Abstract
Black rice production in Indonesia constrained by the bacterial blight disease (BLB) caused by Xanthomonas oryzae pv. oryzae pathotype IV (Xoo). Breeding of BLB resistant cultivars is considered the most sustainable method for BLB disease control, both from an environmental and agricultural perspective. Indonesia has many local black rice varieties that can be used as genes resource to support breeding program producing resistant cultivars. The present research focuses on screening local Indonesian black rice cultivars for resistance against BLB and analyzing the expression of these resistance genes in black rice after inoculation with Xoo. The black rice cultivars Cempo Ireng, Pari Ireng, Melik, Pendek, and Indmira, were inoculated with Xoo while white rice cv. Conde, IRBB21, IR64, and Java14 were used as controls. We assayed the phenotypic performance of the cultivars samples after Xoo inoculation and analyzed their resistance gene expression at 24 and 96 h after Xoo inoculation semiquantitatively. The cultivar showed the best performance was selected for further analysis of the resistance genes using Real-time quantitative PCR. Cempo Ireng was indicated the most resistant cultivar against BLB disease based on the lowest disease intensity and Area Under Disease Progress Curve (AUDPC) value. Cempo Ireng expressed resistant genes xa5, Xa10, Xa21 and RPP13-like after inoculation of Xoo. The expression of xa5, Xa10, and Xa21 was up-regulated while that of RPP13-like was down-regulated in Cempo Ireng.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is one of the main agricultural products in tropical countries, especially in Indonesia. The biodiversity of rice in Indonesia reported being in the third position (7.2%) over the world as the richest country in pigmented rice germplasm, after China (62%) and Sri Lanka (8.6%) (Chaudhary 2003). Among the pigmented rice cultivars, black rice is currently produced in Southeast Asia, including Indonesia (Shinta and Endang 2014). Black rice is widely consumed as a functional food due to its benefits for human health because of its high nutrient and antioxidant content (Chutipaijit et al. 2011). Furthermore, the demand for black rice is increasing. However, its production is still affected by many plant diseases (Cristanti and Endang 2013).
One of the significant diseases of rice plants in Indonesia is bacterial leaf blight (BLB) caused by Xanthomonas oryzae pv. oryzae (Xoo). BLB does not only damage lowland rice crops but also upland rice in Indonesia (Suryadi et al. 2016). Perumalsamy et al. (2010) reported that BLB disease had significantly reduced rice production by 20–80%. However, there is no official report of the BLB in black rice, but still, BLB can be one of the major diseases that contribute to the yield reduction of black rice production. Screening of black rice cultivars which are resistant against BLB could provide potential genetic sources for improving BLB resistance by breeding. A method and strategy for disease control have been developed, which involved analyzing and studying the interactions between pathogens and host plants by determining differences in gene expression (Casassola et al. 2013). Some of the resistance genes against BLB are Xa1, Xa3, xa5, Xa10, Xa21, and RPP13-like. These genes are the most commonly exploited in rice breeding programs because they provide multigenic long-term resistance in commercial rice cultivars (Mew et al. 1992). The expressions of these genes have, however, not yet studied in black rice. Therefore, in this screening study, resistance gene expression as well as the phenotypic performance of some black and, as controls, white rice cultivars after Xoo inoculation, were analyzed.
Materials and methods
Plant materials
The five black rice cultivars, Cempo Ireng, Pari Ireng, Melik, Pendek, and Indmira, were obtained from Institute of Agricultural Technology Yogyakarta and derived from our germplasm collection at Research Center for Biotechnology Universitas Gadjah Mada. The white rice cultivars, Java14, IRBB21, and Conde, were used to represent resistant plants while IR64 was the susceptible control (Susanto and Sudir 2012). The seeds of resistant and susceptible control were obtained from Indonesian Center for Rice Research (ICRR), Subang, West Java.
Xoo inoculation
The Xoo isolate used for this study was the Xoo pathotype IV isolate which a dominant pathotype in Indonesia (Suparyono et al. 2004). This isolate was obtained from the Indonesian Center for Rice Research (ICRR), Subang, West Java. Bacterial culture and preparation were performed according to Sana et al. (2010). Peptone Sucrose Agar (PSA) medium was prepared with 5 g of sucrose composition, 1.25 g peptone, 0.125 g K2PO4, 0.0625 g MgSO4.7H2O and 5 g agar, and 250 ml of dH2O, pH 7.2–7.4. Xoo was cultured on PSA medium and incubated for 72 h at 30 °C. Inoculum preparation bacteria were grown on PSA at 28 ± 2 °C for 48 h and suspended in deionized water. The suspension was adjusted to 108 colony forming units (CFU)/ml from absorbance measurements (A 600 = 0.3, Babu et al. 2003).
Xoo was harvested from the petri dish and transferred to an Erlenmeyer flask containing 200 ml sterile water. The concentration of Xoo suspension was 108 colony forming units (CFU)/ml. Inoculation was done in the afternoon to avoid high environmental heat and evaporation, i.e., between 03.00 and 05.00 p.m. The experimental plant material was divided into four groups including 24 and 96 Hours Post Inoculation (HPI) treatment, mock, and untreated. Each group had three plants with three replications which were used at the same time. All of the plants used were at the transition between tillering stage to heading stage.
The bacterial inoculation was performed according to Kauffman et al. (1973) by the leaf clipping method along 4–5 cm of the leaf tip. Briefly, in the treatment plant, the sterile scissors were dipped into Xoo bacterial suspension and immediately used to cut the rice leaf tip. The mock treatments which used the scissors dipped into the sterile distilled water were also used as a control in respect of the clipping. The untreated plant was unclipped, used as a control. All plants were kept in greenhouse conditions with different compartments for each treatment to prevent the Xoo transmission.
Phenotypic assay of bacterial leaf blight resistance in screened black rice cultivars
Observation of symptoms of Bacterial Leaf Blight (BLB) was conducted by measuring disease intensity (IRRI 1996) and AUDPC (Area Under Disease Progress Curve) according to Djatmiko et al. (2011). In this study, four times of BLB observation were carried out on the first, second, third and fourth week. Three replicates were used in the observation. The plants were at the reproductive stage. Data analysis of phenotypic assay was carried out using one-way analysis of variance (ANOVA) and followed by Duncan test at P < 0.05. For the disease intensity, the regression analysis was also performed to infer the disease progress intensity of the cultivars tested. The selected cultivars which had the most resistant phenotype against BLB were used for quantitative expression and partial sequence analysis of resistance genes.
Expression analysis of resistance genes
Samples were taken at 24 h and 96 h after inoculation by collecting 4–5 cm from the inoculated tip of the leaf (Yu et al. 2014; Peng et al. 2015; Narsai et al. 2013). The samples were then washed with sterile distilled water and stored in plastic zip lock bags, frozen with liquid nitrogen, then stored in a deep freezer at − 80 °C. RNA isolation was performed using the RNeasy Plant Mini Kit (Qiagen). The quality and quantity of RNA were determined using NanoDrop spectrophotometer and agarose gel electrophoresis. One microgram of RNA was subjected to cDNA synthesis using SuperscriptII First-Strand Synthesis System for RT-PCR (Invitrogen). Detection of resistance genes at the RNA level was performed prior to semiquantitative and quantitative resistance gene expression analysis with the housekeeping gene Ubiquitin, as internal standard (Chen and Klebe 1993).
Semiquantitative analysis of resistance gene expression was performed by conventional PCR using Xa and RPP13-like primers (Table 1). The primers used for conventional PCR and quantitative real-time PCR were designed by using Primer3 program (http://bioinfo.ut.ee/primer3-0.4.0/) based on O. sativa Japonica Group sequence data, except the primer of RPP13-like which was obtained from Ghazi et al. (2009). The primers of Ubiquitin gene had sequences of 5′-CTCAGGCTCCGTGGTGGTATG-3′ (Forward) and 5′-GTGATAGTTTTCCCAGTCAACGTC-3′ (Reverse) with amplicon size 200 bp. In the semiquantitative analysis, levels of resistance genes expression, as well as internal standard gene Ubiquitin in screened cultivars, were estimated using ImageJ software via electrophoresis gel image. Estimation of Xa gene expression level was determined by dividing the area of resistance genes by the area of the Ubiquitin gene. The cultivars which showed the resistance character and associated with the expressed genes were selected for further analysis. We selected four resistance genes which were expressed in the selected cultivars and amplified the genes by quantitative real-time PCR (qRT-PCR) with Ubiquitin as an internal standard, and their relative expression levels were analyzed by using Livak Method (Livak and Schmittgen 2001).
DNA sequencing and partial sequence analysis
The cDNA of untreated control samples from Cempo Ireng, Java14, and IR64, are used to amplify xa5, Xa10, Xa21 and RPP13-like genes using conventional PCR. The PCR products were utilized for partial DNA sequencing which was performed by using the BigDye® Terminator v3.1 cycle sequencing kit (Applied Biosystems, USA) at Apical Scientific Sdn. Bhd. located in Selangor, Malaysia. Partial sequence analysis was carried out by using BLAST programs namely BLASTN for similarity analysis (Altschul et al. 1997) and Clustal Omega program (https://www.ebi.ac.uk/Tools/msa/clustalo/) for multiple sequence alignment.
Results and discussion
Plants have developed specific defense mechanism because they are sessile forms. Defense mechanisms can be rapidly activated when plants are attacked by pathogens and can recognize them as invaders, often called as incompatible interactions. However, in susceptible plants, the recognition of the pathogens can be blocked, or the response is too slow so pathogens will spread rapidly and produce widespread symptoms (Aderem and Ulevitch 2000).
Phenotypic assay of bacterial leaf blight resistance in screened black rice cultivars
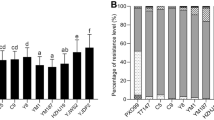
The black rice cultivars analyzed showed Bacterial Leaf Blight (BLB) symptoms as a response to the Xoo inoculation (Fig. 1a). AUDPC can be used to assess quantitative disease resistance in crop cultivars (Jeger and Viljanen-Rollinson 2001). AUDPC values of the screened cultivars, namely IR64, Indmira, Conde, Pendek, IRBB21, Melik, Pari Ireng, Java14 and Cempo Ireng are shown in Fig. 1b. Pari Ireng and Melik were rated as moderately resistant, Conde, Pendek, and Indmira as moderately susceptible, IR64 (the susceptible control) and IRBB21 as susceptible, while Java14 (the resistance control) and Cempo Ireng were resistant against BLB. Interestingly, based on the regression analysis for disease intensity, it can be concluded that the disease development of resistant cultivar was in the same group. Pari Ireng which was characterized to be moderately resistant was in the same group with the resistance cultivar Java14 and Cempo Ireng. According to the regression analysis, it can be inferred that the disease intensity will not develop any further in the resistant cultivars compared to the moderately susceptible and susceptible cultivars.
a Phenotype of BLB resistance in nine screened cultivars (IR64, Pendek, Conde, Indmira, IRBB21, Melik, Pari Ireng, Java14 and Cempo Ireng) 4 weeks after Xoo inoculation. b Area Under Disease Progress Curve (AUDPC) was calculated using disease intensity. c Regression line slopes of disease intensity (indicated by lesion length per leaf length following the criteria of resistance response based on System Evaluation Standard IRRI 1996). *Bars with the same letter do not differ from each other at P < 0.05 (Duncan’s test)
Detection of resistance genes at RNA level in screened black rice cultivars
Resistance gene expression was monitored by a two-step reverse transcription-PCR (RT-PCR), which was chosen to detect rare transcripts or to analyze low-quantity samples due to its reproducibility and ability to detect multiple genes in the same sample (Carding et al. 1992). The results for the tested resistance genes in nine rice cultivars are presented in Fig. 2.
We detected six resistance genes, namely Xa1, Xa3, xa5, Xa10, Xa21, and RPP13. For each cultivar, a different number of expressed resistance genes was observed, as indicated in Fig. 2. The number of expressed genes appears to be associated with resistance level. Pathogens release a factor(s) that can activate the expression of genes known as resistance genes which are responsible for the resistance response (Hummel et al. 2012). Resistance mechanisms such as defensive signaling pathways and resistance gene expression will alert and protect the plant as a response to pathogen attack (Koornneef and Pieterse 2008). However, qualitative data are insufficient to assess corresponding gene expression; this requires quantification of specific RNA transcripts under various experimental conditions using either semiquantitative or quantitative analysis (Marone et al. 2001). For this reason, we conducted a semiquantitative analysis of the resistance genes in selected black rice resistance cultivars.
Semiquantitative expression analysis of resistance gene in screened black rice cultivars
As shown in the Fig. 3a, Xa10 gene expression in cultivars Conde and Melik exhibited a similar pattern. Interestingly in cultivars Indmira and IRBB21, the expression first decreased at 24 h and then increased at 96 h. Pendek and IR64 as susceptible controls both showed a decrease in the expression of Xa10 after 24 and 96 h. Interestingly, the cultivar Cempo Ireng had an increased level of Xa10 expression at 24 and 96 h after Xoo inoculation. The expression of RPP13-like in Melik and Java14 also enhanced at 24 hours and 96 hours while Cempo Ireng showed a decrease after 96 hours (Fig. 3b).
Semiquantitative expression analysis of six resistance genes (Xa1, Xa3, xa5, Xa10, Xa21 and RPP13-like) in nine rice cultivars with the Ubiquitin gene as an internal standard. HPI hours post inoculation (bar heights and the error bars represent the expression level of the gene and standard error in all graphs)
The expression levels of Xa21 were found to have increased at 24, and 96 HPI in all rice cultivars studied except Java14 and Cempo Ireng which have increased only at 96 HPI (Fig. 3c). The Xa3 gene expression level in Fig. 3d showed that Pari Ireng and IRBB21 an increased expression level both after 24 and 96 h, while Java14 and IR64 had an increased expression only after 96 h. The xa5 expression level in the cultivars Conde and Melik (Fig. 3e) increased at 24 as well as 96 HPI, while in Pendek and IR64 the response was slower and only measurable at 96 HPI. In IRBB21 the expression level of xa5 was found to decrease at 24 and 96 HPI. Indmira showed an increased expression level of xa5 at 24 h followed by a decrease at 96 HPI. Meanwhile, Cempo Ireng had an increased expression of xa5 only at 24 h.
Semiquantitative analysis for Xa1 gene expression in Java14 showed that expression increased 24 h and 96 h after inoculation while in Pari Ireng and Melik the expression increased only in 96 h after inoculation (Fig. 3f). The cultivar Pari Ireng had a higher Xa1 expression level compared to Melik at 24 and 96 HPI, while Java14 had the highest level among the black rice cultivars studied. As long as the proper internal standard is all analyzed correctly, these semiquantitative RT-PCR data are reliable (Marone et al. 2001).
Quantitative expression analysis of resistance genes in the selected black rice cultivar
The black rice cultivar Cempo Ireng was selected for quantitative expression analysis using qRT-PCR because of its resistance performance against BLB, while the white rice cultivars Java14 and IR64 were selected as the resistant and susceptible controls, respectively. The results showed that the expression of xa5 was up-regulated in Cempo Ireng as Xoo-treated cultivar and IR64 as susceptible control cultivar but down-regulated in Java14 as resistant control cultivar (Fig. 4a). The level of xa5 expression at 24 HPI in the Cempo Ireng was lower than that found in Java14 but similar to IR64. At 96 HPI, the level of xa5 expression in the Cempo Ireng was the highest compared to Java14 and IR64. The recessive xa5 encodes a mutated TFIIAγ5. This mutation may attenuate TAL effector-activated host gene expression and provides passive resistance (Iyer-Pascuzzi et al. 2008).
The highest relative expression of Xa10 was in Cempo Ireng compared to IR64 and Java14 as susceptible and resistant cultivars, respectively. An increase in the relative expression level of Xa10 could also be observed in Cempo Ireng and Java14, but the Xa10 expression decreased in IR64 when the untreated control and inoculation treatment at 24 and 96 HPI were compared. This indicated that the expression of Xa10 was up-regulated in Cempo Ireng and Java14, but down-regulated in IR64.
The Xa10 gene confers a high level of resistance (Mew et al. 1982), and its expression causes a response that is similar to a hypersensitive response (Kaku and Kimura 1978; Parry and Callow 1986). Xa10 plays a direct role in triggering programmed cell death (PCD) in rice plants through a mechanism similar to that of PCD that occurs in other plant species and animal cells (Tian et al. 2014). The Xa10 protein is located on the endoplasmic reticulum (ER) membrane and affects a decrease in the calcium concentration of the ER and triggers PCD. The ER is an essential organelle that plays a role in various cellular processes including calcium homeostasis, protein secretion, and lipid biosynthesis. ER disruption causes the unfolded protein response, a stress response that triggers apoptosis in the event of severe or prolonged ER dysfunction (Shore et al. 2011). This PCD mechanism probably limits the spread of Xoo invasion in the leaf of the rice plant.
Xa21 gene expression was up-regulated in Xoo-treated Cempo Ireng and IR64 but down-regulated in Java14 (Fig. 4c). This gene expression may enhance the phenotypic performance of Cempo Ireng to be the most resistant among the tested cultivars. The Xa21 gene encodes a receptor-like protein kinase that plays a role in recognition of the cell surface to the pathogen ligand and then activates an intracellular kinase, which ultimately results in a resistance response (Century et al. 1999). Pathogens can infect plants through a site of injury so that the plant responds quickly through defense mechanisms, including the expression of resistance genes (Gomez-Gomez and Boller 2002; Takabatake et al. 2006).
RPP13-like expression in Cempo Ireng was up-regulated and higher than in the other cultivars (Fig. 4d). In transgenic Arabidopsis overexpressing RPP13 are resistant to parasitism by five isolates of Pronospora parasitica (Bittner-Eddy et al. 2000). The results of the present study suggest that RPP-13 like also contributes to the resistance mechanism of black rice.
Analysis of partial sequences of resistance genes in selected cultivars
Analysis of xa5, Xa10, Xa21 and RPP13-like DNA sequences in Cempo Ireng, Java14, and IR64 cultivars, with Oryza sativa Japonica Group as the reference sequence, showed that they had very high levels of identity (Table 2).
Alignment of the partial sequences of the resistance genes showed nucleotide variation in Xa10, Xa21 and RPP13-like sequences between the cultivars analyzed and Japonica as a reference sequence (Fig. 5). The variation in Xa10 consisted of a one-base substitution and a three-base deletion only in IR64. The Xa21 sequences revealed a one-base substitution in Cempo Ireng and Java14 and two substitutions in IR64. The RPP13-like sequence in Cempo Ireng was identical to the reference sequence; however, there were many substitutions and deletions in Java14 and IR64. This nucleotide variation may contribute to the resistance level against BLB in the rice cultivars studied.
The xa5 gene is situated on chromosome 5 and encodes a protein with a change of valine to glutamic acid (V39E) in TFIIAγ5 (Xa5) (Iyer and McCouch 2004), whereas Xa21 and Xa10 are located on chromosome 11 (Nino-Liu et al. 2006). RPP13 is located on chromosome 3 and consists of three alleles, RPP13-Nd, RPP13-Rld, and rpp13-col. These alleles are highly conserved over the first two-thirds of their length and include a leucine zipper, nucleotide-binding site motifs, and the conserved core hydrophobic domain (Bittner-Eddy et al. 2000).
All of the resistance genes examined in this study in the Cempo Ireng cultivar are considered to contribute toward the resistance against BLB. Further elucidation and characterization of the resistance genes will deepen our understanding of the resistance mechanism of black rice toward Xoo.
Conclusions
The Indonesian local black rice cultivar Cempo Ireng was the most resistant cultivar against bacterial leaf blight compared to other cultivars studied. In Cempo Ireng the expression of xa5, Xa10, and Xa21 was up-regulated, while that of RPP13-like was down-regulated.
References
Aderem A, Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 406(6797):782–787
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Babu MR, Sajeena A, Samundeeswari VA, Sreedhar A, Vidhyasekeran P, Reddy MS (2003) Induction of bacterial blight (Xanthomonas oryzae pv. oryzae) resistance in rice by treatment with acibenzolar-S-methyl. Ann Appl Biol 143:333–340
Bittner-Eddy PD, Crute IR, Holub EB, Beynon JL (2000) RPP13 is a simple locus in Arabidopsis thaliana for alleles that specify downy mildew resistance to different avirulence determinants in Peronospora parasitica. Plant J 21(2):177–188
Carding SR, Lu D, Bottomly KA (1992) A polymerase chain reaction assay for the detection and quantification of cytokine gene expression in small number of cells. J Immun Methods 151:277–287
Casassola A, Brammer SP, Chaves MS, Martinelli JA, Grando MF, Denardin NDA (2013) Gene expression: a review on methods for the study of defense-related gene differential expression in plants. Am J Plant Sci 4:64–73
Century KS, Rregina AL, Michael A, John M, Renee T, Keri S, Aubrey S, Jaime L, Pamela CR, Maureen CW (1999) Developmental control of Xa21-mediated disease resistance in rice. Plant J 20(2):231–236
Chaudhary RC (2003) Speciality rices of the world: effect of WTO and IPR on its production. JFAE 1(2):34–41
Chen D, Klebe RJ (1993) Controls for validation of relative reverse transcription-polymerase chain reaction assays. PCR Meth Appl 3:127–129
Chutipaijit S, Chaum S, Sompornpailin K (2011) High contents of proline and anthocyanin increase protective response to salinity in Oryza sativa L. spp. Indica. AJCS 5(10):1191–1198
Cristanti LD, Endang A (2013) Pertumbuhan Padi Hitam dan Serangan Beberapa Herbivor di Sawah Padi Organik Kecamatan Kepanjen. J Biotropika 1(5):221–225
Djatmiko HA, Budi P, Nur P (2011) Penentuan patotipe dan keragaman genetik Xanthomonas oryzae pv. oryzae pada tanaman padi di wilayah karesidenan banyumas. J HPT Tropika 11(1):35–46
Ghazi IA, Prem SS, Vivek D, Kishor G, Ashok KS, Tilak RS, Nagendra KS, Trilochan M (2009) Physical mapping, expression analysis and polymorphism survey of resistance gene analogues on chromosome 11 of rice. J Biosci 34(2):251–261
Gomez-Gomez L, Boller T (2002) Flagellin perception: a paradigm for innate immunity. Trends Plant Sci 7:251–256
Hummel AW, Doyle EL, Bognadove AJ (2012) Addition of transcription activator-like effector binding sites to a pathogen strain-specific rice bacterial blight resistance gene makes it effective against additional strains and against bacterial leaf streak. New Phytol 195:883–893
IRRI (1996) Standard Evaluation System for Rice, 4th edn. International Rice Research Institute, Manila
Iyer AS, McCouch SR (2004) The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol Plant Microb Interact 17:1348–1354
Iyer-Pascuzzi AS, Jiang H, Huang HL, McCouch SR (2008) Genetic and functional characterization of the rice bacterial blight disease resistance gene xa5. Phytopathology 98(3):289–295
Jeger MJ, Viljanen-Rollinson SLH (2001) The use of the area under the disease-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theor Appl Genet 102:32–40
Kaku H, Kimura T (1978) Reaction types office cultivars to strains of Xanthomonas oryzae. Bull Chugoku Nat Agric Exp Stat Ser E 13:17–43
Kauffman HE, Reddya PK, Hiesh SPY, Merca SD (1973) An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis Rep 57:537–541
Koornneef A, Pieterse CM (2008) Cross talk in defense signaling. Plant Physiol 146:839–844
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Marone M, Simona M, Daniela DR, Luca P, Giovanni S (2001) Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol Proced Online 3:19–25
Mew TW, Vera CCM, Reves RC (1982) Interaction of Xanthomonas campestris pv. oryzae and a resistant rice cultivar. Phytopathology 72:786–789
Mew TW, Vera CCM, Medalla ES (1992) Changes in race frequency of Xanthomonas oryzae pv. oryzae in response to rice cultivars planted in the Philippines. Plant Dis 76:1029–1032
Narsai RC, Wang J, Chen J, Wu H, Shou Whelan J (2013) Antagonistic, overlapping and distinct responses to biotic stress in rice (Oryza sativa) and interaction with abiotic stress. BMC Genom 14:93
Nino-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7(5):303–324
Parry RWH, Callow JA (1986) The dynamics of homologous and heterologous interactions between rice and strains of Xanthomonas campestris. Plant Pathol 35:380–389
Peng H, Chen Z, Fang Z, Zhou J, Xia Z, Gao L, Chen L, Li T, Zhai W, Zhang W (2015) Rice Xa21 primed genes and pathways that are critical for combating bacterial blight infection. Scientific Report 5(12165):1–12
Perumalsamy S, Bharani M, Sudah M, Nagarajan P, Arul L, Sarawathi R, Balasubramaninan P, Ramalingam J (2010) Functional marker-assisted selection for bacterial leaf blight resistance genes in rice (Oryza Sativa L.). Plant Breed 129:400–406
Sana TR, Fischer S, Wohlgemuth G, Katrekar A, Jung KH, Ronald PC, Fiehn O (2010) Metabolomic and transcriptomic analysis of the rice response to the bacterial blight pathogen Xanthomonas oryzae pv. oryzae. Metabolomics 6:451–465
Shinta Serafinah I, Endang A (2014) Morphological variation of six pigmented rice local varieties grown in organic rice field in Sengguruh village, Kepanjen district, Malang regency. JTLS 4(2):149–150
Shore GC, Papa FR, Oakes SA (2011) Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol 23:143–149
Suparyono S, Sudir S, Suprihanto S (2004) Pathotype profile of Xanthomonas oryzae pv. oryzae isolates from the rice ecosystem in Java. IJAS 5(2):63–69
Suryadi Y, Samudra IM, Priyatno TP, Susilowati DN, Lestari P, Fatimah F, Kadir TS (2016) Determination of pathotypes from Indonesian Xanthomonas oryzae pv. oryzae population causing bacterial leaf blight and their reactions on differential rice. Makara J Sci 20(3):109–118
Susanto U, Sudir (2012) Ketahanan Genotipe Padi terhadap Xanthomonas oryzae pv. oryzae Patotipe III, IV, dan VIII. JPPTP 31(2):108–116
Takabatake R, Seo S, Ito N, Gotoh Y, Mitsuhara I, Ohashi Y (2006) Involvement of wound-induced receptor-like protein kinase in wound signal transduction in tobacco plants. Plant J 47:249–257
Tian D, Wang J, Zeng X, Gu K, Qiu C, Yang X, Zhou Z, Goh M, Luo Y, Murata-Hori M (2014) The rice TAL effector-dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell 26:497–515
Yu C, Huamin C, Fang T, Jan EL, Chenyang H (2014) Differentially-expressed genes in rice infected by Xanthomonas oryzae pv. oryzae relative to a flagellin-deficient mutant reveal potential functions of flagellin in host-pathogen interactions. Rice J 7(1):1–11
Acknowledgements
This research was partially funded by the Ministry of Research, Technology, and Higher Education Republic of Indonesia by Universities Leading Research Project 2016 to YAP (contract number 863/UN1-P.III/LT/DIT-LIT/2016), World Class Professor Program No. 168.A10/D2/KP/2017 and LPDP research funds to S. We thank Dr. Hans-Joerg Jacobsen, Dr. John Thomas and Dr. Ian Smith for critical reading and improvement of our manuscript. We also thank to Dr. Kristamtini from the Institute of Agricultural Technology Yogyakarta for providing the black rice seeds used in this study, and we express our gratitude to Dr. Sudir from the Indonesian Center for Rice Research (ICRR), Subang, West Java for providing the Xoo isolate.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sutrisno, Susanto, F.A., Wijayanti, P. et al. Screening of resistant Indonesian black rice cultivars against bacterial leaf blight. Euphytica 214, 199 (2018). https://doi.org/10.1007/s10681-018-2279-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-018-2279-z