effects of acclimation to high temperature and its disruption can eliminate such effects, the 18 and 28 °C-acclimated acclimated Daphnia were used in the glutathione experiment and in the BSO experiment, respectively. Both experiments were conducted with both adult and neonate Daphnia exposed to either chemical for 4 days, except for the neonates in the 18 °C glutathione supplement experiment which lasted 8 days. Water was changed at day 4 in this experiment (with fresh GH added), and not changed throughout the other three experiments. Increasing levels of mortality were observed in neonates exposed to various concentrations of BSO, up to 33% at 1mM BSO, so measurements conducted on the remaining individuals suffer from the survivor’s bias. For this reason, and because of the low number of individuals tested at the higher BSO concentrations, statistical analysis for the BSO-treated juveniles was conducted by lumping all non-0 BSO treatments together. Little mortality was observed in adults exposed to BSO or in either age class exposed to GH. Animals were thoroughly washed off the media with supplements prior to LPO and TAC measurements to avoid transferring non-tissue GH and BSO into the samples. Time to immobilization was then measured as described above and LPO and TAC were measured as described in the next paragraph. LPO and TAC were measured in either Daphnia unexposed to the lethal 37 °C, or after the length of exposure resulting in immobilization of the majority of experimental animals: 60 min in 18 °C-acclimated, glutathione-supplemented Daphnia, and 120 min in 18 °C-acclimated, BSO-treated ones.
Lipid peroxidation and total antioxidant capacity
Both LPO and TAC in Daphnia tissues were measured by means of fluorescent determination of BODIPY C11 581/591 dye oxidation (Image-iT kit, ThermoFisher). Developed initially as a probe specifically for LPO (Pap et al. 1999; Drummen et al. 2002; Yoshida et al. 2003), this dye has been subsequently validated for total antioxidant capacity measurement as well (Beretta et al. 2006). In a sample with both hydrophobic and hydrophilic components, BODIPY C11 is rapidly binding to hydrophobic components where it is specifically oxidized by products of lipid peroxidation. Regardless of the presence of such compounds, BODIPY C11 is slowly oxidized by any other oxidants present in the sample, including water-soluble ones, with saturation reached within several hours at 37 °C (Beretta et al. 2006).
For LPO and TAC determination, individual Daphnia were either harvested from their acclimation temperature (18 or 28 °C) directly or after 60-min exposure to 37 °C conducted as described above. The 60-min exposure to 37 °C results in the greatest difference (as compared to 30-min and 120-min exposure) in the percentage of immobilized individuals between the 28 °C-acclimated and 18 °C-acclimated Daphnia: most 28 °C-acclimated individuals still retain swimming ability, while the majority of the or 18 °C-acclimated ones have been immobilized (but are still alive as indicated by heartbeat). Harvested individuals were homogenized in 100 µL of deionized water using a bead-beater homogenizer and the samples centrifuged for 4 min at 8000 rpm, after which samples were kept on ice until measurement on a Biotek plate reader with Ex/Em 485/528 for the oxidized dye and 530/590 for the reduced dye; each well of the 96-well plate containing 50 µL of the sample. After the blank fluorescence reading, 50 µL of BODIPY 581/591 stock was added to the final probe concentration of 10 µM. The plates were incubated in the dark at room temperature for 30 min and measured again with the same Ex/Em filters to obtain the LPO reading. The plates were then incubated for 24 h at room temperature in the dark and the same measurements were repeated again for the TAC reading. The ratio between the oxidized and reduced dye fluorescence intensities (FI; blanks subtracted) was used as the estimates of LPO and TAC:
To calibrate the measurements and to validate the assumption that the measurement after 30-min incubation largely reflects specific oxidation of the dye by hydrophobic peroxides, while the 24-h measurement is largely the inverse of total antioxidants present in the sample, we calibrated both measurements by spiking the samples with cumene hydroperoxide or glutathione. As expected, cumene hydroperoxide increased oxidation of the dye during the first 30 min of exposure, but had a little effect on the oxidation after 24 h of exposure, while the effect of glutathione spikes was the opposite (Supplementary Fig. 1). The obtained calibrations were used to recalculate the measured LPO and TAC values into molar equivalents of cumene hydroperoxide and glutathione, respectively. Note: the glutathione calibration is approximate and should be used for a reference only, as the relationship between glutathione added and TAC estimate is not linear and is monotonous only over a limited range of raw TAC values that do not include values below 25 uM. Because of that the GH equivalent units are shown on a logarithm scale and any values below 25 uM should be interpreted as below detection level. Statistical analysis (see below) was conducted on log-transformed FI485/528/FI530/590 ratios and not on LPO or TAC values calibrated to cumene hydroperoxide or glutathione molar equivalents (does not matter for CumHO units as the transformation is linear, but would have mattered for the GH units). Likewise, upper and lower ranges of the standard error bars shown on TAC figures were calculated from the standard errors around the original measurements.
Lipidomics experiment
Five individuals of one of the clones (RU-RM1-009) from each of the four acclimation temperature X “stay/switch” treatments (see above) were collected, weighed (wet weight) and the egg number and development stage of the clutch they carried were recorded. Lipids were then extracted by chloroform–methanol extraction method using only glass vials and pipettes. One of the “down” transfer samples was lost due to mishandling and could not be replaced; it was replaced by an extra 18 °C “stay” samples, resulting in 6, 5, 4, and 5 replicates in “18, stay”, “28, stay”, “18 switch down”, and “28 switch up” treatments, respectively. Samples were dried under N2 and shipped on dry ice to Kansas State University Lipidomics Center (http://www.k-state.edu/lipid) for phospholipid profiling using API-4000 triple quadrupole mass spectrometer with electrospray and APCI sources (Applied Biosystems). Individual compounds’ signals were normalized by total lipid signal in the sample. Although samples’ protein content data measured using Qubit fluorometer (ThermoFisher) are available, it was not used for normalization due to a significant difference in protein content per unit of wet weight between 18- and 28 °C-acclimated Daphnia.
Fluorescence polarization
To corroborate lipidomics data with predicted effects of thermal acclimation on membrane fluidity (Hazel and Williams 1990), fluorescence polarization (FP) was measured in the non-nuclear fraction of cellular membranes in four replicate individuals from each of the six clones acclimated to either 18° or 28 °C, as described in Kushnareva, 2009. Single Daphnia were homogenized in HEPES/KCl buffer containing 10-mM KCl, 20-mM HEPES, 2-mM EDTA, 250-mM sucrose, and 1-mM DTT, and centrifuged at 8000g for 2 min. Supernatants were incubated with 10-μM 1,6-diphenyl-hexa-1,3,5-triene (100x stock in DMSO) at room temperature for 30 min and their polarized fluorescence was measured on a POLARstar Omega plate reader (LabTech) with 355-nm excitation/460-nm emission filters with mP value set to 250. FP was calculated from parallel and perpendicular FI values as
Statistical analysis
A general linear model was used to analyze the effects of acclimation temperature, recent temperature (where applicable), and/or hypoxia exposure or glutathione or BSO addition on thermal tolerance, LPO, and TAC, using JMP 10 (SAS Institute 2012). Clones were used as a random covariate. When several experiments (dates) were analyzed together, the date was also added to the model as a random block effect. Interactions between random and fixed effects were tested and, when not significant, pooled. Tukey test was used to determine statistical differences between individual treatments. Response variables were log-transformed for the sake of normality (see tables and supplementary tables for details of each analysis). All analyses were done using JMP.
Normalized lipidomics data were analyzed by general linear model as described above (except without clones or dates as random factors, since lipidomics data were obtained for 1 clone in a single block) and by principal component analysis (PCA). Both PCA on covariance and correlation matrices were performed. In general, the covariance matrix (i.e., unscaled data) captures more information contained in the data, but also allows variables with higher averages (i.e., phospholipids with higher occurrence) to have a greater loading on the principal components (Jolliffe 1986). Correlation matrix (scaled data) results in all variables having equal weights, but ignores biologically meaningful effects of high-average variables (i.e., abundant phospholipids). Since the data for all phospholipids are on the same natural scale of % of total signal (even though there is a large variation in means), we chose to report PCA on covariances, while PCA on correlations is reported in the Supplementary materials. Chief conclusions on the loadings of the first 4 PCs do not change regardless of whether we use covariance or correlation matrices.
Results
Long-term and short-term thermal acclimation effects
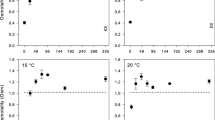
Long-term acclimation to 18 vs. 28 °C had a drastic effect on thermal tolerance measured as time until immobilization (Supplementary Fig. 3, Supplementary Table 2). However, contrary to the expectations, there was no significant difference between standing LPO level in Daphnia measured at the acclimation temperature without exposure to lethal 37 °C (Fig. 1a, Exposure = 0). In addition, the overall significant effect of acclimation temperature (Supplementary Table 3) was due to the differences between the 18- and 28 °C-acclimated Daphnia after such exposure, with the 28 °C-acclimated Daphnia showing higher, not lower LPO, than their less temperature-tolerant 18 °C-acclimated counterparts.
a Lipid peroxidation (LPO, in approximate cumene hydroperoxide molar units) and b total antioxidant capacity (TAC, in approximate glutathione molar units) in Daphnia acclimated to either 18 °C (blue) or 28 °C (red), measured after 0–120 min of exposure to 37 °C. TAC for the 18 °C-acclimated Daphnia were close to the lower bound of detection level in 0- and 30-min exposure. Here and throughout letters on bars are the results of Tukey test (α <0.05): values that share a letter are not significantly different. Total number of biological replicates (each consisting of a single individual) used: 223 (a) and 224 (b). One replicate in A lost due to fluorescence channel overflow. (Color figure online)
In contrast, total antioxidant capacity was significantly higher in 28 °C-acclimated Daphnia as compared to unexposed 18 °C-acclimated individuals (Fig. 1b, Supplementary Table 3) These differences disappeared during the exposure to lethal temperature, apparently as the 28 °C-acclimated Daphnia depleted their antioxidant reserves, while the 18 °C-acclimated Daphnia increased them in response to this acute heat exposure.
These acclimation effects were predictably modified by a short-term (4 days) transfer to the reciprocal temperature (Fig. 2, Supplementary Table 4). Roughly 1/3 of the log-scale difference in time until immobilization attributable to the acclimation temperature was lost during 4 days at 18 °C and roughly 1/3 was gained by the same exposure to 28 °C (Fig. 2a). This pattern was not mirrored by changes in lipid peroxidation after 60 min of heat exposure (Fig. 2b). Instead, the only significant difference observed was between the switched individuals, with the “up”-switched (18 to >28) ones showing disproportionally high level of LPO, while the “down”-switched ones showing a disproportionally low (in fact below reliable detection) level of LPO. Total antioxidant capacity showed, consistently with Fig. 1b, little difference between 18 and 28 °C-acclimated Daphnia with a mildly significant overall (P < 0.02) effect of the temperature treatment with no individual differences surviving the Tukey test.
a Thermal tolerance (T imm, natural log scale), b lipid peroxidation (LPO, in approximate cumene hydroperoxide molar units) and c total antioxidant capacity (TAC, in approximate glutathione molar units) in Daphnia acclimated to either 18 °C (blue) or 28 °C (red) and then either transferred to the reciprocal temperature 4 days prior to the measurement (hatched) or not transferred (solid colors). Both LPO and TAC (b, c) measured after 60 min of exposure to 37 °C. Here and throughout the blue “up” hatching indicates the 18−>28 switch and red “down” hatching–the 28−>18 switch. See Supplementary Table 3 for detailed statistical analysis. Note: LPO for the 28−>18 transfer (b) and TAC for the 18−>28 transfer Daphnia were below linear calibration level. Tukey test results shown only where there were different levels. Total number of biological replicates (each consisting of a single individual) used: 51 (a), 80 (b), and 81 (c). One replicate in B lost due to fluorescence channel overflow. (Color figure online)
Males vs. females comparison
The relationship between thermal tolerance and LPO/TAC is further corroborated by the comparison of males with age- and size-matched females (Fig. 3). As observed earlier, males have a lower thermal tolerance (Fig. 3a) and exhibit a lower, not higher level of LPO than either age- or size-matched females and, predictably, lower TAC (Fig. 3b, c).
a Thermal tolerance (T imm, natural log scale), b lipid peroxidation (LPO, in approximate cumene hydroperoxide molar units) and c total antioxidant capacity (TAC, in approximate glutathione molar units) in male and female Daphnia acclimated to 18 °C. ANOVA p values for the effect of sex/age class <0.0001 for each comparison. Total number of biological replicates (each consisting of a single individual) used: 26
Hypoxia effect on thermal tolerance
Hypoxia had no effect on time to immobilization when measured immediately after Daphnia were transferred into hypoxic medium, but resulted in a significant increase of T imm in 28 °C-acclimated Daphnia if they were allowed a 90-min acclimation to hypoxia at 28 °C (Fig. 4, Supplementary Table 5); no such effect was observed for 18 °C-acclimated Daphnia.
Thermal tolerance (T imm, natural log scale) in Daphnia acclimated to either 18 °C (blue) or 28 °C (red) and then either exposed to hypoxia (checkered bars) or not (controls, solid colors). Time to immobilization measured either immediately after transfer into hypoxic medium, or after a 90-min acclimation. Tukey test conducted separately for no delay and 90-min acclimation experiments (see Supplementary Table 5 for detailed statistical analysis). Total number of biological replicates (each consisting of a single individual) used in the no delay experiment and 90-min delay experiment: 271 and 97, respectively. (Color figure online)
Glutathione manipulations
Adding glutathione to the medium or disrupting glutathione synthesis by adding the BSO inhibitor to the medium predictably changed antioxidant properties of exposed Daphnia (Figs. 5, 6; Supplementary Tables 6, 7). Glutathione supplement has a little effect on lipid peroxidation, except for a roughly twofold decrease in juveniles (Fig. 5a; Tukey test P < 0.05), but, as expected, significantly increased samples’ TAC, particularly in Daphnia juveniles not exposed to 37 °C (Fig. 5c, d, Supplementary Table 6). BSO addition did not significantly modify LPO in either juveniles or adults (Fig. 6a, b; Supplementary Table 7) and did cause a significant reduction of TAC in juveniles not exposed to 37 °C (Fig. 6c).
Lipid peroxidation (a, b) total antioxidant capacity (c, d) in juvenile (a, c) and adult (b, d) Daphnia acclimated to 18 °C, treated with various concentrations of glutathione (GH) and either exposed to 37 °C for 60 min (orange) or not exposed (gray). Tukey test results shown only where there were different levels. Total number of biological replicates (each consisting of a single individual) used: 107 (a, c) and 96 (b, d). (Color figure online)
Lipid peroxidation (a, b) total antioxidant capacity (c, d) in juvenile (a, c) and adult (b, d) Daphnia acclimated to 28 °C, treated with various concentrations of glutathione synthesis inhibitor BSO and either exposed to 37 °C for 60 min (orange) or not exposed controls (gray). LPO was below detectible level in exposed adults in this experiment. For the juveniles (a, c), the Tukey comparisons shown for BSO = 0.1 represent all BSO treatment relative to the control (BSO = 0). Tukey test results shown only where there were different levels. Total number of biological replicates (each consisting of a single individual) used: 105 (a, c) and 95 (b, d). (Color figure online)
The observed changes in LPO or TAC resulted in no detectable change in thermal tolerance (Fig. 7). None of the comparison of GH or BSO level within each temperature had a statistically significant effect on T imm, except the difference between 0.1 and 0.2 mM BSO for juveniles (A). It should be noted that juveniles show much higher LPO (normalized to wet weight) and much lower normalized total antioxidant capacity than adults. Thus, no direct comparisons of juveniles to adults in glutathione manipulation experiments were possible.
Thermal tolerance (T imm) in juvenile (a) and adult (b) Daphnia acclimated to either 18 °C (blue) or 28 °C (red) and treated with various concentrations of glutathione (GH) or glutathione synthesis inhibitor BSO. Total number of biological replicates (each consisting of a single individual) used: 53 (a) and 50 (b). (Color figure online)
Lipidomics experiment
A summary of the data on phospholipid composition in Daphnia acclimated to either 18 or 28 °C with and without short-term (4 days) transfer to the reciprocal temperature (18−>28 °C, “up” and 28−>18 °C, “down” treatments) is shown on Fig. 8. There was little change in phospholipid class composition across the temperature treatments (Fig. 8a), except a mildly significant (P < 0.03; not significant after multiple test correction) change in phosphatidic acid content, appearing to be mostly, if at all, affected by the acclimation rather than current temperature. In contrast, there were significant shifts in the composition of side chains (Fig. 8b), in particular with respect to percentage of compounds with mono-unsaturated side chains and side chains with two double bonds. The share of phospholipids with polyunsaturated side chains (four or more double bonds) decreased in the 28 °C-acclimated Daphnia relative to the 18 °C-acclimated ones. Short-term temperature switches affected the share of phospholipids with 1 or 2 double bonds: it increased after a short-term transfer into a higher temperature (18 to >28 °C, “up”) and decreased after a short-term transfer into a lower temperature (28 to >18 °C, “down”). Phospholipids with three or more double bonds exhibited the opposite pattern: their share dropped after the transfer “up” and increased after the transfer “down”. Nearly all these changes are attributable to the recent (4 day) rather than the previous (long-term acclimation) temperature experienced by Daphnia. All tests for saturation classes (Fig. 8b) survive sequential Bonferroni correction with p value at least 0.05.
Summary of lipidomics experiment results. Phospholipid composition (% of total signal) in Daphnia acclimated to either 18 °C (blue) or 28 °C (red) and then either transferred to the reciprocal temperature (hatched) or not transferred (solid colors; cf. Fig. 2.). a Summarized by phospholipid type: PC phosphatiditcholines, PA phosphatidic acids, PE phosphatidylethanolamines, PG phosphatidylglycerols, PI phosphatidylinositols, PS phosphatidylserines, SM sphingomyelins. Note different scales for PCs and other compounds. b summarized by side chain saturation (number of double bonds in both side chains). *, **, and ***-1-was ANOVA among temperature treatments significance (before multiple test correction), P < 0.05, P < 0.01, and P < 0.0001, respectively. Tukey test results shown only where there were different levels. Total number of biological replicates (each consisting of a single individual) used: 20. (Color figure online)
Supplementary Fig. 5 shows the results for 24 individual compounds with a significant difference among temperature treatments showed with false discovery rate (FDR) <0.15. Nearly, all compounds in this category are PCs (phosphatidylcholines) with two PEs (phosphatidylethanolamines) and one PI (phosphatidylinositol). Most of phospholipids’ composition changes accumulated during long-term acclimation are quickly reversed by current temperature: most comparisons show a stronger effect of recent temperature after a short-term transfer than of the previous acclimation temperature. For some individual compounds, it appears that such reversal occurs quicker after the “down” switch than after an “up” switch [PC(36:0), PC(34,0), PC(38,1), Supplementary Fig. 5]. Fully saturated PE(30:0) shows the opposite pattern: the long-term acclimation differences are easier gained in an “up” switch than lost in the “down” switch. Finally, PE 38:2 shows a pure acclimation effect that is not reversed during a 4-day reciprocal switch (Supplementary Fig. 5, Supplementary Table 8).
The stronger effect of current rather than long-term acclimation temperature on phospholipid composition was further corroborated by principal component analysis (Fig. 9, Supplementary Table 9). This correlation was even stronger for the correlation-based PCA, where PC1 also showed a significant correlation with recent temperature experienced by Daphnia. Note that this correlation exists even in the absence of any significant differences in either PCs or PEs and SMs among treatments, indicating that it is individual phospholipid abundance, not those of combined classes that drive the principal component structure. PC2 (21% of variance) showed a significant leverage by current temperature, while long-term acclimation temperature correlated with PC3 (11% of the variance; Fig. 9, Supplementary Table 9). Finally, PC4 (9%) showed a weak correlation with the presence of microscopically visible ovaries (Supplementary Table 9), possibly indicating differences between somatic and reproductive tissues. PCA on correlations (Supplementary Table 9) showed a similar pattern with current temperature showing leverage on PCs explaining higher percentage of variance than long-term acclimation temperature.
Principal component analysis of phospholipid content in Daphnia acclimated to either 18 °C (blue) or 28 °C (red) and then either maintained at the same temperature (circles) or transferred to the reciprocal temperature (down-pointing triangles: “down” switch, upward-pointing triangles: “up” switch). Fill color represents acclimation temperature, outline color represents current temperature. a Score plot in the planes of the first 3 PCs. b Loading plot of membrane fluidity index (MFI) on PC1 (see Supplementary Table 9 for statistical details). Total number of biological replicates (each consisting of a single individual) used: 20. (Color figure online)
Fluorescence polarization experiment
The 28 °C-acclimated Daphnia showed a significantly slower rate of decrease of FP than those acclimated to 18 °C (Fig. 10a), consistent with the hypothesis of membrane remodeling aimed to reduce membrane fluidity at higher temperatures. Moreover, the slope of FP over measurement temperature estimated for each of the six clones, separately, showed a positive correlation with time until immobilization in 28 °C-acclimated Daphnia, but not in the 18 °C-acclimated ones (Fig. 10b).
Fluorescence polarization (FP) in non-nuclear membranes fraction from Daphnia acclimated to either 18 °C (blue) or 28 °C (red). a Slopes of normalized (FP at 18 °C set to 1) FP over measurement temperature. Heterogeneity of slopes: F = 12.5, P < 0.0005. b Correlations between clone-specific FP slope over measurement temperature and clones’ average time until immobilization. Regressions: 28 °C, F = 22.3, P < 0.01; 18 °C, F = 0.49, P < 0.5; heterogeneity of slopes, F = 9.91; P < 0.015. Total number of biological replicates (each consisting of a single individual) used: for clonal means of T imm—356; for clonal means of FP slopes—39. (Color figure online)
Discussion
Oxidative stress in general and lipid peroxidation (LPO) in particular has long been recognized as proximal causes of tissue damage in the pathogenesis of numerous respiratory and cardiovascular conditions (Mylonas and Kouretas 1999; Halliwell 2000; Zinellu et al. 2016). Yet, even in the medical literature, there has been a long-standing disagreement about the effects of oxidative damage intervention (for example through dietary antioxidants) on clinical outcomes (Halliwell 2000), largely due to uncertainties similar to those described above. Similar concerns, however, are not frequently voiced in the context of oxidative damage as a bioindicator of environmental stress, and antioxidant capacity and lipid peroxidation are routinely used as biomarkers of such stress in a variety of organisms (de Pinto et al. 2015; de Almeida et al. 2009; Libralato et al. 2016; Bhat et al. 2016).
There is little doubt that plastic response to elevated temperatures is correlated with increased resistance to oxidative damage (Abele et al. 1998; Suzuki and Mittler 2006; Monaghan et al. 2009; Bedulina et al. 2010; Lushchak et al. 2011; Zhou et al. 2011; de Pinto et al. 2015; Bae et al. 2016; Klumpen et al. 2016). While very well characterized in plants due to its importance for crops’ heat tolerance (Suzuki and Mittler 2006; de Pinto et al. 2015), the role of ROS scavenging in protecting cells and tissues at elevated temperatures is less well understood in animals. Directly relevant for the oxidative damage theory of aging (e.g., Zhou et al. 2011), antioxidant capacity is thought to also protect organisms from the elevated oxidative damage at high temperatures, but direct and unequivocal evidence of this is surprisingly scarce. In Daphnia, total antioxidant capacity (TAC) and/or levels of lipid peroxidation (LPO) are known to change with age (Barata et al. 2005a) and temperature (Becker et al. 2011; Bae et al. 2016) and interact with exposure to ROS-producing substances and other toxicants (Barata et al. 2005b; Bae et al. 2016). Thermal tolerance shows significant acclimation effects that are consistent with changes of antioxidant capacity (Zeis et al. 2009, 2013; Becker et al. 2011; Klumpen et al. 2016), including hemoglobin expression (Williams et al. 2011; Becker et al. 2011). Likewise, there is also evidence of local adaptation and rapid evolution in Daphnia thermal tolerance (Yampolsky et al. 2014; Geerts et al. 2015) that is mirrored by heritable changes in antioxidant defenses (Oexle et al. 2016). Thus, one can hypothesize that plastic and heritable changes in antioxidant capacity may be the basis of, respectively, plastic and selective response to environmental temperatures. The predictions for the levels of LPO are more complex. On one hand, one should expect LPO to increase with temperature due to an accelerated rate of free radical production. On the other hand, poikilothermic organisms often decrease polyunsaturated fatty acid (PUFA) content at higher temperatures (Hazel and Williams 1990; Martin-Creuzburg et al. 2012). Because PUFA is one of the principle targets of lipid peroxidation (Barata et al. 2005a; Gueraud et al. 2010), one should expect a decrease, not increase of LPO. Either way, more temperature-tolerant genotypes and/or physiological states should show lower LPO, either due to better antioxidant protection or to more radical changes in lipid composition eliminating LPO targets. Reduction of PUFA content at higher temperature may, therefore, have a twofold beneficial effect: reduction of membrane fluidity (Hazel and Williams 1990) and reduced production of toxic LPO products.
In contrast to these expectations, our results indicate that neither lipid peroxidation (LPO) nor abundance of major phospholipid species is good predictors of heat tolerance. We observed a higher, not lower LPO after 60-min exposure to the lethal 37 °C in the more temperature-tolerant 28 °C-acclimated Daphnia than in less temperature-tolerant 18 °C-acclimated ones (Fig. 1a). This is a robust effect, also observed in similar experiments using a more traditional TBARS-based LPO measurement (data not reported). What may be the reason for this paradoxical observation? As outlined above, LPO measured during exposure to a stressful environment may turn out to be higher in more tolerant than in less tolerant individuals, if it serves as a protective or signal mechanism. We hypothesize that LPO may play such a role in heat-acclimated Daphnia during exposure to lethally high temperature. The unexpected antioxidant role of polyunsaturated fatty acids, the likely LPO targets, has been proposed based on in-vivo and in-vitro experiments (Richard et al. 2008; Giordano and Visioli 2013), including both direct and regulatory mechanisms of PUFA effects on ROS (Giordano and Visioli 2013). Possibly, the changes in TAC we report here are manifestation of the organism’s response to the elevated levels of LPO playing a role of a signaling mechanism. Alternatively, elevated levels of LPO in 28 °C-acclimated Daphnia may be a by-product of higher hemoglobin expression, as reduction of heme-bound iron is known to drive lipid peroxidation (Sadrzadeh et al. 1984). Thus, one may hypothesize that 28 °C-acclimated Daphnia sustain a higher oxidative damage to lipids during lethal heat exposure as a cost of increased hemoglobin-mediated oxygen availability and antioxidant capacity that protect other oxidation targets more essential for survival at high temperature.
Indeed, the total antioxidant capacity (TAC) did show a consistent pattern of being higher in more heat tolerant organisms both in comparisons between acclimation temperatures (Figs. 1b, 2b) and between males vs. females (Fig. 3). The roles of antioxidants in increased thermal tolerance are indirectly corroborated by the observed increase in Timm in Daphnia exposed to mild hypoxia (Fig. 4) known to up-regulate antioxidant pathways, in particular the glutathione cycle (Becker et al. 2011; Klumpen et al. 2016). Consistently, this increase is observed in 28 °C-acclimated Daphnia but not in the 18 °C-acclimated ones and only after a 90 min exposure to hypoxic conditions, indicating an organismal response to hypoxia, not the hypoxic conditions themselves that had an effect on heat tolerance. However, our attempts to disrupt or mimic thermal acclimation by inhibiting the glutathione pathway or by supplementing Daphnia with reduced glutathione, altered TAC levels in the predicted manner (Figs. 5, 6), but failed to produce a corresponding change in thermal tolerance (Fig. 7). This means that either other, non-glutathione antioxidant pathways, such as thioredoxin and catalase pathways, play a role in tolerance to higher temperatures. These pathways, although linked to the glutathione pathway through common regulation (Gorrini et al. 2013), are not directly affected by glutathione concentration and manipulation of all of these pathways would be needed to unequivocally demonstrate the role of ROS scavenging in heat tolerance.
We, therefore, propose the following model of the interplay between LPO and temperature acclimation in Daphnia. In the absence of acute exposure to lethal temperature, LPO is maintained at some acceptable homeostatic level regardless of the acclimation temperature (Fig. 1, exposure = 0). During such acute exposure, the heat-acclimated Daphnia allows their LPO level to increase, providing antioxidant protection either directly by scavenging free radicals or indirectly by redirecting other antioxidants for protection of more essential oxidation targets. The homeostatic levels of LPO may be maintained by adjustment of unsaturated fatty acids abundance (Barata et al. 2005b; Schlechtriem et al. 2006). Indeed, LPO appears to change rapidly after a short-term reciprocal temperature switch (Fig. 2a), increasing in the 18 to >28 switched Daphnia and dropping below detection level in the 28 to >18 switched ones, possibly indicative of an imbalance between previous and current oxidative environments experienced by the organisms. Such opposing trends in LPO in short-term switches follow those observed in some of phospholipids with unsaturated side chains such as PC(38:5), PC(38:6), PC(38,4), and PC(37:2) (Supplementary Fig. 5). It is possible that a rapid increase of these targets for LPO in response to the change in temperature, followed by slow return to homeostatic level after a long-term acclimation, results in the observed spikes in LPO following short-term switches.
The lipidomics experiment indicated that currently experienced temperature (4-day reciprocal switch) has a greater impact on lipid composition than previously experienced long-term acclimation temperature (Figs. 8, 9; Supplementary Fig. 5). This quick loss of lipid composition memory is consistent with the intermediate levels of thermal tolerance in short-term switched Daphnia (Fig. 2a), but falls short of explaining the entire thermal tolerance difference between acclimation temperatures, since the reciprocal switches still retain some level of tolerance observed in their non-switch counterparts. However, we found two indications that phospholipid composition variation affected membrane fluidity in our experiments. First, there was a strong correlation (Fig. 9b) between the 1st principal component of phospholipid abundances (that explains 43% of variance) and phospholipid fluidity index, a composite index that is equal to the ratio between the abundance of PCs and combined abundance of PEs and SMs and that correlates well with membrane fluidity measured by fluorescence polarization (Fajardo et al. 2011). Second, and, perhaps, more importantly, the results of fluorescence polarization measurements (Fig. 10) strongly suggested that changes in membrane fluidity at higher temperatures (Hazel and Williams 1990) and thus, presumably, the underlying changes in phospholipid composition, may be a proximal cause for the increased thermal tolerance in 28 °C-acclimated Daphnia. Thus, lipid composition plasticity, possibly involving changes in some minor polyunsaturated phospholipid species, appears to play a dual role in Daphnia temperature tolerance: by adjusting LPO targets abundance on a shorter timescale and by modulating membrane fluidity on a longer timescale. These effects are not mutually exclusive with the possible role of changes in total antioxidant capacity between acclimated and non-acclimated individuals during an acute exposure to lethally high temperature, although the degree to which these mechanisms contribute to thermal acclimation is still unknown.
Data accessibility
All data necessary to recreate the analysis are available in Supplementary Materials and on http://www.datadryad.org.
Abbreviations
- BSO:
-
Buthionine sulfoximine
- CumHO:
-
Cumene hydroperoxide
- DPH:
-
1,6-diphenyl-hexa-1,3,5-triene
- DTT:
-
Dithiotreitol
- FDR:
-
False discovery rate
- FI:
-
Fluorescence intensity
- FP:
-
Fluorescence polarization
- GH:
-
Glutathione (reduced)
- LPO:
-
Lipid peroxidation
- TAC:
-
Total antioxidant capacity
- PA:
-
Phosphatidic acids
- PC:
-
Phosphatiditcholines
- PC[n]:
-
Principal component #n
- PCA:
-
Principal component analysis
- PE:
-
Phosphatidylethanolamines
- PFI:
-
Phospholipid fluidity index
- PG:
-
Phosphatidylglycerols
- PI:
-
Phosphatidylinositols
- PS:
-
Phosphatidylserines
- PUFA:
-
Polyunsaturated fatty acid
- ROS:
-
Reactive oxygen species
- SM:
-
Sphingomyelins
- T imm :
-
Time until immobilization
References
Abele D, Burlando B, Viarengo A, Pörtner H (1998) Exposure to elevated temperatures and hydrogen peroxide elicits oxidative stress and antioxidant response in the Antarctic intertidal limpet Nacella concinna. Comp Biochem Physiol Part B 120:425–435
Anderson ME (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111–112:1–14
Bae E, Samanta P, Yoo J, Jung J (2016) Effects of multigenerational exposure to elevated temperature on reproduction, oxidative stress, and Cu toxicity in Daphnia magna. Ecotoxicol Environ Saf 132:366–371
Barata C, Navarro JC, Varo I, Riva MC, Arun S, Porte C (2005a) Changes in antioxidant enzyme activities, fatty acid composition and lipid peroxidation in Daphnia magna during the aging process. Comp Biochem Physiol B Biochem Mol Biol 140:81–90
Barata C, Varo I, Navarro JC, Arun S, Porte C (2005b) Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comp Biochem Physiol C Toxicol Pharmacol 140:175–186
Becker D, Brinkmann B, Zeis B, Paul R (2011) Acute changes in temperature or oxygen availability induce ROS fluctuations in Daphnia magna linked with fluctuations of reduced and oxidized glutathione, catalase. Biol Cell 103:351–363
Bedulina DS, Zimmer M, Timofeyev MA (2010) Sub-littoral and supra-littoral amphipods respond differently to acute thermal stress. Comp. Biochem. Physiol B-Biochem Mol Biol 155:413–418
Beretta G, Aldini G, Facino RM, Russell RM, Krinsky NI, Yeum KJ (2006) Total antioxidant performance: a validated fluorescence assay for the measurement of plasma oxidizability. Anal Biochem 354:290–298
Bhat HA, Kaur T, Bhat R, Vyas D (2016) Physiological and biochemical plasticity of Lepidium latifolium as ‘sleeper weed’ in Western Himalayas. Physiol Plant 156:278–293. doi:10.1111/ppl.12362
Canale CI Henry PY (2010) Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic unpredictability. Clim Res 43:135–147. doi:10.3354/cr00897
Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S. (2013) Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radical Biol Med 65:978–987
de Almeida EA, Bainy ACD, Loureiro APM, Martinez GR, Miyamoto S, Onuki J e al (2009) Oxidative stress in Perna perna and other bivalves as indicators of environmental stress in the Brazilian marine environment: antioxidants, lipid peroxidation and DNA damage. Comp Biochem Physiol A-Mol Integr Physiol 146:588–600. doi:10.1016/j.cbpa.2006.02.040
de Pinto MC, Locato V, Paradiso A, De Gara L (2015) Role of redox homeostasis in thermo-tolerance under a climate change scenario. Ann Bot (Lond) 116:487–496
Drummen GP, van Liebergen LC, Op den Kamp JA, Post JA (2002) C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (Micro)Spectroscopic characterization and validation of methodology. Free Radic Biol Med 33:473–490
Fajardo VA, McMeekin L, LeBlanc PJ (2011) Influence of phospholipid species on membrane fluidity: a meta-analysis for a novel phospholipid fluidity index. J Membrane Biol 244:97–103
Farmer EE, Mueller MJ (2013) ROS-Mediated Lipid Peroxidation and RES-Activated Signaling. Annu Rev Plant Biol 64:429–450
Geerts AN, Vanoverbeke J, Vanschoenwinkel B, Van Doorslaer W, Feuchtmayr H, Atkinson D, Moss B, Davidson TA, Sayer CD, De Meester L (2015) Rapid evolution of thermal tolerance in the water flea Daphnia. Nat Clim Change 5:665–668
Giordano E, Visioli F (2013) Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fatty Acids (PLEFA) 90:1–4. doi:10.1016/j.plefa.2013.11.002
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12:931–947. doi:10.1038/nrd4002
Gueraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K (2010) Chemistry and biochemistry of lipid peroxidation products. Free Radical Res 44:1098–1124. doi:10.3109/10715762.2010.498477
Halliwell B (2000) Lipid peroxidation, antioxidants and cardiovascular disease: how should we move forward? Cardiovasc Res 47:410–418
Hazel JR, Williams E (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–277. doi:10.1016/0163-7827(90)90002-3
Hoffmann AA, Willi Y (2008) Detecting genetic responses to environmental change. Nat Rev Genet 9:421–432
Jolliffe IT (1986) Principal component analysis. Springer-Verlag. p. 487
Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L (1998) COMBO: A defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147–159
Klumpen E, Hoffschröer N, Zeis B, Gigengack U, Dohmen E, Paul RJ (2016) Reactive oxygen species (ROS) and the heat stress response of Daphnia pulex: ROS-mediated activation of hypoxia-inducible factor 1 (HIF-1) and heat shock factor 1 (HSF-1) and the clustered expression of stress genes. Biol Cell. In press, 2016, Aug 12. doi:10.1111/boc.201600017. (Epub ahead of print)
Kushnareva Y (2009) Membrane Fluidity Measurements Using UV Fluorescence Polarization and the POLARstar Omega. LabTech Application note. Available: http://www.bmglabtech.com/media/35216/1043850.pdf
Libralato G, Prato E, Migliore L, Cicero AM, Manfra L (2016) A review of toxicity testing protocols and endpoints with Artemia spp. Ecol Indic 69:35–49. doi:10.1016/j.ecolind.2016.04.017
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Martin-Creuzburg D, Wacker A, Ziese C, Kainz MJ (2012) Dietary lipid quality affects temperature-mediated reaction norms of a freshwater key herbivore. Oecologia 168:901–912. doi:10.1007/s00442-011-2155-1
Maulucci G, Daniel B, Cohen O, Avrahami Y, Sasson S (2016) Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells. Mol Aspects Med 49:49–77
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92
Morris M, Rogers SM. (2014) Integrating phenotypic plasticity within an ecological genomics framework: Recent insights from the genomics, evolution, ecology, and fitness of plasticity. In: Landry CR, Aubin-Horth N, eds. Ecological Genomics: Ecology And The Evolution Of Genes And Genomes. Advances In Experimental Medicine And Biology 781:73–105
Mylonas C, Kouretas D (1999) Lipid peroxidation and tissue damage. In Vivo 13:295–309
Oexle S, Jansen M, Pauwels K, Sommaruga R, De Meester L, Stoks R (2016) Rapid evolution of antioxidant defence in a natural population of Daphnia magna. J Evol Biol 29:1328–1337. doi:10.1111/jeb.12873
Oomen RA, Hutchings JA (2015) Genetic variability in reaction norms in fishes. Environ Rev 23:353–366. doi:10.1139/Z86-007 10.1139/er-2014-0077
Pap EH, Drummen GP, Winter VJ, Kooij TW, Rijken P, Wirtz K.W, Op den Kamp JA, Hage WJ, Post JA (1999) Ratio-fluorescence microscopy of lipid oxidation in living cells using C11-BODIPY(581/591). Febs Lett 453:278–282
Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP (2010) Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol 25:459–467. doi:10.1016/j.tree.2010.05.006
Podosinovikova NP, Petrov VV, Dolgo-Saburov VB (2005) Daphnia magna Straus: a new model for evaluating the antioxidant action of water-soluble preparations in vivo. Exper Clin Pharmacol 68:68–70 (Russian)
Richard D, Kefi K, Barbe U, Bausero P, Visioli F (2008) Polyunsaturated fatty acids as antioxidants. Pharmacol Res 57:451–455. doi:10.1016/j.phrs.2008.05.002
Rodriguez-Martinez MA, Ruiz-Torres A (1992) Homeostasis between lipid peroxidation and antioxidant enzyme activities in healthy human aging. Mech Ageing Dev 66:213–222
Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton JW (1984) Hemoglobin. A biologic fenton reagent. J Biol Chem 259:14354–14356
SAS Institute (2012) JMP Statistical software. SAS Institute, Inc, Cary
Schlechtriem C, Arts MT, Zellmer ID (2006) Effect of temperature on the fatty acid composition and temporal trajectories of fatty acids in fasting Daphnia pulex (Crustacea, Cladocera). Lipids 41:397–400
Sgro CM, Terblanche JS, Hoffmann AA (2016) What can plasticity contribute to insect responses to climate change? Annu Rev Entomol 61:433–451. doi:10.1146/annurev-ento-010715-023859
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
van Kleunen M, Fischer M (2005) Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol 166:49–60
Williams PJ, Dick KB, Yampolsky LY (2011) Heat tolerance, temperature acclimation, acute oxidative damage and canalization of haemoglobin expression in Daphnia. Evol Ecol 26:591–609
Yampolsky LY, Glazko GV, Fry JD (2012) Evolution of gene expression and expression plasticity in long-term experimental populations of maintained under constant and variable ethanol stress. Mol Ecol 21(17):4287–4299
Yampolsky LY, Schaer TMM, Ebert D (2014) Adaptive phenotypic plasticity and local adaptation for temperature tolerance in freshwater zooplankton. Proc R Soc Ser B Biol Sci 281:20132744
Yoshida Y, Shimakawa S, Itoh N, Niki E (2003) Action of DCFH and BODIPY as a probe for radical oxidation in hydrophilic and lipophilic domain. Free Radic Res 8:861–872
Zeis B, Lamkemeyer T, Paul RJ, Nunes F, Schwerin S, Koch M, Schütz W, Madlung J, Fladerer C, Pirow R (2009) Acclimatory responses of the Daphnia pulex proteome to environmental changes. I. Chronic exposure to hypoxia affects the oxygen transport system and carbohydrate metabolism. BMC Physiol 9:7
Zeis B, Becker D, Gerke P, Koch M, Paul RJ (2013) Hypoxia-inducible haemoglobins of Daphnia pulex and their role in the response to acute and chronic temperature increase. Biochim Biophys Acta 1834:1704–1710.
Zhou KI, Pincus Z, Slack FJ (2011) Longevity and stress in Caenorhabditis elegans. Aging-US 3:733–753
Zinellu E, Zinellu A, Fois AG, Carru C, Pirina P (2016) Circulating biomarkers of oxidative stress in chronic obstructive pulmonary disease: a systematic review. Respir Res 17:150. doi:10.1186/s12931-016-0471-z
Acknowledgements
We are grateful to Dhirendra Kumar for equipment use, to Anthony C. Tharp and Irina Kaverina for assistance with fluorescence polarization measurements and to Joe Bidwell, Dieter Ebert, Karl Joplin, Aruna Kilaru, Dominik Martin-Creuzburg, and Roberto Arbore for many useful suggestions. This work was partly supported by NSF DEB-1136706 to LYY and ETSU Student-Faculty Collaboration grants to BLC and JWC.
Author contributions
LYY designed the study, participated in the experimental work, analyzed the data, and wrote the manuscript. BLC, JC, and KJH did the experimental work and participated in data analysis and writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
B. L. Coggins, J. W. Collins, and K. J. Holbrook contributed equally to the work and are listed alphabetically.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coggins, B.L., Collins, J.W., Holbrook, K.J. et al. Antioxidant capacity, lipid peroxidation, and lipid composition changes during long-term and short-term thermal acclimation in Daphnia . J Comp Physiol B 187, 1091–1106 (2017). https://doi.org/10.1007/s00360-017-1090-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1090-9