Abstract
Environmental light conditions are of general importance in predator–prey interactions. In aquatic systems, prey individuals experience different levels of predation risk depending on the properties of the visual environment, such as structural complexity or water transparency. To reduce the threat of predation, prey should move to habitats providing better protection against visual predators. We studied the role of UV wavelengths in habitat choice behaviour under predation risk in a fish, the three-spined stickleback (Gasterosteus aculeatus) that uses UV signals in different contexts of intraspecific communication. In a laboratory experiment sticklebacks were exposed to a predatory threat and given the choice between two escape habitats, one providing full-spectrum conditions including UV light (UV+) and one without UV wavelengths (UV−). Fish from two rearing treatments were tested, one group had been raised under natural lighting conditions (UV+), the other group under UV-deficient lighting conditions (UV−). Sticklebacks from the UV+ group preferred the UV− habitat as a refuge which suggests that predator avoidance behaviour is UV-related in this species with UV− conditions presumably being advantageous for prey fish. However, individuals from the UV− treatment group were equally attracted to both presented light habitats. It is possible that these fish could not discriminate between the two light habitats due to physiological limitations caused by their rearing conditions. Further control trials with neutral-density filters revealed that the UV− habitat preference of UV+ fish in the main experiment was rather not influenced by a difference in achromatic brightness between the UV+ and UV− habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of vision in aquatic systems is greatly influenced by constraints of environmental light. For instance, ambient light conditions are of crucial importance in behavioural interactions such as predator–prey encounters (Emery 1973; Hobson 1979). In fishes, prey individuals experience different levels of predation risk depending on their visual environment, which is especially affected by water transparency or structural complexity (Crowder and Cooper 1982; Miner and Stein 1996). Thus, one tactic to reduce predation risk in prey fish is to choose habitats according to favourable visual conditions. For example, Snickars et al. (2004) showed that juvenile perch (Perca fluviatilis L.) when exposed to predation preferred structured instead of open water habitats and Skov et al. (2007) found that an increase in turbidity leads to an increased use of vegetated habitats in the same species. Numerous further studies described the relationship between structural refuges, water transparency and anti-predator behaviour (Werner et al. 1983; Gregory and Levings 1996; Mattila 1992; Macia et al. 2003), whereas direct evidence of habitat preferences based on wavelength-dependent properties of the light environment is lacking in fishes. However, spectral composition of light in aquatic habitats underlies strong variation due to the combination of water depth and the relative abundance of dissolved and suspended matter (Lythgoe 1972). For example, the penetration of short-wave light (UV-A: 300–400 nm), which many fishes can perceive, is attenuated with depth caused by a stronger absorbance and scattering of downwelling light (Losey et al. 1999). In communication, higher scattering and an increased optical path of UV wavelengths compared to longer wavelengths allow effective signalling of UV signals only at close range. Nevertheless, in many aquatic habitats the amount of UV at moderate depths lies above the visual threshold of most UV-sensitive fishes (Losey et al. 1999) and UV wavelengths are of particular recent interest in studies on visual signalling in fishes (i.e. Smith et al. 2002; Cummings et al. 2003; Losey 2003; Siebeck 2004; Boulcott and Braithwaite 2005; Modarressie et al. 2006; Rick et al. 2006).
We studied the influence of UV wavelengths on habitat preferences under predation risk in the three-spined stickleback (Gasterosteus aculeatus), a species using UV wavelengths in intraspecific signalling during female mate choice (Boulcott and Braithwaite 2005; Rick et al. 2006; Rick and Bakker 2008a), male mate choice (Rick and Bakker 2008b), male–male interactions (Rick and Bakker 2008c) as well as shoaling decisions (Modarressie et al. 2006). In a dichotomous choice experiment, we investigated whether the presence of a potential predator affects the choice of solitary sticklebacks when offered two escape habitats, one providing full-spectrum light conditions including UV (UV+) and one with UV wavelengths being blocked (UV−). Moreover, to test whether previous rearing conditions had an effect on habitat selection in sticklebacks we compared the choice behaviour of individuals that had been raised under natural lighting conditions (UV+) with those raised under artificial lighting conditions without UV light (UV−).
Methods
Three-spined sticklebacks used in the experiment were laboratory-bred first generation offspring of anadromous fish that had been caught during spring migration in April 2005 on the island of Texel, The Netherlands. In March 2006, clutches from random mating pairs of wild-caught fish were taken out from their nests after fertilisation. Subsequently, eggs from the same nest were split into two equally-sized sub-groups of maximally 40 eggs. Eggs were placed in small one litre plastic aquaria with air ventilation. One sub-group was maintained under full-spectrum light conditions (UV+) by covering the holding aquaria with a UV− and visible light-transmitting Plexiglas partition (GS-2458, Röhm, Darmstadt, Germany), whereas the other sub-group was kept under UV-deficient conditions (UV−) by using an UV-opaque Plexiglas partition (GS-233, Röhm, Darmstadt, Germany, Fig. 1). Thus, two light habitats were created differing in their spectral transmission of UV wavelengths as well as overall brightness (Fig. 2a). Full-spectrum illumination including UV wavelengths was provided by fluorescent tubes (True Light, Natural Daylight 5500, 36 W, 1,200 mm) that produce a proportion of UV similar to natural skylight and were hanging 30 cm above the water surface. Fish were kept in these aquaria for up to 4 weeks after hatching. Then they were moved into bigger group aquaria (40 × 20 × 25 cm, 40 l), while light conditions were left unchanged. Visual contact between groups was prevented by placing opaque partitions between holding aquaria. The fish were kept at standardized summer conditions (daylength 16L:8D, temperature 17 ± 2°C) until the age of 6 months. Thereafter light settings were switched to winter conditions (daylength 10L:14D). Juvenile fish were fed with Artemia nauplii and subadult fish with frozen chironomids ad libitum. All aquaria were aerated and cleaned regularly to allow sufficient light penetration.
The predators, four live perch (Perca fluviatilis) caught from the river Rhine, were maintained in the laboratory for 2 years in two large holding aquaria (100 × 40 × 40 cm, 160 l) under standardized summer conditions (daylength 16L:8D, temperature 17 ± 2°C). Illumination was provided by fluorescent tubes (True Light, Natural Daylight 5500, 36 W, 1,200 mm). The perches had a mean standard length of 16 cm. They were fed with frozen chironomids ad libitum. Two days before being used in an experiment, feeding was stopped to enhance motivation to attack prey during the experimental trials.
In November 2006, 26 subadult sticklebacks (13 per light treatment) from two different families for the UV+ treatment and three different families for the UV− treatment with a standard length of 2.3 ± 0.2 cm were removed from their group aquaria and isolated into single aquaria (30 × 20 × 20 cm, 12 l). Fish from only five families were chosen for the experimental trials because these individuals fulfilled the criteria of similar body length, age, and group size in their former holding tanks. Illumination of the single aquaria conformed to the previous holding conditions. Opaque partitions between aquaria prevented visual contact between individuals. Before being used in the choice experiment, fish were held in their single aquaria for at least 2 days.
The test aquarium designated to test light habitat preferences consisted of a 128-l aquarium (80 × 40 × 40 cm) divided into two compartments, one holding the test individual, the other the stimulus predator (Fig. 3). The uncovered predator compartment was divided from the test-fish compartment by UV-transparent Plexiglas (GS-2458), so that the test fish could see the predator in full-spectrum light. The waterproof divider between compartments prevented olfactory influences on predator–prey interactions. Although olfactory stimuli are important cues involved in predator avoidance behaviour in fish (Wisenden 2000) we solely focused on visual cues and excluded further confounding variables. One lateral half of the test-fish compartment was covered with UV-transparent Plexiglas (GS-2458, Röhm, Darmstadt, Germany) provided with a layer of a UV-blocking filter (Lee Filter No. 229), whereas the other half was only covered by UV-transparent Plexiglas (GS-2458, Fig. 1). Thus, analogous to the rearing conditions two light habitats were created that differed in their spectral UV content as well as in achromatic brightness (Fig. 2b). On top of the test fish section between the two filters, a narrow opaque plastic plate (5 × 40 cm) was placed in order to minimize the area of mixed light in the central test-fish compartment. Up to a height of 18 cm the inner walls of the whole test-fish compartment were laminated with grey insulating tape (fix-o-moll Powerband), which is highly reflective over the UV and visible wave range, and covered by an additional layer of UV-transparent Plexiglas (GS2458). The whole set-up was illuminated by a fluorescent tube (True Light, Natural Daylight 5500, 36 W, 1,200 mm) positioned 38 cm above the water surface. The test aquarium was surrounded by opaque grey plastic partitions up to a height of 30 cm. A black curtain encased the whole set-up. The aquarium was placed on a polystyrene plate on which lines marked two zones in the test-fish compartment. One zone was located close (27–45 cm) to the predator compartment and one at a greater distance (45–63 cm). A small electric pump was installed in the predator compartment to create a constant water flow that should stimulate swimming activity in the perch predator.
Transmission spectra of the optical filters used for the two different rearing conditions and the experimental setup: ultraviolet-transmitting filter (UV+, thin black), ultraviolet-blocking filter for the rearing tanks (UV−, thick grey), ultraviolet-blocking filter for the main experiment (UV−, thin grey) and fullspectrum neutral density filters for the control experiment (ND1, broken black; and ND2, broken grey). Spectra were taken from Rick et al. (2006) except for the ultraviolet-blocking filter (UV−) used in the main experiment. Measurements were taken with an Avantes AVS-USB2000 spectrophotometer and an Avantes DH-2000 deuterium–halogen lightsource. Transmission was determined by measuring reflectance relative to a 98% white standard with the reflection probe attached at a 90° angle to the filter located on the white reference
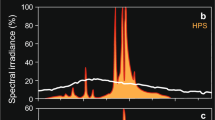
Irradiance measurements of the lighting conditions for the two different rearing treatments and the experimental setup: (a) rearing conditions (UV+, black; UV−, grey), (b) main experiment (UV+, black; UV−, grey) and (c) control experiment (ND1, black; ND2, grey). In a, b the irradiance between 400 and 700 nm is virtually identical for both filters (UV+, UV−) whereas the amount of UV between 300 and 400 nm considerably differs. In c the difference in achromatic brightness transmission between the ND1 and ND2 filter is reflected in a consistent difference in achromatic brightness for the wavelength range between 300 and 700 nm. Irradiance was measured with an Avantes AVS-USB2000 spectrophotometer connected to an Avantes CC-UV/VIS cosine corrector located in the tank and centered under the relevant optical filter. Irradiance calibration was performed versus an Avantes NIST traceable irradiance application standard
Plan view of the experimental set-up which consisted of a divided rectangular aquarium: (A) Stimulus compartment, which held the perch predator; (B) test fish compartment; (C) UV transparent partition dividing both compartments; (shaded areas) UV transparent (bright) and UV blocking filters (dark) covering the test fish compartment; (dashed lines) borders of the two zones differing in distance to the predator; (black area) opaque plastic plate dividing light habitats
Prior to the start of each experimental trial, the perch was transferred to its compartment. Then a test fish was enclosed in a small rectangular plastic box (7.5 × 5 × 18 cm) with open top and bottom sides, which was located in the test-fish compartment 5 cm in front of the predator compartment. The side of the box facing away from the predator consisted of UV-transparent Plexiglas (GS2458), whereas the other three sides were made of opaque grey plastic. During a 15 min acclimation phase, the test fish could observe the two light environments without recognizing the presence of a predator. The subsequent test phase was started by lifting the box by a string from behind the curtain, so that the fish could now perceive the predator and freely move in the test-fish compartment. After 15 min, the experimental trial was stopped and both fish were returned to their holding tanks. In the following trial, test fish and predator were replaced by new individuals and the experimental procedure was repeated, but with exchanged optical filters over the test-fish compartment. Test fish were only used once and each predator was only used once a day in order to minimize stress caused by excessive handling. We replaced tank water after each trial. All trials were done in normal daylight hours.
Stickleback behaviour was recorded from above with a webcam (Creative CT6840, Creative Labs, Dublin, Ireland). Filmed trials were analysed without knowledge of filter positions. The time a stickleback spent in the UV+ and UV− environment during the 15 min test phase was measured as well as the time spent in the two zones at different distances from the predator. Filming from above did not allow for recording an individual’s position under the narrow opaque plastic plate between the two filter sections. Furthermore, observation of the zone close to the predator (0–27 cm) revealed that fish from both treatment groups only spent short time in this zone with an average time of 30.0 s (range = 0–54 s). Hence, movements into this zone were regarded as predator inspection behaviour and were not included in the analyses. Filming did also not allow to record predator behaviour. Nevertheless, it can be assumed that predators represented a considerable threat during all trials which became evident by an increased swimming activity and attack behaviour (I. P. Rick, personal observation).
As the two filters differed in quantal flux (UV+ to UV−: mean 21% reduction; Fig. 1), we performed an additional control experiment with neutral density filters ND1 (Lee 209, Zilz, Germany) and ND2 (Cotech 298, Zilz, Germany) instead of the UV+ and UV− filters to determine whether sticklebacks’ habitat selection was rather based on the wavelength composition of the two light environments instead of brightness differences. Neutral density filters differed in quantal flux uniformly between 300 and 700 nm without altering spectral composition. The difference in quantitative transmission between the two filters (ND1 and ND2) was 34% for the waveband between 300 and 700 nm (Rick et al. 2006) and the proportional quantitative transmission relative to the UV+ filter was 44% for the ND1 filter and 68% for the ND2 filter, respectively (Fig. 1). Hence, both ND conditions were darker than the fullspectrum lighting conditions under which the predator stimulus appeared (Fig. 2b, c).
The control trials were carried out immediately after the main experiment. Twenty new fish (ten per light treatment) were chosen to ensure that individuals were not familiar with the experimental conditions particularly with regard to the predator stimulus. Fish originated from two different families for the UV+ treatment group and three different families for the UV− group so that, analogous to the main experiment, similarity in body size, age and group size during former holding conditions between the test individuals was provided. The control experiment was performed and analysed as described for the main experiment. Due to the perceivable difference in brightness between the ND1 and ND2 filters, films could not be analysed without knowledge of filter position.
Statistics
Before statistical analyses, we tested the relative amount of the time prey fish spent in various parts of the test-fish compartment for normality using the Kolmogorov–Smirnov test. Normally distributed data were tested with parametric statistical tests; otherwise nonparametric statistics were used. We used a paired t test to analyse the relative amount of time UV+ and UV− fish spent in the UV+ and UV− habitats as well as the relative amount of time UV− fish spent in the ND1 and ND2 habitats during the control experiment, respectively. The relative amount of time UV+ fish spent in the ND1 and ND2 habitats was analysed using a Wilcoxon matched-pairs signed-ranks test. Treatment groups were compared using an independent samples t test for the UV experiment, whereas a Mann–Whitney U test was used for the control experiment. We used parametric t tests and, if required, Wilcoxon matched-pairs signed-ranks tests to analyse the relative amount of time spent in the two zones at different distance from the predator. Because multiple individuals per family were tested during the experimental trials and in order to minimize the risk of pseudoreplication, we introduced family identity as a random factor and performed linear mixed models (LME) implemented in R 2.6.1 statistical package (R Development Core Team 2007) on the difference in the relative amount of time the test fish spent in each light habitat. Tests of significance were based on likelihood-ratio tests (“LRT”) that follow a χ 2-distribution. To approximate normality, we had to transform data for the control experiment using an exponential transformation. The test-probabilities are two-tailed throughout. Family identity had no significant influence on habitat choice behaviour of both treatment groups in the UV experiment as well as in the control experiment (LRT, all χ 2 < 1.196, all P > 0.274).
Results
When exposed to a predatory threat, UV+ fish significantly preferred the UV− habitat (mean ± SD = 63.9 ± 16.9%) over the UV+ habitat as a refuge (mean ± SD = 36.1 ± 16.9%; paired t test: t 11 = −2.853, P = 0.016; Fig. 4). The relative time UV− fish spent in the UV+ (mean ± SD = 49.5 ± 15.7%) or the UV− habitat (mean ± SD = 50.5 ± 15.7%) did not differ significantly (paired t test: t 12 = − 0.123, P = 0.904; Fig. 4) with the UV+ filter being located seven times on the left side and six times on the right side of the experimental setup. When comparing both treatment groups, habitat preference of UV+ fish was significantly different from that of UV− fish (independent samples t test: t = −2.091, df = 23, P = 0.048; Fig. 4).
Mean relative time ± SD spent by 12 individuals from the UV+ treatment group (grey bars) and 13 individuals from the UV− treatment group (white bars) within the light habitat under each filter during the test phase of the main experiment. The filters blocked (UV−) or transmitted (UV+) UV radiation between 300 and 400 nm. *P < 0.05
In the UV+ habitat fish from the UV+ as well as the UV− treatment group spent a significantly larger proportion of time in the zone more distant from the predator (UV+ group: mean ± SD = 77.9 ± 15.0%; UV− group: mean ± SD = 76.4 ± 15.6%) than in the zone closer to it (UV+ group: mean ± SD = 22.1 ± 15.0%; paired t test: t 11 = −6.437, P < 0.001; UV− group: mean ± SD = 23.6 ± 15.6%; paired t test: t 12 = −6.104, P < 0.001). In addition, under the UV− filter fish from both treatment groups spent a significantly longer time more distant from the predator (UV+ group: mean ± SD = 66.7 ± 20.5%; UV− group: mean ± SD = 70.1 ± 18.9%) than closer to it (UV+ group: mean ± SD = 33.3 ± 20.5%; paired t test: t 11 = −2.807, P = 0.017; UV− group: mean ± SD = 29.9 ± 18.9%; paired t test: t 12 = −3.842, P = 0.002). Treatment groups did not significantly differ in this respect for the UV+ side (independent samples t test: t = −0.220, df = 23, P = 0.828) and the UV− side (independent samples t test: t = 0.437, df = 23, P = 0.666).
In the control experiment, fish from the UV+ treatment group did not spent relatively more time in the darker (ND1) (median = 50.8%; inter-quartile range = 45.9–52.0%) than in the brighter habitat (ND2) (median = 49.2%; inter-quartile range = 48.0–54.1%; Wilcoxon matched-pairs signed-ranks test: z = −0.153, N = 10, P = 0.878). UV− fish spent relatively more time, although not significantly so, in the brighter habitat (mean ± SD = 68.8 ± 33.1%) than in the darker one (mean ± SD = 31.2 ± 33.1%; paired t test: t 9 = 1.801, P = 0.105). Treatment groups did not significantly differ in their habitat preference in the control experiment (Mann–Whitney U test: N 1 = 10, N 2 = 10, z = −1.817, P = 0.106).
Under the brighter ND condition fish from the UV− treatment group spent a significantly larger proportion of time in the zone more distant from the predator (median = 98.3%; inter-quartile range = 92.7–100%) than closer to it (median = 1.7%; inter-quartile range = 0–7.2%; Wilcoxon matched-pairs signed-ranks test: z = −2.821, N = 10; P = 0.005) whereas fish from the UV+ treatment did not stay significantly longer in the more distant zone (median = 70.2%; inter-quartile range = 62.4–80.4%) than closer to the predator (median = 29.8%; inter-quartile range = 19.6–37.6%; Wilcoxon matched-pairs signed-ranks test: z = −0.773, N = 9; P = 0.440). In the darker ND habitat fish from both treatment groups did not stay significantly longer in either the more distant zone (UV+ group: mean ± SD = 58.3 ± 27.9%; UV− group: mean ± SD = 62.3 ± 33.8%) or the zone closer to the predator (UV+ group: 41.7 ± 27.9%; paired t test: t 8 = −0.939, P = 0.372; UV− group: mean ± SD = 37.7 ± 33.8%; paired t test: t 5 = 0.966, P = 0.371). When comparing both treatment groups for the brighter ND condition UV− fish spent a significantly greater proportion of time in the zone more distant from the predator than UV+ fish (Mann–Whitney U test: N 1 = 9, N 2 = 10, z = −2.308, P = 0.021). None of the treatment groups did significantly differ in this respect for the darker ND condition (independent samples t test: t = 0.272, df = 15, P = 0.789).
Discussion
The results of the UV experiment showed that individual sticklebacks raised under full-spectrum light had a preference for UV− over UV+ lighting conditions in the presence of a predatory threat. Thus, we can conclude that habitat selection in the three-spined stickleback is affected by the amount of available UV radiation. Other studies on the impact of UV wavelengths on stickleback habitat preferences yielded different results although the experimental light conditions were rather similar to the ones in the present study. For instance, Modarressie et al. (2006) did not find a significant preference for light conditions either including or lacking UV wavelengths in non-reproductive adult sticklebacks from a local freshwater population. In addition, reproductively active stickleback males from the same population chose nest sites independent of the portion of environmental UV light (Modarressie and Bakker 2006). The discrepancy between these and the present study may be due to differences between populations with regard to the use of UV wavelengths in visual tasks. Moreover, stickleback lighting preferences may be context dependent. Fish in the present study experienced habitat differences and the simultaneous presence of a predator, whereas in the former studies only a general habitat preference was tested. However, in order to better rule out that sticklebacks in the present study chose light habitats independent of the presence of a predator further control experiments without a predator stimulus would be helpful.
Sticklebacks prefer conspecifics viewed in UV+ instead of UV− conditions (Modarressie et al. 2006; Rick et al. 2006; Rick and Bakker 2008b, c). Accordingly, when choosing mates, some birds also prefer UV-containing light environments, which was demonstrated for European starlings (Sturnus vulgaris) and blue tits (Parus caeruleus) (Hunt et al. 1999; Maddocks et al. 2002). Without a social context, Maddocks et al. (2002) found no preference of individuals of both species for environments either with or without UV wavelengths. Thus, a preference for certain UV lighting conditions might also be context-specific in such a way that individuals or groups involved in intraspecific interactions exhibit preferences that are different or absent in solitary individuals.
The test fish used in our experiment were raised under predator-free conditions in the laboratory and thus were confronted with a live predator for the first time during the experimental trials. Nevertheless, sticklebacks preferred to stay at a greater distance from the predator suggesting an unlearned response to recognize that the visual appearance of the predator poses a substantial threat.
Which benefit may a prey individual have from preferring a refuge providing UV-deficient light conditions instead of full-spectrum light? On the one hand, the test fish may have been attracted to shelter in the UV− condition, because it chose the light environment that was different from that of the predator compartment. Alternatively, the UV− light habitat could simulate increased water depth which may be preferred by the test fish to escape predation. However, although this may represent a strategy to escape terrestrial predators (Power 1984) it seems rather unlikely with regard to fish predators which often inhabit less shallow waters (Crowder and Cooper 1982) so that predation risk should be enhanced in deeper water.
In general, the visibility of visual signals depends on the ambient light conditions and the contrast they generate against their natural background (Endler 1993; Vorobyev and Osorio 1998). According to Lythgoe (1979) colour and brightness contrast against a visual background are reduced with distance in the UV due to stronger absorption and veiling effects compared to longer wavelengths. Consequently, prey fish should prefer UV-rich environments as a refuge which contradicts the preference for UV-deficient lighting conditions found in the present study. But, when considering the rather short signalling distance in our experiment, the UV+ lighting conditions combined with the artificial background of intense broadband reflectance may have produced an increased overall background contrast for the prey fish (Losey et al. 1999). Thus, sticklebacks may have preferred the UV− habitat as it may provide better background matching and thereby reduced discriminability for potential receivers. However, to make more safe assumptions about how visual signalling occurs in a predator–prey interaction such as the one presented here a further step should be to measure reflectance properties of the involved visual signals and the background as well as the predator’s perceptual capacities (Endler 1992) in order to calculate the conspicuousness of prey individuals against the visual background under different lighting conditions (see Hastad et al. 2005).
It is important to note that the fish predator used in our experiment is not capable of UV vision since only juvenile perch possess UV cones during the planktivorous foraging mode which disappear when they become benthivorous (reviewed in Bowmaker 1990). Hence, both experimental lighting conditions as well as prey fish appearances in the main experiment may have looked identical from the predator’s perspective which on the other hand largely rules out that predator behaviour had a direct effect on habitat choice decisions of the test fish. The perch predator should rather exclusively represent a threat stimulus in order to induce avoidance behaviour in the experimental prey fish.
In contrast to fish from the UV+ treatment group, UV− fish did not significantly prefer either light habitat in the choice experiment. Nevertheless, UV− fish also showed predator avoidance behaviour in that they preferably stayed at a greater distance from the predator. Hence, our results demonstrate that rearing conditions with respect to the spectral composition of environmental light affected habitat preferences under predation risk. In contrast to this, Greenwood et al. (2002) found in starlings that UV-deficient lighting conditions during ontogenesis did not affect light habitat preferences compared to individuals raised in full-spectrum light suggesting that the preferences existed regardless of whether birds were familiar with the provided light conditions or not. Sticklebacks in our experiment also did not choose their habitat according to familiarity.
Physiological constraints may rather be responsible for the difference in behaviour between the two rearing groups. For instance, it cannot be ruled out that, compared to UV+ fish, UV− fish were not able to sufficiently discriminate between the two presented light habitats due to a lack in UV sensitivity caused by the UV-deficient rearing conditions. McDonald and Hawryshyn (1995) observed visual perception in three-spined sticklebacks using compound action potentials from ganglion cells and found spectral sensitivity to vary between populations from habitats with differing spectral light compositions. Additionally, when comparing bluefin killifish populations from different habitats, Fuller et al. (2004) found cone opsin expression to be related to variation in the frequency of cone classes. More recently, Shand et al. (2008) showed that the rearing environment of fish may affect adult vision as well. They compared individuals of black bream (Acanthopagrus butcheri) reared under fullspectrum aquarium conditions with individuals reared under short wavelength-reduced conditions and found an increased proportion of long wavelength-sensitive double cones in the latter one which indicates a response to changes in environmental light by altering opsin expression. In contrast, in a cichlid species, the blue acara (Aequidens pulcher), a reduction in the frequency of the blue-sensitive single cone class was found as an effect of rearing under monochromatic blue light (Kröger et al. 1999). Rearing under differing lighting conditions also had a considerable impact on regulatory mechanisms at higher levels of visual processing (Kröger et al. 2001a) combined with behaviour (Kröger et al. 2003). In a further study on Astatotilapia burtoni, another cichlid species, spectral rearing conditions had an effect on the properties of the lens optics as well (Kröger et al. 2001b). Whether similar mechanisms of plasticity can be assumed for the visual system of sticklebacks raised under conditions varying in UV content requires further investigation.
The results of the control experiment showed that sticklebacks from the UV+ rearing group did not significantly prefer either the darker (ND2) or brighter (ND1) full-spectrum conditions. Thus, in the UV experiment, these fish may rather have discriminated between the two light habitats based on the differences in wavelength composition instead of achromatic brightness differences. This is in accordance to data on UV and female as well as male visual preferences (Rick et al. 2006; Rick and Bakker 2008b). However, both ND lighting conditions chosen in the present study were considerably darker than the predator environment illuminated by fullspectrum light so that they could have provided equal visual protection for the test fish. Consequently, the results from our control experiment cannot completely rule out that the UV+ fish preferred the UV− environment in the main experiment due to a preference for darker fullspectrum conditions.
The treatment groups in the control experiment differed, even though not significantly so, in their habitat preference with UV− fish tending to show a stronger association to the brighter light habitat. Additionally, UV− fish spent significantly more time at a greater distance from the predator than UV+ fish. UV+ fish did not stay significantly longer more distant from the predator than closer to it, which raises the question to what extent predator-avoidance behaviour occurred in the experimental trials with UV+ fish. In summary, the results from the control experiment cannot be interpreted sufficiently which is also based on a lack of information on stickleback achromatic processing and due to the rather low sample size.
In a previous study on the effects of predation on habitat selection in sticklebacks, fish moved from the open water into the littoral zone when exposed to increased predation (Jakobsen et al. 1988). Accordingly, Ibrahim and Huntingford (1989) showed that non-breeding males prefer to forage in weed beds when suffering predation risk. Furthermore, Candolin and Voigt (1998) demonstrated that the presence of predators forced reproductively active stickleback males to choose vegetated nest sites over open ones. The use of vegetated and more complex habitats may enhance an individual’s survival and future reproduction, since structured environments reduce foraging success in piscivorous predators (Persson and Eklöv 1995). Whether the presence or absence of UV radiation in the habitat affects predator avoidance behaviour in sticklebacks in other contexts, such as foraging, awaits further study.
In conclusion, fish raised in full-spectrum conditions preferred the UV-deficient lighting conditions instead of full-spectrum light including UV wavelengths in the presence of a predator. However, it is important to note that the experimental lighting conditions used here were rather artificial which may especially be the case for the UV-deficient conditions. For example, spatial variation of spectral transmission in aquatic environments is not restricted to the UV waveband alone. In fact, in clear water both the short and long wavelength end of the spectrum are attenuated with depth (Jerlov 1976), whereas in nutrient-rich freshwater habitats light transmission is shifted to longer wavelengths (560–750 nm) due to a high absorption of short wavelengths by dissolved organic matter (Jerlov 1968). Nevertheless, our results provide first evidence for a potential UV-dependent habitat preference in a fish under predation risk. Surprisingly, individuals from the UV− treatment group were equally attracted to both presented light habitats. Whether these fish could not discriminate between the two lighting conditions as a result of developmental constraints caused by their rearing conditions requires further investigation.
References
Boulcott PD, Braithwaite VA (2005) The role of ultraviolet wavelengths in the mate-choice decisions of female three-spined sticklebacks. J Exp Biol 208:1453–1458. doi:10.1242/jeb.01569
Bowmaker JK (1990) Visual pigments of fishes. In: Douglas RH, Djamgoz MBA (eds) The visual system of fishes. Chapman and Hall, London, pp 81–107
Candolin U, Voigt HR (1998) Predator-induced nest site preference: safe nests allow courtship in sticklebacks. Anim Behav 565:1205–1211. doi:10.1006/anbe.1998.0892
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63:1802–1813. doi:10.2307/1940122
Cummings ME, Rosenthal GG, Ryan MJ (2003) A private ultraviolet channel in visual communication. Proc R Soc Lond B Biol Sci 270:897–904. doi:10.1098/rspb.2003.2334
Emery AR (1973) Preliminary comparisons of day and night habits of freshwater fish in Ontario lakes. J Fish Res Board Can 30:761–774
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat 139:125–153. doi:10.1086/285308
Endler JA (1993) Some general-comments on the evolution and design of animal communication-systems. Philos Trans R Soc B 340:215–225. doi:10.1098/rstb.1993.0060
Fuller RC, Carleton KL, Fadool JM, Spady TC, Travis J (2004) Population variation in opsin expression in the bluefin killifish, Lucania goodei: a real-time PCR study. J Comp Physiol [A] 190:147–154
Greenwood VJ, Smith EL, Cuthill IC, Bennett ATD, Goldsmith AR, Griffiths R (2002) Do European starlings prefer light environments containing UV? Anim Behav 64:923–928. doi:10.1006/anbe.2002.1977
Gregory RS, Levings CD (1996) The effects of turbidity and vegetation on the risk of juvenile salmonids, Oncorhynchus spp., to predation by adult cutthroat trout, O. clarkii. Environ Biol Fishes 3:279–288. doi:10.1007/BF00000500
Hastad O, Victorsson J, Odeen A (2005) Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc Natl Acad Sci USA 102:6391–6394. doi:10.1073/pnas.0409228102
Hobson ES (1979) Interactions between piscivorous fishes and their prey. In: Clepper H (ed) Predator–prey systems in fishery management. Sport Fishery Institute, Washington, pp 231–242
Hunt S, Cuthill IC, Bennet ATD, Griffiths R (1999) Preferences for ultraviolet partners in the blue tit. Anim Behav 58:809–815. doi:10.1006/anbe.1999.1214
Ibrahim AA, Huntingford FA (1989) Laboratory and field studies of the effect of predation risk on foraging in three-spined sticklebacks (Gasterosteus aculeatus). Behaviour 109:46–57. doi:10.1163/156853989X00150
Jakobsen PJ, Johnsen GH, Larsson P (1988) Effects of predation risk and parasitism on the feeding ecology, habitat use, and abundance of lacustrine threespine stickleback (Gasterosteus aculeatus). Can J Fish Aquat Sci 45:426–431
Jerlov NG (1968) Optical oceanography. Elsevier, Amsterdam, p 194
Jerlov NG (1976) Marine optics. Elsevier, Amsterdam, p 231
Kröger RHH, Bowmaker JK, Wagner H-J (1999) Morphological changes in the retina of A. pulcher (Cichlidae) after rearing in monochromatic light. Vision Res 39:2441–2448. doi:10.1016/S0042-6989(98)00256-9
Kröger RHH, Braun SC, Wagner H-J (2001a) Rearing in different photic and chromatic environments modifies spectral responses of cone horizontal cells in adult fish retina. Vis Neurosci 18:857–864. doi:10.1017/S0952523801186025
Kröger RHH, Campbell MCW, Fernald RD (2001b) The development of the crystalline lens is sensitive to visual input in the African cichlid fish, Haplochromis burtoni. Vision Res 41:549–559. doi:10.1016/S0042-6989(00)00283-2
Kröger RHH, Knoblauch B, Wagner H-J (2003) Rearing in different photic and spectral environments changes the optomotor response to chromatic stimuli in the cichlid fish Aequidens pulcher. J Exp Biol 206:1643–1648. doi:10.1242/jeb.00337
Losey GS (2003) Crypsis and communication functions of UV-visible coloration in two coral reef damselfish, Dascyllus aruanus and D. reticulatus. Anim Behav 66:299–307. doi:10.1006/anbe.2003.2214
Losey GS, Cronin TW, Goldsmith TH, Hyde D, Marshall NJ, McFarland WN (1999) The UV visual world of fishes: a review. J Fish Biol 54:921–943. doi:10.1111/j.1095-8649.1999.tb00848.x
Lythgoe JN (1972) The adaptation of visual pigments to their photic environment. In: Dartnall HJA (ed) Handbook of sensory physiology, VII/1: photochemistry of vision. Springer, Berlin, pp 566–603
Lythgoe JN (1979) The ecology of vision. Clarendon Press, London
Macia A, Abrantes K, Paula J (2003) Thorn fish Terapon jarbua (Forskål) predation on juvenile white shrimp Penaeus indicus H. Milne Edwards and brown shrimp Metapenaeus monoceros (Fabricius): the effect of turbidity, prey density, substrate type, and pneumatophore density. J Exp Mar Biol Ecol 291:29–56. doi:10.1016/S0022-0981(03)00097-2
Maddocks SA, Bennett ATD, Hunt S, Cuthill IC (2002) Context-dependent preferences in starlings and blue tits: mate choice and light environment. Anim Behav 63:69–75. doi:10.1006/anbe.2001.1868
Mattila J (1992) The effect of habitat complexity on predation efficiency of perch Perca fluviatilis L. and ruff Gymnocephalus cernuus (L.). J Exp Mar Biol Ecol 157:55–67. doi:10.1016/0022-0981(92)90074-K
McDonald CG, Hawryshyn CW (1995) Intraspecific variation of spectral sensitivity in threespine stickleback (Gasterosteus aculeatus) from different photic regimes. J Comp Physiol [A] 176:255–260. doi:10.1007/BF00239927
Miner JG, Stein RA (1996) Predator detection and habitat choice by small bluegills: effects of turbidity. Trans Am Fish Soc 125:97–103. doi:10.1577/1548-8659(1996)125<0097:DOPAHC>2.3.CO;2
Modarressie R, Bakker TCM (2006) No evidence for UV-based nest-site selection in sticklebacks. Front Zool 3:17. doi:10.1186/1742-9994-3-17
Modarressie R, Rick IP, Bakker TCM (2006) UV matters in shoaling decisions. Proc R Soc Lond B Biol Sci 273:849–854. doi:10.1098/rspb.2005.3397
Persson L, Eklöv J (1995) Prey refuges affecting interactions between piscivorous perch and juvenile perch and roach. Ecology 76:70–81. doi:10.2307/1940632
Power ME (1984) Depth distribution of armored catfish: predator-induced resource avoidance? Ecology 65:523–528. doi:10.2307/1941414
R Development Core Team (2007) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Rick IP, Bakker TCM (2008a) Color signaling in conspicuous red sticklebacks: do ultraviolet signals surpass others? BMC Evol Biol 8:189. doi:10.1186/1471-2148-8-189
Rick IP, Bakker TCM (2008b) UV wavelengths make female three-spined sticklebacks (Gasterosteus aculeatus) more attractive for males. Behav Ecol Sociobiol 62:439–445. doi:10.1007/s00265-007-0471-6
Rick IP, Bakker TCM (2008c) Males do not see only red: UV wavelengths and male territorial aggression in the three-spined stickleback. Naturwissenschaften 95:631–638. doi:10.1007/s00114-008-0365-0
Rick IP, Modarressie R, Bakker TCM (2006) UV wavelengths affect female mate choice in three-spined sticklebacks. Anim Behav 71:307–313. doi:10.1016/j.anbehav.2005.03.039
Shand J, Davies WL, Thomas N, Balmer L, Cowing JA, Pointer M et al (2008) The influence of ontogeny and light environment on the expression of visual pigment opsins in the retina of the black bream, Acanthopagrus butcheri. J Exp Biol 211:1495–1503. doi:10.1242/jeb.012047
Siebeck UE (2004) Communication in coral reef fish: the role of ultraviolet colour patterns in damselfish territorial behaviour. Anim Behav 68:273–282. doi:10.1016/j.anbehav.2003.11.010
Skov C, Nilsson PA, Jacobsen L, Brönmark C (2007) Habitat-choice interactions between pike predators and perch prey depend on water transparency. J Fish Biol 70:298–302. doi:10.1111/j.1095-8649.2006.01255.x
Smith EJ et al (2002) Ultraviolet vision and mate choice in the guppy (Poecilia reticulata). Behav Ecol 13:11–19. doi:10.1093/beheco/13.1.11
Snickars M, Sandstroem A, Mattila J (2004) Antipredator behaviour of 0 + year Perca fluviatilis: effect of vegetation density and turbidity. J Fish Biol 656:1604–1613. doi:10.1111/j.0022-1112.2004.00570.x
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B Biol Sci 265:351–358. doi:10.1098/rspb.1998.0302
Werner EE, Gilliam JF, Hall DJ, Mittelbach GG (1983) An experimental test of the effects of predation risk on habitat use in fish. Ecology 64:1540–1548. doi:10.2307/1937508
Wisenden BD (2000) Olfactory assessment of predation risk in the aquatic environment. Philos Trans R Soc B 355:1205–1208. doi:10.1098/rstb.2000.0668
Acknowledgments
We are grateful to Ricarda Modarressie, Sebastian Baldauf, Timo Thünken, Joachim Frommen for discussion and comments on the manuscript, and Dorothee Breuer and Marie Fengler for their help with data collection. We thank Jan Hottentot for catching sticklebacks in the field. Manuscript preparation was supported by a grant from the Deutsche Forschungsgemeinschaft (Ba 2885/1-3). The study conforms to the legal requirements of Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rick, I.P., Bakker, T.C.M. Ultraviolet light influences habitat preferences in a fish under predation risk. Evol Ecol 24, 25–37 (2010). https://doi.org/10.1007/s10682-008-9287-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-008-9287-8