Abstract
Common bacterial blight (CBB) is an economically important disease of common beans and causes significant yield losses worldwide, including Ethiopia. Therefore, development and use of resistant cultivars are important options to manage the disease. Thus, field reactions of common bean genotypes to CBB and their agronomic performances were conducted at two locations in Southern Ethiopia, during 2018/2019. The experiment was arranged in a randomized complete block design with three replications. Analysis of variance revealed significant (P < 0.01) genotypic variation for disease and yield parameters at both locations. The results showed wide ranges of variation in incidence (26.05–82.75%), severity (23.75–70.70%), AUDPC (773.9–2544%-days) and disease progress rate (0.024–0.142 unit day−1) on average for both locations. And also, remarkable genotypic variation for quantitative growth and yield characters such as plant height, days to 50% flowering, days to 90% maturity, number of pods, number of seeds, seed weight and grain yield were observed. Based on mean severity and R8_severity score, genotypes were grouped as 20% resistant, 55% moderately resistant and 25% susceptible at JARC, and 15% resistant, 80% moderately resistant and only 5% susceptible at Debub Ari. Variable relationships were found among and between parameters studied. The overall results indicated that SER119 and MALB135 showed consistent resistance reaction to CBB, and SER119 also gave high yield across fields. It is recommended to use SER119 with proper management practices for bean production and MALB135 as parent for CBB resistance development in plant breeding programs of the country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulse crops are the most fundamental in the Ethiopian agriculture in providing staple diet to the population (Ali et al. 2006). Among the legumes, common bean (Phaseolus vulgaris L.) is the world’s second most important pulse crop after soybean (Parades et al. 2009) and constitutes a significant part of human diet in Ethiopia (Buruchara et al. 2010). The crop is an important source of nutrients for more than 300 million people in parts of Eastern Africa and Latin American, representing 65% of total protein consumed, 32% of energy and a major source of micronutrients and folic acid (Petry et al. 2015). Common bean has been cultivated as a field crop for a very long time and is the most important food legume produced in the country, particularly in Southern and Eastern parts of Ethiopia (Fininsa 2003). Its production all over the world also approves its high commercial and nutrient values, and consumer use (Popovic et al. 2012a, b).
The world total production of common bean was 22.8 million metric tons of dry beans in 2014 (FAOSTAT 2014). The crop is potentially grown in Eastern, Southern, Southwestern and the Rift valley areas of Ethiopia (Fininsa and Yuen 2001). It is cultivated in wide range of agro-ecologies and farming systems including drought-stressed areas (Asrat et al. 2009). In this regard, common bean was covering 0.3 million ha of the grain crop area and production and productivity of 0.52 million ton and 1.69 t ha−1, respectively, during the 2017/2018 cropping season. Similarly, it covered 0.103 million ha of land and contributed 1.62 t ha−1 of grain yields in Southern Ethiopia (CSA 2018). However, common bean productivity is even below the national average of 1.69 t ha−1 (CSA 2018).
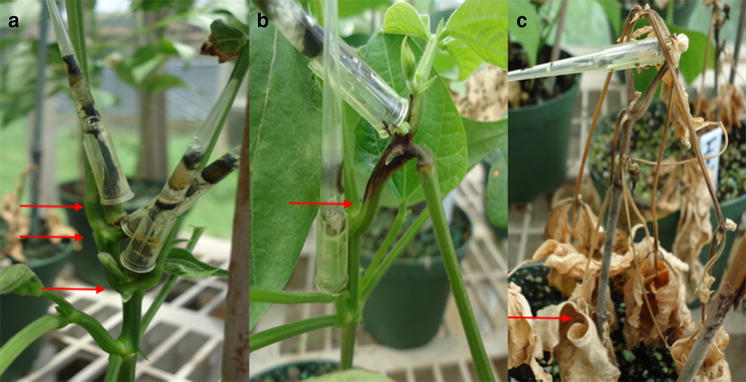
Low productivity of the crop is attributed to both abiotic and biotic constraints (Kimino et al. 2016; Ribeiro et al. 2017). Common bacterial blight (Xanthomonas axonopodis pv. phaseoli) is the most economically important disease and is widely distributed in many parts of Ethiopia (Fekadu 2013; Gudero and Terefe 2018). Common bacterial blight (CBB) affects foliage, pods and seeds of common bean and during extended period of warm and humid weather, the disease can be highly destructive and causes losses in both yield and seed quality of bean (Fininsa 2003). Viteri and Singh (2014) reported yield loss of up to 50% in tropical and subtropical regions of the world. Up to 22.4% yield reduction of bean is reported in eastern parts of the country (Fininsa 2003). And, yield losses of between 22 and 40% have been reported for infected susceptible cultivars (Gillard et al. 2009; Cuervo et al. 2012). Of course, average yield losses may range from 20 to 100% depending on the occurrence and other collective factors; and it could reduce seed quality, germinability and market value (Singh and Schwartz 2010). The situation calls for designing of effective management schemes.
Disease management options such as use of pathogen-free seeds, plant debris management, crop rotation, and cleaning seed containers before storage are recommended to minimize the damage caused by CBB. Disease pressure can also be reduced by the use of intercropping and varietal mixtures (Fininsa 2003; Fikire 2004). Seed treatment with antibiotics has been recommended to disinfest external contamination of seeds (McMullen and Lamey 2000). Foliar applications of copper-based bactericides could also control the disease before it establishes (Selamawit 2004). However, cultural practices and chemicals could not provide complete control of CBB. Thus, use of resistant varieties is the best alternative to lower the damage caused by the disease (Mutlu et al. 2005; Osdaghi et al. 2009; Singh and Schwartz 2010; Maniruzzaman 2015). Host resistance is supposed to reduce yield loss, pesticide use and production costs, increase profitability, seed quality and minimize environmental impacts (Singh and Schwartz 2010).
On the other hand, although the use of resistant varieties is considered the most simple and economical means of control, the practice is sometimes impaired by pathogen variability along with other factors (Arnaud-Santana et al. 1994). In this regard, most of the improved varieties were found susceptible to CBB in Ethiopia. The implication is that lack of improved disease resistance bean genotypes is the bottle neck that contributes to lower yield of common bean in the study areas (Tekle et al. 2014). That is, continuous screening of bean genotypes to identify new bean varieties with resistance to major foliar diseases and high yield is necessary (Todorovic et al. 2008; Ruphine 2018). Moreover, breeding bean genotypes that are resistant to major foliar diseases play a vital role in improving the livelihoods of subsistence bean growers through long-term control of the disease (Durham 2011; Fourie et al. 2011). Therefore, the objective of the study was to evaluate resistant reactions of common bean genotypes to CBB and their agronomic performance in Southern Ethiopia, during the 2018/2019 main cropping season.
Materials and methods
Description of study areas
The study was carried out at two different hotspot locations in the 2018/19 main cropping season. One of the field experiments was conducted at Jinka Agricultural Research Center (JARC), on station. JARC is located at geographic coordinates of 36o54.42′’E and 05o78.55″N and at an altitude of 1403 m.a.s.l. The monthly average temperature ranged between 16.8 and 30.4 °C. The mean annual rainfall for the area is 129 mm during the experimental period. The second field experiment was executed at Debub Ari district (on farm), which is located in between 36o30′ and 37o 73′E and 05o67′–6o19′N and at an altitude of 1605 m.a.s.l. The district is characterized by mean monthly temperature of 13.6–28.6 °C. Mean annual rainfall and monthly temperature ranges are organized from National Meteorology Agency, Southern branch.
Planting materials, procedures and design
The treatments used in the experiment were consisting of 20 common bean genotypes with the objective to evaluate the response of genotypes to resistance reaction against CBB and their agronomic performances (Table 1). The genotypes included 9 commercially available varieties collected from Melkassa Agricultural Research Center and 11 pipeline genotypes obtained from Hawassa Agricultural Research Center. There existed prior information regarding the response of commercial varieties to CBB around Melkassa areas. The pipeline genotypes are candidate genotypes, which were evaluated for yield potential around Hawassa areas. However, both genotype groups were not evaluated for their reaction to CBB in the study areas. The variety Dinknesh was used as a susceptible check based on suggestion of Tumsa et al. (2015).
In the experimental setup, a plot size of 1.6 × 2 m2 consisting of four rows of bean with 0.4 m space between rows was used. A gross area of 6.8 × 49.5 m2 was used at each location. Common bean planting was made in August 2018. Inorganic (NPS) fertilizer was uniformly applied for each plot according to recommendations (100 kg ha−1) in the study areas, and all other necessary agronomic crop management practices were carried out properly for all treatments. The experiment was laid out in a randomized complete block design (RCBD) with three replications.
Disease assessment
Disease incidence and severity were recorded and subsequently, area under disease progress curve (AUDPC) and disease progress rate were computed to evaluate the resistance reactions of common bean genotypes against CBB. To determine disease incidence and severity, ten plants per plot were randomly taken from the central rows and marked to collect data. Disease incidence was rated as mean percentage of number of diseased plants per total number of plants considered as in the following formula:
Disease severity was scored from each plot using a 1–9 severity scoring scale (CIAT 1987), where 1 = no visible disease symptom observed, and 9 = more than 25% of the leaf surface area with large coalescing and generally necrotic lesions resulting in defoliation. Severity scoring was done on weekly basis (for six times) starting from the appearance of the first typical disease symptom. Severity scores were converted into percentage severity index (PSI) using the formula suggested by Wheeler (1969) for statistical data analysis.
Areas under disease progress curve (AUDPC) values were calculated from disease severity data from each date of disease assessment as described by Campbell and Madden (1990).
where n is the total number of assessments, ti is the time of the ith assessment in days from the first assessment date and xi is the percentage of disease severity at ith assessment. AUDPC was expressed in %-days, because severity was expressed in percent and time (t) in days.
The resistance reaction type categories were determined from the averages of disease severity scores for each genotype per plot. Furthermore, severity scores at R8 (50% of the plants began to fill the seeds in their first pods or pod filling growth stage) were also used for classifying the genotypes into reaction groups as suggested by van Schoonhoven and Pastor-Corrales (1987) and CIAT (1987). Average scores of ≤ 3, 4–6 and ≥ 7 were considered as resistant, moderately resistant and susceptible, respectively (CIAT 1987).
Growth and yield assessment
Growth parameters
Growth parameters such as days to 50% flowering, plant height and days to 90% physiological maturity were recorded. Days to flowering was recorded by counting the number of days after emergence when 50% of the plants per plot open flower. Days to maturity was recorded by counting the number of days required from emergence up to days when 90% of pods per plant matured per plant. Plant height (cm) was measured using a caliper at physiological maturity stage from the harvestable rows of five randomly taken plants. Mean of five randomly measured plants were used for analysis.
Yield and yield components
Two central rows were harvested to determine grain yield (g) and recorded on a plot basis. Grain yield was corrected at 12% based on seed moisture content, and it was converted to t ha−1. Seed counter and sensitive balance were used to determine hundred seed weight (g) as random sample of the total adjusted yield. Five plants were randomly taken from central rows to calculate yield-related components, which consisted of number of pods per plant (NPP) and number of seeds per pod (NSPP). Pod number per plant was determined by counting pods of the five randomly selected plants, while NSPP was recorded by counting the total number of seeds in a pod from ten randomly sampled pods taken from previously considered five plants.
Data analyses
Disease incidence, severity, AUDPC, growth and yield traits data were subjected to analysis of variance (ANOVA) using SAS GLM procedure (SAS 2014) to determine the response of bean genotypes. Mean separation among genotypes were performed using DMRT (Gomez and Gomez 1984) at 0.05 probability level. Correlation analyses were used to examine associations between and among disease and yield parameters. Logistic [ln(y/1-y)] (Van der Plank 1963) and Gompertz [-ln(-ln(y))] (Berger 1981) epidemiological models were used to determine the disease progress rate from the linear regression for each separate treatment (Van der Plank 1963). The fitness of the models was tested based on the magnitude of coefficient of determination (R2) and residuals standard error (SE) obtained per model (Campbell and Madden 1990). Gompertz model showed higher R2 and lower SE than Logistic model at JARC. Thus, rate of increase in CBB was estimated and compared using Gompertz model at JARC and the Logistic model at Debub Ari. The two locations were different agro-ecologies because of heterogeneity of variance as tested by Bartlett’s test (Gomez and Gomez 1984. Thus, separate analyses were used per location.
Results
Disease incidence
Combined analysis of disease parameters, yield and yield related components data showed significant variation between experimental locations (Table 2). Typical characteristic symptom of CBB was first appeared on genotypes MALB131, MALB17, MALB44, MALB132 and Nasir at 42 days after planting (DAP) at JARC and 44 DAP at Debub Ari on the same genotype. Analysis of variance showed that there were highly significant (p < 0.001) differences among tested genotypes for disease incidence (Tables 2 and 3). At JARC, the highest (85.5%) disease incidence was recorded from genotype MALB104, while the lowest incidence was observed from SER119 (24.4%) and MALB135 (25.5%) (Table 3). SER119 and MALB135 genotypes reduced disease incidence by 71.46% and 70.17%, respectively, compared with MALB104 genotype. At Debub Ari, the highest (80%) disease incidence was recorded from MALB17, followed by MALB131 (76.6%), MALB110 (68.8%), MALB106 (67.7%), MALB122 (65.5%), MALB173 (63.3%); and the lowest (27.7% and 31.1%) disease incidence was recorded on genotypes MALB135 and SER119, respectively (Table 3).
Disease severity
The mean CBB severity on bean genotypes revealed different levels of damage (Table 3). Analysis of variance showed that the highest (74.5% and 66.9%) mean disease severity was recorded from MALB17 at JARC and Debub Ari, respectively. However, it was statistically on par with MALB104 (70.4%), MALB131 (69.7%) and MALB44 (66.7%) at JARC and MALB106 at Debub Ari. The lowest mean disease severity was observed from MALB135 and SER119 at both locations (Table 3). MALB135 and SER119 reduced mean disease severity by 73.4% and 70.8%, respectively, as compared to MALB17 at JARC. Similarly, at Debub Ari, mean severity was reduced by 62.9% (SER119) and 63.4% (MALB135) compared with level of mean severity registered on genotype MALB17. The overall disease pressure was relatively higher in Debub Ari (on farm) than in JARC (on station) in the cropping season.
Area under disease progress curve (AUDPC)
According to ANOVA results, the highest AUDPC value was recorded from MALB17 at JARC (2700.2%-days) and 2387.8%-days at Debeb Ari, while the lowest values were noted from MALB135 and SER119 genotypes at both experimental locations (Table 3). However, the values were not significantly different from KATB9, Dimtu, Dinknesh and SER125 at Debub Ari; and KATB9 at JARC (Table 3). At JARC, planting SER119 and MALB135 reduced AUDPC by 67.97%-days and 70.68%-days, respectively, and at Debub Ari, 67.7%-days and 68.6%-days in that order during the epidemic periods.
Disease progress rate (r)
Disease development showed different rates of progression among common bean genotypes at both experimental locations (Table 4). Planting genotype MALB135 reduced disease progress rate by 87.59% and 79.59% as compared to MALB17 at JARC and Debub Ari, respectively. And also, SER119 reduced disease progress rate by 77.37% at JARC and 67.35% at Debub Ari compared with MALB17. At JARC, the highest (0.137 units day−1) disease progress rate was computed from MALB17, followed by MALB131 (0.128 units day−1) and MALB104 (0.122 units day−1). On the other hand, the lowest (0.017 units day−1) disease progress rate was calculated from KATB9, followed by MALB135 (0.017 units day−1) and SER119 (0.02 units day−1) at JARC. Closely similar trends were noticed at Debub Ari. As compared to Dinknesh (susceptible check), MALB17, MALB131, MALB104, MALB110, Nasir and Hawassa dume had the highest disease development at both locations. On the contrary, genotypes MALB135, SER119, SER125 and KATB9 had the lowest disease development at both experimental sites (Table 4).
Reaction of common bean genotypes to CBB disease
The combined analysis revealed that bean genotypes had highly significant (p < 0.001) mean squares for disease parameters at both locations (Table 2). Thus, the study suggested that the genotypes showed variable response to CBB. The variation in reaction observed among genotypes for CBB could indicate the existence of high genetic variability among the tested genotypes, implying that genotypes could be used to genetically improve resistance to CBB. Accordingly, evaluated genotypes recorded mean severity scores ranged from 1.78 to 7.03 and R8_severtity of 2.0–8.0 at JARC and 2.23–6.56, and R8_severity of 3.0–8.0 at Debub Ari. However, none of the evaluated genotypes and/or lines was immune to CBB at both experimental locations (Table 5).
Based on the reaction group categories of CIAT (1987), van Schoonhoven and Pastor-Corrales (1987) and results obtained, genotypes showing resistant reaction were SER119, MALB135, KATB9 and Dimtu (scores ranging from 2.0 to 3.0), whereas MALB17, MALB131, MALB44, MALB104 and MALB173 (scores ranging from 7.0 to 8.0) were found susceptible at JARC. Similarly, the most resistant genotypes were Dimtu, SER119 and MALB135 (scores of 3.0), while genotype MALB17 was qualified into susceptible groups, which score 8.0 at Debub Ari. The remaining genotypes were classified moderately resistant. Consistency was also observed between mean severity scores of six repeated assessments and R8_severity scores (Table 5).
Growth and yield related components
Number of pods per plant, seeds per pod, 100 seed weight, plant height, days to 50% flowering and days to 90% maturity were significantly varied at both experimental sites (Tables 2, 6 and 7). At JARC, the highest (86 cm) mean value for plant height was measured on the genotype Dimtu, while the lowest (31 cm) height was recorded from Ado(SAB736). Nasir, Dinknesh and Awash 2 were ranked in the same mean height class but statistically on par with MALB44, MALB17 and MALB135, and the remaining genotype also varied each other. Dinknesh took the longest mean days to 50% flowering, while the shortest duration was on SER125, SER119, Ado(ASB736), Awash 2 and MALB131, which was significantly different from MALB132, MALB115, Dimtu and Dinknesh genotypes. The longest mean days to 90% maturity were recorded on Dinknesh, but it was not statistically different from MALB135 and MALB104. On the other hand, the shortest mean values were recorded on Ado(SAB736), MALB17 and MALB110 genotypes (Table 6).
Genotype MALB115 had the highest number of pods per plant. Conversely, the lowest mean number of pods was counted from MALB17. Genotype Awash 2 and KATB9 had the highest and the lowest mean number of seeds per pod, respectively (Table 6). Genotypes recorded different plant height, days to 50% flowering, days to 90% physiological maturity, number of pods per plant and seeds per pod at Debub Ari (Table 7). At both locations, heavier (49.6 g) seed weight was obtained from genotype KATB9 than others. But, the lowest (22.6 g) seed weight was harvested from genotype Awash 2 in the cropping season (Tables 6 and 7).
Grain yield
Analysis of variance indicated that there was highly significant (p < 0.001) difference among tested genotypes for grain yield (Tables 6 and 7). About 57.04% yield gap was recorded between the resistant (SER119) and the susceptible (MALB17) genotypes at JARC. The highest mean grain yield was obtained from SER119 (4.33 t ha−1) and MALB106 (4.33 t ha−1) at JARC, which were not significantly different from the mean grain yield obtained from MALB115, KATB9, Awash 2 and MALB135. The lowest seed yield was recorded from the genotype MALB17 (1.86 t ha−1), which had significant grain yield reduction among tested genotypes (Table 6) and severely infected by CBB during the cropping season (Tables 3 and 4).
At Debub Ari experimental site, the highest (2.76 t ha−1) mean grain yield was harvested from genotype SER119; but it was statistically on par with nearly half of the tested genotypes (Table 7). Genotype SER119 maintained consistent production potential (Tables 6 and 7) and disease resistant reaction at both locations (Tables 3, 4, 5). About 38.04% and 31.15% grain yield reduction was recorded from MALB106 and MALB17, respectively, in comparison with genotype SER119. The lowest (1.71 t ha−1) grain yield was obtained from genotype MALB110 though it was not significantly different from genotypes Nasir, Ado(SAB 736), Awash 2, MALB104 and MALB17 (Table 7).
Association of disease, growth and yield parameters
The associations between disease parameters (PSI, AUDPC and disease progress rate), growth, yield and yield-related components were examined using simple correlation analysis. Variable levels of relationships were found among disease, growth and yield parameters at both experimental locations (Table 8). At JARC, association results showed very strong positive correlation (r = 0.99***) and very highly significant (p < 0.0001) association between severity and AUDPC values. Severity and AUDPC also had positive and very significant (p < 0.01) correlation with disease progress rate, where r = 0.76**. Grain yield was highly significantly (p < 0.01) and negatively correlated (r = – 0.54**, – 0.55** and – 0.46**) with severity, AUDPC and disease progress rate, respectively (Table 8).
Plant height had significant and positive correlation with days to 50% flowering, days to 90% physiological maturity, number of pods per plant and hundred seed weight. Days to 50% flowering showed significant and positive correlation with days to 90% physiological maturity. Grain yield had highly significant (p < 0.01) and positive correlation (r = 0.51**) with number of pods per plant and significant positive correlation (r = 0.30*) with number of seeds per pod (Table 8). Closely similar trends were traced on the associations between and among disease and growth and yield parameters at Debub Ari (Table 8).
Discussion
Common bean diseases cause accountable quantitative and qualitative losses (20–100%) of common bean worldwide (Singh and Schwartz 2010; Karavina et al. 2011). Among which CBB is the most severe and devastating bean disease in Ethiopia (Gudero and Terefe 2018). Thus, host resistance is considered as a cost-effective and eco-friendly method of bean disease management (Popovic et al. 2012a, b; Bhat et al. 2017). In this regard, the response of 20 common bean genotypes to CBB and their agronomic performances were evaluated under field conditions in Southern Ethiopia. Genotypes attained significantly different levels of reaction to CBB where severity scores of 2.0–8.0 at JARC and 3.0–8.0 at Debub Ari were recorded.
Genotypes showing resistant reactions were SER119, MALB135, KATB9 and Dimtu, while MALB17, MALB131, MALB44, MALB104 and MALB173 were grouped susceptible at JARC. Dimtu, SER119 and MALB135, and MALB17 were resistant and susceptible at Debub Ari, respectively. The remaining genotypes (55% and 80%) were in the category of moderately resistant at JARC and Debub Ari, respectively. This finding was in agreement with several studies which confirmed the presence of variable degrees of resistance, susceptibility, tolerance or other classes regarding bean genotypes against CBB both under controlled and field conditions (Todorovic et al. 2008; Perry and Pauls 2011; Donmez et al. 2013; Alladassi et al. 2018).
The significant differences in reaction observed among genotypes for CBB could indicate the existence of high genetic variability in the tested genotypes. That is, genotypes SER119, MALB135, KATB9 and Dimtu could be used to genetically improve resistance to CBB in the study areas and other related agro-ecologies. Similarly, Markell et al. (2009) and Pastor-Corrales et al. (2010) noted that variable responses of genotypes to CBB showed the possibility of obtaining genes for resistance and confirmed the presence resistant genes in the donor parents studied. It could also imply the potential for developing tolerant/resistant cultivars with acceptable varietal characters (Bhat et al. 2017).
Characteristic disease symptom was first spotted on genotypes MALB131, MALB17, MALB44, MALB132 and Nasir on average at 43 days after planting. However, the highest (85.5%) disease incidence was recorded from genotype MALB104 and the lowest disease incidence was observed from SER119 (24.4%) and MALB135 (25.5%) at JARC. Similarly, at Debub Ari, the highest (80%) disease incidence was recorded from MALB17, followed by MALB131 (76.6%), MALB110 (68.8%), MALB106 (67.7%), MALB122 (65.5%), MALB173 (63.3%); and the lowest (27.7% and 31.1%) disease incidence was recorded on MALB135 and SER119, respectively. Comparable trends were observed for disease severity, AUDPC and disease progress rate at both locations.
For instance, MALB135 and SER119 reduced mean disease severity by 73.4% and 70.8%, respectively, compared with MALB17 at JARC. Similarly, mean severity was reduced by 62.9% (SER119) and 63.4% (MALB135) compared with level of mean severity observed from genotype MALB17 at Debub Ari. Regarding AUDPC, genotypes SER119 and MALB135 reduced AUDPC values by 67.97 and 70.68%-days, respectively, at JARC; and 67.7 and 68.6%-days in that order at Debub Ari. Such variability among genotypes could be explained by the genetic status of the host, along with other factors such as environmental adaptability.
Other related studies also noted that common bean genotypes showed variable responses to different diseases. For example, Bhat et al. (2017) reported that evaluation of 41 genotypes to CBB showed significantly different response in terms of disease intensity to the pathogen. Thirty four bean genotypes, with different backgrounds, were evaluated for angular leaf spot at three sites of Kenya in 2014; and entries revealed significant variability with respect to disease severity and between sites (Kimno et al. 2016). And also, Ruphine (2018) screened 24 common bean genotypes for disease resistance and agronomic performance at two sites in Kenya for two consecutive years. In the study, genotypes revealed significant variations for diseases and agronomic traits. Of the evaluated genotypes, genotypes Red34, KKBC05/32, KK071, GLP2, Ciankui, RWR2245 and Cal6 were found resistant to CBB (Ruphine 2018).
Amongst the sites, the overall disease pressure was relatively higher in JARC than in Debub Ari areas. Of course, bean planting at JARC increased disease severity and AUDPC by 9.92% and 10.29% as compared to planting at Debub Ari, respectively, in the cropping season. Even though plant disease epidemic is influenced by availability of host tissue, temperature, moisture and plant resistance levels, and other factors (Campbell and Madden 1990), the disease pressure in the former location could be mainly attributed to the presence of plant debris from previous seasons as the field history confirmed the case and it is a trial site for long years. Crop debris could result in inoculum buildup in the area and aggravate CBB epidemics. Gudero and Terefe (2018) noticed that accumulation of crop debris could increase CBB severity by up to 44.49% in a comprehensive survey study in Southern Ethiopia. In addition, Gilbertson et al. (1990) indicated that Xanthomonas axonopodis pv. phaseoli could survive longer on crop residues left on the soil surface, which might serve as source of primary inoculum.
Analysis of variance also pointed out a considerable genotypic variation for number of pods per plant, seeds per pod, hundred seed weight, plant height, days to flowering, days to physiological maturity and grain yield at both JARC and Debub Ari experimental sites. Various previous studies also confirmed the presence of genotypic variations among common bean genotypes for growth and yield parameters at different experimental conditions for many reasons (Chavez-Servia et al. 2016; Kimno et al. 2016; Ersulo and Dana 2018). In this regard, a related field study, using six improved common bean varieties, in Southern Omo of Southern Ethiopia in 2015, indicated that responses of varieties significantly varied for number of pods per plant, seeds per pod, 1000-seed weight, plant height, days to flowering, days to maturity and grain yield (Mitiku and Mesera 2017).
In terms of grain yield and disease reaction, the best performing genotypes were SER119, MALB106, MALB115, KATB9, Awash 2 and MALB135, which recorded mean grain yield of 4.33, 4.33, 4.10, 3.86, 3.90 and 3.90 t ha−1, respectively, at JARC. High yielder genotypes SER119 and MALB135 recorded a yield gain of 57.04% compared with the least performer genotype MALB17, which gave only 1.86 t ha−1. Closely similar trends were observed at the second experimental site though the lowest (1.71 t ha−1) grain yield was obtained from genotype MALB110. Genotype SER119 maintained consistent production potential and disease resistant reaction and low yielding genotypes were severely infected by CBB at both locations.
A study by Ersulo and Dana (2018) on the performance of bean varieties on yield and yield-related components in Segen Zone of Southwestern Ethiopia in 2015 and 2016, found significant difference among genotypes for the studied traits. The difference is attributed to the existence of genetic variability among genotypes (De Lima et al. 2012). Also, Fekadu (2013), Emishaw (2007) and Mitiku & Meresa (2017) reported that grain yield of common bean was affected by the background of the genotypes under Ethiopian conditions. Furthermore, Szilagyi (2003) conducted a study on yield and yield components using Bulgarian common bean genotypes and discovered a wide variation among bean cultivars for different yield components.
Moreover, genotypes produce relatively higher (3.21 t ha−1) overall mean grain yield at JARC than at Debub Ari (2.27 t ha−1), which negated with disease progression in the cropping season. This could be explained by its favorable environmental condition for crop growth and high soil fertility status in the area (as it is continuously used to serve as evaluation experimental site). Low seed yield obtained from Debub Ari might not be associated only to the disease development but also adaptability of genotypes to the area could also be another factor which might contribute to reduced yield of genotypes. Similarly, Daniel et al. (2014) noted that variations in yield among bean genotypes were from adaptability potential of the crop in addition to biotic constraints of production. A landrace by environment study in Spain indicted the existence of significant effects of both environment and environment by landrace interactions for growth and yield traits of common bean landraces (Casquero et al. 2006).
The correlation study implied positive and highly significant association among disease parameters. Yield parameters also had a positive correlation among themselves. However, it was studied that disease parameters maintained negative and highly significant relationship with yield traits. For example, susceptible genotypes recorded higher AUDPC values which resulted in severe grain yield reductions computed to be nearly by half as compared to the resistant genotypes at both locations. Several other studies also concluded that disease and yield parameter have negative associations (Kassahun 2008; Lemessa et al. 2011; Boersman et al. 2015) for various reasons and could result in recognizable yield reductions.
Conclusions
The present study investigated the resistance reaction of 20 common bean genotypes to CBB and their agronomic performance. The study demonstrated that the test genotypes showed significantly varying response to severity of CBB, which ranged from 19.8 to 74.5%. This could imply the existence of resistance sources against the disease. During the study, genotypes were grouped as 20% resistant, 55% moderately resistant and 25% susceptible at JARC and 15% resistant, 80% moderately resistant and only 5% susceptible at Debub Ari. Also, remarkable genotypic variation for quantitative growth and yield characters such as plant height, days to 50% flowering, days to 90% physiological maturity, number of pods, number of seeds, seed weight and grain yield were noticed. Among evaluated genotypes, SER119, KATB9, Dimtu and MALB135 showed resistant reaction at JARC and except KATB9 at Debub Ari. SER119 had high production potential in addition to disease resistance and gave 4.3 t ha−1 at JARC and 2.76 t ha−1 at Debub Ari. Results indicate that there is a potential for disease resistance coupled with important agronomic traits to develop superior genotypes in the bean breeding program. Thus, it is recommendable to use SER119 genotype with proper cultural practices for reducing CBB damages and grain yield losses in the study areas, and MALB135 genotype as sources of resistance to CBB. However, it is important to evaluate common bean genotypes for more seasons across locations to confirm their consistency for CBB resistance reaction and agronomic characters, along with other diseases in searching for multiple disease resistance.
References
Ali K, Kenneni G, Ahmed S, Malhotra R, Beniwal S (2006) Food and forage legumes of ethiopia: progress and prospects. Proceeding of the Workshop on Food and Forage Legumes, 22–26 September 2003, Addis Ababa, Ethiopia. ICARDA, Aleppo, Syria
Alladassi BME, Nkalubo ST, Mukankusi C, Kayaga HN, Gibson P, Edema R, Urrea CA, Kelly JD, Rubaihayo PR (2018) Identification of common bean genotypes with dual leaf and pod resistance to common bacterial blight disease in Uganda. Afr Crop Sci J 26(1):63–77
Arnaud-Santana E, Coyne DP, Eskiridge KM, Vidaver AK (1994) Inheritance, low correlations of leaf, pod, and seed reactions to common blight disease in common beans, and implications for selection. J Am Soc Hortic Sci 119:116–121
Asrat A, Blair MW, Almekinders C (2009) Genetic diversity and population structure of common bean (Phaseolus vulgaris L.) landraces from the East African highlands. Theor Appl Genet 120:1–12
Berger RD (1981) Comparison of the Gompertz and Logistic equations to describe plant disease progress. Phytopathology 71:716–719
Bhat HA, Khurshid A, Rayees AA, Sajad HW, Arif H, Showket AD (2017) Resistant resources and transmission of bacterial blight of common beans (Phaseolus vulgaris L.). J. Pharmacogn Phytochem. 6(4):380–383
Boersman JG, Hou A, Gillard CL, McRae KB, Conner RL (2015) Impact of common bacterial blight on the yield, seed weight and seed discoloration of different market classes of dry beans (Phaseolus vulgaris L.). Can J Plant Sci 95:703–710
Buruchara R, Mukankusi C, Ampofo K (2010) Bean disease and pest identification and management: Handbooks for small-scale seed producers. Handbook four. CIAT publication No. 371. International center for Tropical Agriculture, Kampala, Uganda
Campbell CL, Madden LV (1990) Introduction to Plant Epidemiology. John Wiley and Sons, New York, USA
Casquero PA, Lema M, Santalla M, De Ron AM (2006) Performance of common bean (Phaseolus vulgaris L.) landraces from Spain in the Atlantic and Mediterranean environments. Genet Resour Crop Evol 53:1021–1032
Chávez-Servia JL, Heredia-García E, Mayek-Pérez N, Aquino-Bolaños EN, Hernández-Delgado S, Carrillo-Rodríguez JC, Gill-Langarica HR, Vera-Guzmán AM (2016) Diversity of common bean (Phaseolus vulgaris L.) landraces and the nutritional value of their grains. Grain Legum. https://doi.org/10.5772/63439
CIAT (1987) Standard systems for the evaluation of bean germplasm. International Center for Tropical Agriculture, CIAT, Cali, Colombia
CSA (2018) Report on area production of major crops (private peasant holdings) in Meher Season: Agricultural sample survey. Volume I, Statistical Bulletin 586, Addis Ababa, Ethiopia
Cuervo M, Medina C, Ramirez JL, Balcazar S, Martinez J, Debouck D (2012) Bacteria of common bean. http://cropgenebank.sgrp.cgiar.org/index.php?option_com_content and view_article&id_412 and itemid_597. Accessed on 13 July 2019
Daniel T, Teferi A, Tesfaye W, Assefa S (2014) Evaluation of improved varieties of haricot bean in West Belessa. Northwest Ethiopia Int J Sci Res 3(12):2319–7064
de Lima MS, Carneiro JE, Carneiro JES, Carneiro PCS, Pereira CS, Vieira RF, Cecon PR (2012) Characterization of genetic variability among common bean genotypes by morphological descriptors. Crop Breed. Appl Biotechnol 12:76–84
Donmez MF, Sahin F, Elkoca E (2013) Identification of bean genotypes from Turkey resistance to common bacterial blight and halo blight diseases. Acta Sci Pol Hortorum Cultus 12(4):139–151
Durham KM (2011) Evaluation of common bacterial blight resistance in a resistant inter-cross of population of common bean. Master’s thesis. University of Gelph, Ontario, Canada
Emishaw W (2007) Comparison of the growth, photosynthesis and transpiration of improved and local varieties of common bean (Phaseolus vulgaris L.) at Haramaya. Master’s thesis. Haramaya University, Haramaya, Ethiopia
Ersulo D, Dana A (2018) Evaluation of common bean varieties for yield and yield component in Segen Area Peoples Zone, SNNPRS. Ethiopia Food Sci Qual Manag 72:11–14
FAOSTAT (2014) Dry Bean. Statistical database. http://faostat.fao.org/site/567/default.aspx#ancor. Accessed on 22 June 2019
Fekadu G (2013) Assessment of farmers’ criteria for common bean variety selection: the case of Umbullo water shade in Sidamo Zone of the southern region of Ethiopia. Ee J Res Innov Fore 5(2):4–13
Fikire L (2004) Effect of intercropping and cultivar mixtures on bean diseases and yield. Pest Mgt J Eth 8:71–81
Fininsa C (2003) Relationship between common bacterial blight severity and bean yield loss in pure stand and maize bean intercropping. Int J Pest Manag 49:177–185
Fininsa C, Yuen J (2001) Association of bean rust and common bacterial blight epidemics with cropping systems in Hararghe highlands Eastern Ethiopia. Int J Pest Manag 47:211–219
Fourie D, Herselman L, Mienie C (2011) Improvement of common bacterial blight resistance in South African dry bean cultivar Teebus. Afr Crop Sci J 19(4):377–386
Gilbertson RL, Rand RE, Hagedorn DJ (1990) Survival of Xanthomonas campestrispv. phaseoli and pectolytic strains of Xanthomonas campestris in bean debris. Plant Dis 74:322–327
Gillard CL, Conner RL, Howard RJ, Pauls KP, Shaw L, Taran B (2009) The performance of dry bean cultivars with and without common bacterial blight resistance in field studies across Canada. Can J Plant Sci 89:405–410
Gomez KA, Gomez AA (1984) Statistical Procedures for Agricultural Research. John Wiley and Sons, Singapore
Gudero G, Terefe H (2018) Distribution and association of factors influencing bean common bacterial blight (Xanthomonas axonopodis pv. phaseoli) epidemics in Southern Ethiopia. Arch Phytopathol Plant Prot. 51(19–20):1066–1089
Karavina C, Mandumbu R, Parwada C, Zivenge E (2011) Epiphytic survival of Xanthomonas axonopodispv. phaseoli (EFSM). J Anim Plant Sci. 9(2):1161–1168
Kassahun A (2008) Reaction of common bean cultivars to Xanthomonas axonopodis pv. phaseoli strains and integrated management of common bacterial blight in Eastern Amhara Region, Ethiopia. Master’s thesis. Haramaya University, Haramaya, Ethiopia
Kimno SK, Kiplagat OK, Arunga EE, Chepkoech E (2016) Evaluation of selected French bean (Phaseolus vulgaris L.) genotypes for resistance to angular leaf spot (Pseudocercospora griseola) in Western Kenya. Am J Exp Agric 13(4):1–6
Lemessa F, Sori W, Wakjira M (2011) Association between angular leaf spot (Phaeoisariopsis griseola (Sacc.) Ferraris) and common bean (Phaseolus vulgaris L.) yield loss at Jimma Southwestern Ethiopia. Plant Pathol J. 10(2):57–65
Maniruzzaman (2015) Evaluation of genetic resistance to common bacterial blight in dry Edible bean. Master’s thesis, North Dakota State University of Agriculture and Applied Sciences, Fargo, North Dakota
Markell SM, Pastor-Corrales MA, Jordahl JG, Lampa RS, Mathew FB, Osorno JM, Goswami RS (2009) Virulence of Uromyces appendiculatus to the resistance gene Ur-3 identified in North Dakota in 2008. Annu Rep Bean Improv Coop 52:82–83
McMullen MP, Lamey HA (2000) Seed treatment for disease control. Disease management guide. Northern Dakota State University, North Dakota
Mitiku M, Mesera T (2017) Performance evaluation of common bean (Phaseolus vulgaris (L.)) varieties at Benatsemay Woreda of South Omo Zone, SNNPR. Ethiopia. Int J Agric Biosci 6(6):277–280
Mutlu N, Miklas P, Reiser J, Coyne D (2005) Backcross breeding for improving resistance to common bacterial blight in pint beans (Paseolus vulgaris L.). Plant Breed 124:282–287
Osdaghi E, Alizadeh A, Shams-Bakhsh M, Lak MR (2009) Evaluation of common bean lines for their reaction to the common bacterial blight pathogen. Phytopathol Mediterr 48:461–468
Parades MC, Becerra V, Tay JU (2009) In organic nutritional composition of common bean (Phaseolus vulgaris L.) genotypes race Chile. Chil J Agric Res 69:486–491
Pastor-Corrales MA, Wright EM, Markel SG, Awale HE, Kelly JD, Jordahl JG, Lamppa RS, Mathew FM, Osorno JM, Goswami RS (2010) Comparing the virulence of new races of the common bean rust pathogen from Michigan and North Dakota. Annu Rep Bean Improv Coop 53:128–129
Perry G, Pauls KP (2011) Common bacterial blight in Phaseolus vulgaris. In: Hendriks BP (Ed.) Agricultural Research Updates - Volume 2. Nova Science Publishers Inc., New York, USA
Petry N, Boy E, Wirth JP, Hurrell RF (2015) The potential of the common bean (Phaseolus vulgaris L.) as a vehicle for iron biofortification. J Nutr 7:1144–1173
Popovic T, Starovic M, Aleksic G, Zivkovic S, Josic D (2012a) Response of different beans against common bacterial blight disease caused by Xanthomonas axonopodispv. phaseoli. Bulg J Agric Sci 18:701–707
Popovic T, Starovic M, Aleksic G, Zivkovic S, Josic D, Ignjatov M, Milovanovic P (2012b) Response of different beans against common bacterial blight disease caused by Xanthomonas axonopodis pv phaseoli. Bulg J Agric Sci 18(5):701–707
Ribeiro T, Azebado CVG, Esteves JAdeF, Carbonell SAM, Ito MF, Chiorato AF (2017) Reaction of common bean lines to Xanthomonas axonopodis pv. phaseoli and Curtobacteria flaccumfaciens pv. flaccumfaciens Crop Breed. Appl Biotechnol 17:40–46
Ruphine NB (2018) Resistance of common bean genotype to foliar and bacterial diseases. Master’s thesis. University of Nairobi, Kenya
SAS Institute (2014) SAS/STAT user’s guide, version 9.3. Cary. SAS Institute Inc., New York
Selamawit C (2004) Occurrence of common bacterial blight strains and its effect on quality of bean seeds in Ethiopia. Master’s thesis. Haramaya University, Haramaya, Ethiopia
Singh SP, Schwartz HF (2010) Breeding common bean for resistance to disease. Crop Sci 50:2200–2223
Szilangyi L (2003) Influence of drought on seed yield components in common beans. Bulg J Plant Physiol 320–330 (Special Issue)
Tekle Y, Getachew G, Wondewosen S, Tibebu S, Ermias M (2014) Evaluation of common bean (Phaseolus vulgaris L.) varieties for yield and yield components. J Biol AgricHealthcare 4(17):22–25
Todorovic J, Vasic M, Todorovic J (2008) Bean and green beans. Institute for Vegetable and Field Crops, Faculty of Agriculture, Novi Sad, Serbia
Tumsa K, Negash K, Amsalu B, Ayana G, Rezene Y, Tsegaye D (2015) Resistance breeding against major disease of common bean in Ethiopia. In: National bean research Programs, Ethiopian Institutes of Agricultural Research, Addis Ababa, Ethiopia
Van der Plank JE (1963) Plant diseases: epidemics and control. Academic Press, New York
van Schoonhoven A, Pastor-Corrales AA (1987) Standard system for the evaluation of bean germplasm. Centro Internacional de Agricultura Tropical, Cali, Colombia. https://doi.org/10.1007/s13398-014-0173-7.2
Viteri DM, Singh SP (2014) Response of 21 common beans of diverse origins to two strains of the common bacterial blight pathogen Xanthomonas campestris pv. phaseoli. Euphytica 200:379–388
Wheeler JBEJ (1969) An introduction in plant diseases. Wiley, London
Acknowledgements
We would like to thank Southern Agricultural Research Institute for financing the study. Melkassa and Hawassa Agricultural Research Centers are well acknowledged for providing seeds of common bean genotypes for the study. We are very grateful to staff members of Jinka Agricultural Research Center for their all rounded support during the field study. We would also like to appreciate growers of Debub Ari district who involved in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Adila, W., Terefe, H. & Bekele, A. Common bacterial blight (Xanthomonas axonopodis pv. phaseoli) resistance reaction in common bean genotypes and their agronomic performances in Southern Ethiopia. J. Crop Sci. Biotechnol. 24, 387–400 (2021). https://doi.org/10.1007/s12892-021-00087-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-021-00087-4