Abstract
Plant height determines plant biomass yield, harvest index and economic yield. We analyzed quantitative trait loci (QTL) and gene action controlling plant height. We generated the maternal and paternal testcrossing (TC/M and TC/P) populations based on a recombinant inbred line population. Data for plant height at t1, t2, t3, t4 or t5 stages were collected over 2 years from 3 TC/M field trials and 2 TC/P field trials. At single-locus level, 32 QTLs at five stages and 24 conditional QTLs at four intervals were detected, and 14 QTLs shared in different years or populations or stages. Plant height displayed dynamic characteristics through expression of QTLs. A total of 21 novel QTLs were detected and 11 QTLs validated the previous results. And 19 QTLs explained over 10% of phenotypic variation, such as qPH-Chr9-2, qPH-Chr19-4 and qPH-Chr22-4. The region of NAU5330-NAU1269 on chromosome 19 may be a desired target for genetic improvement of plant height in Upland cotton. In addition, five and eight heterotic loci were identified in TC/M and TC/P populations, respectively. Additive, partial dominance and overdominance effects were observed in both TC populations. We also identified 43 epistatic QTLs and QTLs by environment interactions by inclusive composite interval mapping method. Taken together, additive, partial dominance and overdominance effects together with epistasis explained the genetic basis of plant height in Upland cotton.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hybrid has superior performance over its parents with diverse genetic basis in growth speed, stress resistance, fitness, quality improvement and yield potential, this phenomenon is termed as heterosis or hybrid vigor. Heterosis was exploited in many crops in agriculture, while the mechanism of heterosis is vague up till now. Three hypotheses tried to explain the phenomenon, including dominance, over-dominance and epistasis (Chen 2013; Li et al. 2015). The dominance hypothesis describes that the better performance of F1 over both of its parents was contributed by dominant alleles masking deleterious recessive alleles (Bruce 1910; Jones 1917; Xiao et al. 1995). The over-dominance hypothesis illustrated the superiority of heterozygote with interaction between dominant allele and recessive allele (Krieger et al. 2010). The pseudo-overdominance referred to obvious overdominance effect, which wasn’t accurate in single locus due to linked loci located in repulsion phase (Jones 1917; Li et al. 2015). The epistasis hypothesis assumed that interactions among non-allelic QTLs or genes contributed to heterosis (Yu et al. 1997; Hua et al. 2002, 2003).
Plant height refers to the sum of internode lengths above ground, reflecting the status of vegetative growth in crop plants (Shang et al. 2016a). It directly affects planting density in crop production, which plays an essential role in determining plant architecture, the resistance to lodging, and key technological links for machine harvesting. The Green Evolution, in association with chemical fertilizers, pesticides, controlled irrigation and new methods of cultivation, including mechanization, was accomplished by projecting plant height using the high-yielding semi-dwarf rice variety (Farmer 1986; Sasaki et al. 2002). More than 1300 QTLs were detected underlying plant height in rice, maize, soybean, triticale, cotton and so on (http://www.gramene.org/qtl; http://www2.cottonqtldb.org:8081/index). A total of 15 QTLs with partial dominance effect were detected for plant height for 15 varied chromosome segment substitution lines (CSSLs) in rice; and interactions of additive × additive (AA) and additive × dominance (AD) were observed by segregating at the four major QTLs with the largest effects on plant height (Shen et al. 2014). These researches demonstrated that dominance and epistasis were the major genetic basis of plant height.
Previous studied detected plant height for heterosis differed in several crops, such as 42.0% of mid-parent heterosis (MPH) in maize (Larièpe et al. 2012), 35.9% in rice (Shen et al. 2014), 20.6% in wheat (Zhang et al. 2007) and 8.5% in Upland cotton (Shang et al. 2016a). Nine heterotic loci for plant height were identified from 203 single segment substitution lines (SSSLs), resulting that QTLs with over-dominance effect were main contributors to heterosis for plant-related traits at the single-locus level in maize (Wei et al. 2015). Another study discovered that heterosis on plant height generated by pseudo-overdominance using a recombinant inbred line (RIL, hereinafter same) population in sorghum by dissecting different height components of the known auxin transporter Dw3 gene (Li et al. 2015). Recently, the heterozygosity for plant height increased gibberellins (GA) levels yields by genome-wide association studies (GWAS) using BC1-derived doubled haploid lines in maize (Hu et al. 2017), consistent with result that GA were up-regulated in wheat hybrids (Zhang et al. 2007). A total of 14 environmentally common QTLs with overdominance effect were identified for plant height and ear height using a RIL based design III population in an elite maize hybrid (Li et al. 2017).
The ‘immortalized’ testcross (TC) populations based on a RIL population allowed repeated experiments and analyses by creating heterozygotes, as the immortalized F2 population (Hua et al. 2002; Mei et al. 2005). Previous studies underlying heterosis were reported by the permanent BC populations in rice (Xiao et al. 1995; Li et al. 2001, 2008), maize (Frascaroli et al. 2007), rapeseed (Radoev et al. 2008) and cotton (Shang et al. 2015, 2016a, b, c). However, few reports on QTL analysis controlling dynamic plant height were performed in Upland cotton. QTLs controlling plant height were differently expressed at developing stages; and the genetic basis of quantitative traits only at final maturity is not representative in Upland cotton (Shang et al. 2015). Another dynamic analysis for plant height in our lab demonstrated that QTLs mainly showed partial dominance effect at the early stage and mostly displayed overdominance effect at the later stage (Shang et al. 2016a). Plant height is a representative dynamic trait related to heterosis, which is an accurate measured trait to explore heterosis. But no study reported on dynamic QTLs and heterotic loci for plant height using two corresponding parental TC populations in cotton and other crops. In the present study, both maternal TC population (TC/M population) and paternal TC population (TC/P population) were simultaneously developed based on one RIL population to explore dynamic QTLs and dynamic heterotic loci controlling plant height at multiple developmental stages in Upland cotton.
Materials and methods
Plant materials
The RIL population was developed by single seed descent method derived from an Upland cotton hybrid ‘Xinza 1’ (GX1135 × GX100-2) in previous work (Shang et al. 2015, 2016a, b, c). Two experimental populations were developed based on RIL population consisting of 177 lines of F14 generations: (1) the maternal testcrossing population (hereafter TC/M population): 177 hybrids originated from 177 F14 RILs testcrossed by original female parent GX1135, respectively; (2) the paternal testcrossing population (hereafter TC/P population): 177 hybrids originated from 177 F14 RILs testcrossed by original male parent GX100-2, respectively. The inbred seeds of 177 RI lines seeds and 354 hybrid accession seeds were obtained in Sanya, Hainan in 2015 and 2016. The control set was planted for four repeats in every field trial as: GX1135, ‘Xinza 1’, GX100-2, and a local commercial hybrid as a competition control. The commercial hybrid ‘Ruiza 816’ was regarded as the competition control in Yellow River Region (E1 and E2, see details below), and ‘Ezamian 10’ in Yangtze River Region (E3, see details below).
Field trials and trait evaluation
A total of five field trials were sown in 2015E1, 2015E3, 2016E1 and 2016E2 following randomized complete block design with two replications. Two TC/M trials were conducted at first at final stage in 2015E1 and 2015E3, containing RIL population (hereafter RIL-M population), corresponding TC/M population, GX1135 as a common testcrossing male parent, and the control set. Then, the third TC/M trial was arranged for plant height trait at five development stages in 2016E2. Two TC/P field trials were performed including RIL population (hereafter RIL-P population), TC/P population, GX100-2 as another common testcrossing male parent, and the control set at five development stages in 2016E1 and 2016E2. Three locations mentioned above see details in Ma et al. (2017). Each BC1F14 progeny was inter-planted in the middle of its female parent and its common testing-male parent GX1135 (original female parent of ‘Xinza 1’) or GX100-2 (original male parent of ‘Xinza 1’) for one replication. Totally, 904 plots with two rows per plot (18 plants each) were planted including four control sets in every trial. The field management was performed by the local routine method.
Data for plant height were recorded by measuring the main-stem height of individuals before the cotton plants were removed the shoot apex (Li et al. 2015; Shang et al. 2015, 2016a). The height measuring unit was centimeter (cm). Eight scored plants without the marginal effect were chosen to evaluate in every plot. The data were collected over the period of 2 years. A total of 4520 plots in five field trials were evaluated at multiple stages. The TC/M trial in 2015E1 was measured at the final stage (t5: September 1); and the TC/M trial in 2015E3 was measured at t5 stage in September 6. Plant height in both TC/M and TC/P trials in 2016E2 were measured for five stages at intervals of 12 days from June 9 to July 27, respectively (t1: June 9, t2: June 21, t3: July 3, t4: July 15 and t5: July 27). Plant height in TC/P trial in 2016E1 was measured just at two early development stages, t1: June 9, and t2: June 21, with three sets of missing data due to the hailstone disaster. The data at a certain stage were used to map QTL and the incremental values during four intervals were used to map conditional QTL.
Genetic map and data analysis
The genetic map of simple sequence repeats (SSR) markers based on the RIL population and the genotype data of TC/M population have been published before (Shang et al. 2016b). A total of 623 loci were distributed on 31 linkage groups, which anchored on 26 chromosomes. The map covered 3889.9 cM (88.20%) of Upland cotton genome with interval of 6.2 cM on average. The genotype data of TC/P population were deduced by that of RIL population based on genetic mating designs (See Table S7).
Mid-parent heterosis value (MPH, hereinafter same) of each TC progeny was deduced by phenotypic values of its parents planting both sides of the hybrid. Heterotic loci referred to QTL detected by MPH datasets (Hua et al. 2003; Mei et al. 2005), which defined as follows: a = (P1P1 − P2P2)/2; MPH = d = [F1 − (P1P1 + P2P2)/2]; F1 = (a + d) (P1 and P2, alleles from female and male parents of F1, respectively). Datasets in single environment and the best linear unbiased estimates (BLUEs) across the environments assuming fixed effects for the genotype were used to map QTLs. The statistical analyses were performed using R package of lsmeans (version, 2.27-61; Russell 2016; Liu et al. 2016) assuming a full random model as follows: Y = genotype + environment + genotype × environment + block, where block involved two replicates in each environment. At single locus level, we mapped single-locus QTLs in the confidence interval of 95% and estimated genetic effects by the software QTL Cartographer (Version 2.5) (Zeng 1994; Wang et al. 2007). The composite interval mapping (CIM) method was used for QTL mapping for multiple datasets. Estimating by 1000 permutation times, the threshold of LOD declared a significant QTL at significant level of P < 0.05, whereas the QTL with at least LOD 2.0 was considered as a common QTL in another environment or population (Shang et al. 2016b). The degree of dominance was estimated for common QTLs derived from different populations or datasets (Radoev et al. 2008). Three types of genetic effect for single-locus QTLs were defined: additive effect loci just detected in TC population, complete or partial dominant effect loci with d/a ≤ 1, over-dominant effect loci with d/a > 1 or QTLs just detected by MPH data (Luo et al. 2009; Shang et al. 2016a). The QTL was identified by a set of phenotypic values at one development stage t(n); The conditional QTL was identified by increment dataset during a period from stage t(n) to stage t(n + 1) (Shang et al. 2016a). Common QTLs were defined QTLs flanking the position linked and shared common marker(s) in different populations or stages (Shao et al. 2014).
At two-locus level, the software of QTL IciMapping 4.1 (www.isbreeding.net) had proved to be more efficient for controlling background by detection of QTL × environment interaction (Meng et al. 2015; Shang et al. 2016a). Thus, we conducted the two-locus analysis using inclusive composite interval mapping (ICIM) method. A threshold LOD 2.5 and 5 scores were used to declare significant main effect QTLs and QTL × environments (M-QTLs and QEs), and epistatic QTLs and QTL × environments (E-QTLs and QQEs) (Shang et al. 2016a).
Results
Phenotypic performance for plant height at multiple stages
Table 1 presented phenotypic performance for plant height in RIL, TC/M, TC/P, MPH-M and MPH-P datasets at five stages in three environments. The original female parent GX1135 displayed higher plant height than the original male parent GX100-2 on average. At t5 stage, the average plant height was greater in 2015E3 in Yangtze River Region than that in 2015E1 in Yellow River Region in RIL-M and TC/M populations. It was attributed to different plant architectures at the two locations because of different local photo-thermal conditions and cultivation strategies. Plant height showed hybrid vigor with wide ranges from − 13.54 to 19.54% on MPH datasets, similar to the tendency in rice from − 7.40 to 14.40% of MPH (Shen et al. 2014). The increment of growth rate was larger at early stages (∆t1–2, ∆t2–3 and ∆t3–4) than that at the last development interval (∆t4–5). The mid-parent heterosis (MPH) showed a dynamic character from t1 to t5 in both TC populations (Shang et al. 2015). In the same environment (Hejian, 2016E2), mean values in TC/M population were superior to that in RIL-M population. On the contrary, the plant height heterosis on TC/P progenies decreased rather than RIL-P population due to inferior performance of the current male parent GX100-2 with recessive homozygotes. Mean values of MPH datasets increased by two or three times in TC/M population than that in TC/P population. The results indicated that average performance of two parents determined the performance of their hybrid.
Variance analysis was performed for replicates across multiple environments or at multiple stages or intervals for RIL, TC and MPH datasets in TC/M and TC/P trials (Table 2). For the majority of plant height datasets, genotype and environment variances showed significant difference at 0.01 or 0.001 significance levels. On the contrary, genotype × environment and environment × replicate variation of the majority of datasets showed non-significant except in TC/M trials at t5 stages. The correlation presented in Table S1 between RIL, TC and MPH datasets in two TC trials. Highly positive correlations were observed between RIL and TC performance at five stages in TC/M and TC/P trials. Similarly, correlations showed highly positive between TC and MPH performance, consistent with the previous study (Shang et al. 2016a). In both TC trials, negative or non-significant relationships were observed between RIL and MPH datasets at all of development stages for plant height.
QTLs, conditional QTLs and heterotic loci at single locus level
In the present study, a total of 42 QTLs and conditional QTLs were identified from TC/P, TC/M, RIL, MPH-P and MPH-M datasets (Tables 3, 4).
A total of 32 QTLs were detected at five development stages of t1, t2, t3, t4 and t5 (Table 3). Overall, 11, 14, nine, nine and 13 QTLs were detected at t1, t2, t3, t4 and t5 stages, respectively. Sixteen common QTLs verified each other at multiple stages, environments or populations. In TC/P trials, 12 QTLs were identified in TC/P population including three common QTLs of qPH-Chr1-1, qPH-Chr2-1 and qPH-Chr19-5. From MPH-P datasets, 7 heterotic loci were detected including three common QTLs of qPH-Chr1-1, qPH-Chr12-1 and qPH-Chr9-2. The qPH-Chr1-1 shared in TC/P and MPH-P datasets, which was also resolved at t3, t4 and t5 stages at the same time (Fig. 1). Two common heterotic loci named qPH-Chr1-1 and qPH-Chr9-2 were detected simultaneously at t3, t4 and t5 stages using MPH-P datasets. The qPH-Chr1-1 was also detected in TC/P dataset. However, over-dominant qPH-Chr1-1 displayed negative genetic effect and the over-dominant qPH-Chr9-2 showed positive genetic effect. The detected qPH-Chr19-5 in TC/P population showed additive effect at t1, t2, t4 and t5 stages. A total of 14 additive QTLs, 3 partial dominant QTLs and 11 over-dominant QTLs were estimated in TC/P trials (Table 5). In TC/M trials, six and four QTLs were observed using TC/M and MPH-M datasets, respectively (Fig. 1). In both datasets, four, four, four, three and seven QTLs were detected at stages t1, t2, t3, t4 and t5, respectively. Both qPH-Chr19-2 and qPH-Chr19-4) were detected at the early development stages (t1, t2 and t3). The qPH-Chr19-2 showed partial dominant effect in the three stages in TC/M population, explaining 12.30% of PV on average. The QTL qPH-Chr19-4 explained 11.72–20.93% of phenotypic variation (PV), and increased 1.10-2.29 cm plant height providing alleles by the current female parent. However, qPH-Chr25-1 and qPH-Chr26-1 were detected at the later stages just in TC/M population. The qPH-Chr25-1 was simultaneously identified at t3, t4 and t5 stages in TC/M population in 2016E2, which showed additive effect. The four QTLs (qPH-Chr2-1, qPH-Chr14-1, qPH-Chr19-2 and qPH-Chr19-4) were detected repeatedly in TC/P trials. The qPH-Chr20-1 displayed apparent over-dominant effect, which was detected in both TC/M and MPH-M datasets. A total of 5 additive QTLs, 3 partial dominant QTLs and 6 over-dominant QTLs were estimated in TC/M population (Table 5).
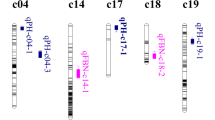
Locations of QTLs controlling plant height identified at five stages in two parental TC populations * and ** (# and ##), marker showed respectively segregation distortion significant at P = 0.05 and 0.01 levels; markers with * and ** skewed toward the GX1135 alleles, and markers with # and ## skewed toward the GX100-2 alleles. t1–t5 refer to five development stages
Together, eight and 18 QTLs were identified in the TC/M and TC/P populations, respectively. Only the qPH-Chr19-2 shared in both TC populations. All of six common QTLs showed stable genetic effects, indicating high accuracy of these QTLs and be valuable to MAS breeding. For example, qPH-Chr2-1 with additive effect was simultaneously detected at four stages t1, t2, t3, t4 and t5 in TC/P population, respectively. Three common QTLs (qPH-Chr19-1, qPH-Chr19-2 and qPH-Chr19-4) explained 11.53–20.93% of PV and showed positive genetic effect.
Table 4 presented 24 conditional QTLs which were identified during four development intervals including ∆t1–2, ∆t2–3, ∆t3–4 and ∆t4–5. Totally, 5 common conditional QTLs were observed across more than one interval or environment such as qPH-Chr1-1, qPH-Chr6-1, qPH-Chr9-2, qPH-Chr19-2, and qPH-Chr20-2. In TC/M trials, one, one, two and three QTLs were detected in four periods of ∆t1–2, ∆t2–3, ∆t3–4, and ∆t4–5. In TC/P trials, 6, 5, 6 and 6 QTLs were detected at ∆t1–2, ∆t2–3, ∆t3–4, and ∆t4–5, respectively. The common QTL qPH-Chr9-2 was simultaneously identified during two growth periods (∆t1–2 and ∆t4–5), explaining 9.12% and 26.76% of PV, respectively. The qPH-Chr6-1 was detected at ∆t2–3, ∆t3–4 and ∆t4–5 at the same time in 2016E2 based on TC/P population. Among these 24 QTLs, 14 conditional QTLs validated QTLs from five stages (Tables 3, 4). Among these QTLs and conditional QTLs, we identified five and eight heterotic loci in TC/M and TC/P populations, respectively.
Genetic effect at single locus level
In two testcross experiments, we identified 17 QTLs in both TC populations and 12 heterotic loci using mid-parent heterosis (MPH) datasets by CIM method. Additive, partial dominance and overdominance effect were observed for single QTLs (Table 5). In TC/M population, genotypes of individuals contain heterozygous P1P2 alleles and homozygous P1P1 dominant alleles providing by maternal parent GX1135. And five additive QTLs and six over-dominant QTLs contributed much to heterosis, following three partial dominant QTLs. In TC/P population, genotypes of individuals contain heterozygous P1P2 alleles and homozygous P2P2 recessive alleles from paternal parent GX100-2. And 14 additive QTLs, 3 partial dominant QTLs and 28 over-dominant QTLs were identified. The results indicated that additive, partial dominance and overdominance effect explained the genetic basis of plant height and the heterosis in Upland cotton. Relationship between whole-genome heterozygosity and dynamic performances.
We examined the correlations between whole genome marker heterozygosity of 653 loci and mean values underlying plant height at five stages in TC/M, MPH-M, TC/P, and MPH-P datasets (Table S3). No significant relationship was observed between dynamic performances for plant height and overall genome marker heterozygosity at all of the five development stages. Majority of the correlation showed negative but non-significant in the TC/M and MPH-M datasets, as well as in the TC-P and MPH-P datasets.
Gene actions controlling plant height by environments
At the two-locus level, 31 main effect QTLs and QTLs × environment interaction (M-QTLs and QEs) and 25 epistatic QTLs and QTLs × environment interaction (E-QTLs and QQEs) were identified at t1, t2, and ∆t1–2 stages in TC/P trials across 2016E1 and 2016E2 (Table 6, S4, S5). Totally, 15, 14, two M-QTLs and QEs, and 16, 7, two E-QTLs and QQEs were detected from three datasets in RIL-P, TC/P and MPH-P datasets, respectively. And 83.33% of identified M-QTLs by ICIM method (Table S4) were common to single locus detected QTLs by CIM method (Tables 3, 4). In RIL population, five M-QTLs and QEs were identified at t1 stage; and two were simultaneously observed at t1 and t2 stages. In the TC/P population, four and seven M-QTLs and QEs were detected at t1 and t2 stages, with 4.99% and 3.59% of phenotypic variation (PV) on average, respectively. The region of Gh616-CIR139 was expressed repeatedly, explaining 12.72% and 3.95% of PV in the RIL and TC/P populations, respectively. Taken together, 22 M-QTLs and QEs explained less phenotypic variation than that by the detected E-QTLs and QQEs. Twelve E-QTLs interacted in multiple stages or populations, such as DPL0894-SWU10800 which were observed at t2 stage in the RIL-P population, as well as at t1 stage in the TC/P population.
In TC/M trials, we detected 16 M-QTLs and QEs, and 17 E-QTLs and QQEs at t5 stage across 2015E1, 2015E3 and 2016E2 at the two-locus level (Table S6, S7). A total of 11, four, one M-QTLs and 11, five, one E-QTLs were detected under more than one environments by three datasets in RIL-M, TC/M and MPH-M datasets, respectively (Table 6). Two stable M-QTLs and QEs were simultaneously identified both on chromosome 14 in RIL-M population and on chromosome 22 in TC/M population.
We also dissected the genetic types of gene actions by the relationship between M-QTLs and E-QTLs (Table S8). In TC/P trials, five pairs of E-QTLs caused between either of M-QTLs (Type II), 20 E-QTLs caused between neither of M-QTLs (Type III) and no E-QTLs caused between both of M-QTLs (Type I). In TC/M trials, five E-QTLs were repeatedly detected. They located on Chr 9, Chr 11 and Chr 12. Sixteen E-QTLs belonged to Type III, one E-QTLs and QQEs belonged to Type II, no Type I was observed. The results indicated that E-QTLs mainly contributed to phenotype by Type III in multiple populations of both TC trials.
Discussion
Comparison among two parental TC populations
Previous studies on QTL mapping for plant height provided information at the final development stages in other crops (Shen et al. 2014; Wei et al. 2015). A total of 47 dynamic QTLs for plant height were explored using TC/M populations in Upland cotton in previous study (Shang et al. 2016a). However, no paternal testcross population (TC/P) was exploited to explore dynamic plant height at multiple development stages in Upland cotton. In the present study, two permanent parental testcrossing populations were developed for the first time to explore dynamic QTLs and heterotic loci for plant height in Upland cotton. Superior performance and MPH values by two to three times were observed in TC/M population than that in TC/P population at all of 5 stages in the same environment (Hejian, 2016E2). The result was attributed to the superior performance of GX1135 in comparison with GX100-2 because the mean performances of both parents were essential to the superiority of their hybrid. However, a total of 18 and 30 QTLs including heterotic loci were detected in TC/M and TC/P experiments, respectively. The result indicated large power to map QTLs using the TC/P population. Similar to the previous study, 98 and 105 QTLs for fiber quality and yield-related traits were detected in TC/M and TC/P populations in Upland cotton, respectively (Fang et al. 2016).
Common QTLs controlling dynamic plant height across multiple stages, populations or years
Experimental design in two parental TC trials made it available to validate QTLs across multiple populations with high accuracy. In the present study, 35 common QTLs (50%) for dynamic plant height were detected in two parental TC trials across 2015 and 2016 in E1, E2 and E3. A total of 14 QTLs were detected by best linear unbiased estimates (BLUEs) for the replicated datasets in more than one environment for validating the accuracy of the QTLs controlling plant height (Table S2). Seven common QTLs were same to the QTLs by single environment mentioned above, such as qPH-Chr2-1, qPH-Chr19-1, qPH-Chr19-2, qPH-Chr19-4 and qPH-Chr24-1 (Table S2). Here, we also detected 32 QTLs and 24 conditional QTLs were detected in RIL, TC/M, and TC/P populations derived from the cross ‘Xinza1’ (Table 3). A total of 50 conditional QTLs (71.43%) for plant height were detected at eight successive times in rapeseed (Brassica napus) (Wang et al. 2015). A total of 11 QTLs in the present study were same to the previous results in 2012 (Table S9) (Shang et al. 2016a). Particularly, the region of NAU5330-NAU1269 was detected for 21 times at most in the same RIL population at early stages (t1, t2, t3) across 2 years at two locations. The region on chromosome 19 explained 20.93% of PV on average. The flanking marker PGML0695 of qPH-Chr22-2 in the present study was common to a hotspot including qFE24.1, qFM24.1 and qFS24.1 (Tang et al. 2015).
A total of 65,412 SSRs from CottonGen were mapped to six sets of genome sequences for three Gossypium species to define the physical locations, respectively (Zhu et al. 2017). We verified physical locations of flanking markers such as HAU1332 flanking with qPH-Chr4-1 in order to explore QTLs or genes controlling plant height. In addition, two GWAS loci (Hd3a and Hd1) controlled plant height on chromosome 6 in rice and Hd3a displayed strong over-dominant effects (Huang et al. 2015). In Upland cotton, the homologous sequences of Hd3a and Hd1 were located on chromosome 2, 3, 4, 5, 8, 12, and 13 in reference genome of “TM-1” (Zhang et al. 2015). On these seven chromosomes, a total of 16 QTLs for plant height were detected in the present study, providing insight for further research. A high density new map involving in SNP and SSR markers will be available to validate the important regions of these QTLs with high accuracy.
Genetic basis of dynamic plant height and the heterosis in Upland cotton
In the present study, plant height showed dynamic at different stages in TC/M and TC/P trials not only for the number of QTLs but also the portion of genetic effects (Tables 3, 4, 5). Over-dominant QTLs was the most prevalent than additive and partial dominance QTLs in TC/P population at single locus level, same as in TC/M population. In rice, plant height locus, named Hd3a, also showed strong over-dominant effect (Huang et al. 2015). Here, partial dominant and over-dominant QTLs were more than additive QTLs at t1, t2 and t3 stages in TC/M population, similarly at t1, t3 and t4 stages in TC/P population. Nevertheless, more QTLs showed additive effect at t5 stage in TC/M population, similar at t2 stage in TC/P population. No partial dominant conditional QTL was estimated in the present study (Table 5). Then, all of 10 QTLs detected in TC/M populations in the region of NAU5330-NAU1269 showed partial dominant effect, whereas all of 2 QTLs detected in TC/P populations showed additive effect. The results indicated that different genetic factors controlled dynamic plant height in Upland cotton between TC/M and TC/P populations as well as at different stages.
In rice, 15 heterotic loci (HL) contributed to heterosis acting in dominance for plant height in rice (Shen et al. 2014). In maize, 9 HL with dominant and over-dominant effects were mainly affected for plant height (Wei et al. 2015). In this study, the experimental design is also valuable to identify heterotic loci. Eight HL were repeatedly identified at multiple stages. Six common HL shared at multiple stages. Over-dominant qPH-Chr1-1 and qPH-Chr9-2 were identified at t3, t4 and t5 stages in TC/P and TC/M populations, respectively. In both TC populations, additive, partial dominance and over-dominant effects played roles for dynamic plant height. The results were consistent with previous studies in cotton and wheat (Shang et al. 2016a; Wang et al. 2010). We also found that majority of the correlation showed non-significant between the TC/M and MPH-M datasets, as well as between the TC-P and MPH-P datasets. The result was consistent with the previous analyses in maize (Xiao et al. 1995), rice (Hua et al. 2002; Yu et al. 1997), and cotton (Shang et al. 2016a). It might be attributed to just a few heterozygous loci, which explained a large proportion of the advantage in hybrids (Huang et al. 2015). Moreover, we detected 30 and six epistatic QTLs in both TC and their MPH datasets by ICIM method. Corresponding QTLs × environment interaction explained phenotypic variation in multiple populations. The result was consistent with the previous study (Shang et al. 2016a), too. But no epistatic QTLs were detected at t1–2 interval by MPH-P datasets. However, the majority of average M-QTLs or E-QTLs explained a larger proportion of phenotypic variation than did the QTL by environment interaction. It was concluded that additive, partial dominant and overdominant effects determined heterosis for plant height in Upland cotton, together with epistasis and QTL by environment interaction.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article represented fully within the article and in the Table S10.
References
Bruce AB (1910) The Mendelian theory of heredity and the augmentation of vigor. Science 32:627–628
Chen ZJ (2013) Genomic and epigenetic insights into the molecular bases of heterosis. Nat Rev Genet 14:471–482
Fang D, Wang H, Huang C, Zhao W, Dai B, Shen C, Zhang B, Li D, Lin Z (2016) Identification of QTL for fiber quality and yield traits using two immortalized backcross populations in Upland cotton. PLoS ONE 11:e0166970
Farmer BH (1986) Perspectives on the ‘Green Revolution’ in South Asia. Mod Asian Stud 20:175–199
Frascaroli E, Cane MA, Landi P, Pea G, Gianfranceschi L, Villa M, Morgante M, Pe ME (2007) Classical genetic and quantitative trait loci analyses of heterosis in a maize hybrid between two elite inbred lines. Genetics 176:625–644
Hu S, Wang C, Sanchez DL, Lipka AE, Liu P, Yin Y, Blanco M, Lubberstedt T (2017) Gibberellins promote brassinosteroids action and both increase heterosis for plant height in maize (Zea mays L.). Front Plant Sci 8:1039
Hua J, Xing Y, Xu C, Sun X, Yu S, Zhang Q (2002) Genetic dissection of an elite rice hybrid revealed that heterozygotes are not always advantageous for performance. Genetics 162:1885–1895
Hua J, Xing Y, Wu W, Xu C, Sun X, Yu S, Zhang Q (2003) Single-locus heterotic effects and dominance by dominance interactions can adequately explain the genetic basis of heterosis in an elite rice hybrid. Proc Natl Acad Sci USA 100:2574–2579
Huang X, Yang S, Gong J, Zhao Y, Feng Q, Gong H, Li W, Zhan Q, Cheng B, Xia J, Chen N, Hao Z, Liu K, Zhu C, Huang T, Zhao Q, Zhang L, Fan D, Zhou C, Lu Y, Weng Q, Wang ZX, Li J, Han B (2015) Genomic analysis of hybrid rice varieties reveals numerous superior alleles that contribute to heterosis. Nat Commun 6:6258
Jones DF (1917) Dominance of linked factors as a means of accounting for heterosis. Genetics 2:466–479
Krieger U, Lippman ZB, Zamir D (2010) The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat Genet 42:459–463
Larièpe A, Mangin B, Jasson S, Combes V, Dumas F, Jamin P, Lariagon C, Jolivot D, Madur D, Fievet J, Gallais A, Dubreuil P, Charcosset A, Moreau L (2012) The genetic basis of heterosis: multiparental quantitative trait loci mapping reveals contrasted levels of apparent overdominance among traits of agronomical interest in maize (Zea mays L.). Genetics 190:795–811
Li Z, Luo L, Mei H, Wang D, Shu Q, Tabien R, Zhong D, Ying C, Stansel JW, Khush GS, Paterson AH (2001) Overdominant epistatic loci are the primary genetic basis of inbreeding depression and heterosis in rice. I. Biomass and grain yield. Genetics 158:1737–1753
Li H, Ribaut JM, Li Z, Wang J (2008) Inclusive composite interval mapping (ICIM) for digenic epistasis of quantitative traits in biparental populations. Theor Appl Genet 116:243–260
Li X, Li X, Fridman E, Tesso TT, Yu J (2015) Dissecting repulsion linkage in the dwarfing gene Dw3 region for sorghum plant height provides insights into heterosis. Proc Natl Acad Sci USA 112:11823–11828
Li H, Yang Q, Fan N, Zhang M, Zhai H, Ni Z, Zhang Y (2017) Quantitative trait locus analysis of heterosis for plant height and ear height in an elite maize hybrid zhengdan 958 by design III. BMC Genet 18:36
Liu W, Maurer HP, Leiser WL, Tucker MR, Weissmann S, Hahn V, Würschum T (2016) Potential for marker-assisted simultaneous improvement of grain and biomass yield in triticale. Bioenergy Res 10:449–455
Luo X, Fu Y, Zhang P, Wu S, Tian F, Liu J, Zhu Z, Yang J, Sun C (2009) Additive and over-dominant effects resulting from epistatic loci are the primary genetic basis of heterosis in rice. J Integr Plant Biol 51:393–408
Ma L, Zhao Y, Wang Y, Shang L, Hua J (2017) QTLs analysis and validation for fiber quality traits using maternal backcross population in Upland cotton. Front Plant Sci 8:2168
Mei HW, Li ZK, Shu QY, Guo LB, Wang YP, Yu XQ, Ying CS, Luo LJ (2005) Gene actions of QTLs affecting several agronomic traits resolved in a recombinant inbred rice population and two backcross populations. Theor Appl Genet 110:649659
Meng H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:269–283
Radoev M, Becker HC, Ecke W (2008) Genetic analysis of heterosis for yield and yield components in rapeseed (Brassica napus L.) by quantitative trait locus mapping. Genetics 179:1547–1558
Russell VL (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) Green revolution: a mutant gibberellins-synthesis gene in rice. Nature 416:701–702
Shang L, Liu F, Wang Y, Abduweli A, Cai S, Wang K, Hua J (2015) Dynamic QTL mapping for plant height in Upland cotton (Gossypium hirsutum). Plant Breed 134:703–712
Shang L, Ma L, Wang Y, Su Y, Wang X, Li Y, Abduweli A, Cai S, Liu F, Wang K, Hua J (2016a) Main effect QTL with dominance determines heterosis for dynamic plant height in upland cotton. G3: genes. Genom Genet 6:3373–3379
Shang L, Liang Q, Wang Y, Zhao Y, Wang K, Hua J (2016b) Epistasis together with partial dominance, over-dominance and QTL by environment interactions contribute to yield heterosis in upland cotton. Theor Appl Genet 129:1429–1446
Shang L, Wang Y, Cai S, Wang X, Li Y, Abduweli A, Hua J (2016c) Partial dominance, overdominance, epistasis and QTL by environment interactions contribute to the heterosis in two Upland cotton hybrids. G3: genes. Genom Genet 6:499–507
Shao Q, Zhang F, Tang S, Liu Y, Fang X, Liu D, Liu D, Zhang J, Teng Z, Paterson AH, Zhang Z (2014) Identifying QTL for fiber quality traits with three upland cotton (Gossypium hirsutum L.) populations. Euphytica 198:43–58
Shen G, Zhan W, Chen H, Xing Y (2014) Dominance and epistasis are the main contributors to heterosis for plant height in rice. Plant Sci 215–216:11–18
Tang S, Teng Z, Zhai T, Fang X, Liu F, Liu D, Zhang J, Liu D, Wang S, Zhang K, Shao Q, Tan Z, Paterson AH, Zhang Z (2015) Construction of genetic map and QTL analysis of fiber quality traits for Upland cotton (Gossypium hirsutum L.). Euphytica 201:19–213
Wang S, Basten CJ, Zeng ZB (2007) Windows QTL cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh
Wang Z, Wu X, Ren Q, Chang X, Li R, Jing R (2010) QTL mapping for developmental behavior of plant height in wheat (Triticum aestivum L.). Euphytica 174:447–458
Wang X, Wang H, Long Y, Liu L, Zhao Y, Tian J, Zhao W, Li B, Chen L, Chao H, Li M (2015) Dynamic and comparative QTL analysis for plant height in different developmental stages of Brassica napus L. Theor Appl Genet 128:1175–1192
Wei X, Wang B, Peng Q, Wei F, Mao K, Zhang X, Sun P, Liu Z, Tang J (2015) Heterotic loci for various morphological traits of maize detected using a single segment substitution lines test-cross population. Mol Breed 35:1–13
Xiao J, Li J, Yuan L, Tanksley SD (1995) Dominance is the major genetic-basis of heterosis in rice as revealed by QTL analysis using molecular markers. Genetics 140:745–754
Yu S, Li J, Xu C, Tan Y, Gao Y, Li X, Zhang Q, Saghai Maroof MA (1997) Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc Natl Acad Sci USA 94:9226–9231
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Zhang Y, Ni Z, Yao Y, Nie X, Sun Q (2007) Gibberellins and heterosis of plant height in wheat (Triticum aestivum L.). BMC Genet 8:40
Zhang T, Hu Y, Jiang W, Fang L, Guan X, Chen J, Zhang J, Saski CA, Scheffler BE, Stelly DM, Hulse-Kemp AM, Wan Q, Liu B, Liu C, Wang S, Pan M, Wang Y, Wang D, Ye W, Chang L, Zhang W, Song Q, Kirkbride RC, Chen X, Dennis E, Llewellyn DJ, Peterson DG, Thaxton P, Jones DC, Wang Q, Xu X, Zhang H, Wu H, Zhou L, Mei G, Chen S, Tian Y, Xiang D, Li X, Ding J, Zuo Q, Tao L, Liu Y, Li J, Lin Y, Hui Y, Cao Z, Cai C, Zhu X, Jiang Z, Zhou B, Guo W, Li R, Chen ZJ (2015) Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat Biotechnol 33:531–537
Zhu T, Liang C, Meng Z, Sun G, Meng Z, Guo S, Zhang R (2017) CottonFGD: an integrated functional genomics database for cotton. BMC Plant Biol 17:101–109
Acknowledgements
We thank Dongyong Xu and Huaiyu Lu (Guoxin Seed Company Ltd, Cangzhou, Hebei Province) for their contributions on field experiments and data acquisition in E2. Thanks to Lianguang Shang and Xiaocui Wang (China Agricultural University) for the outstanding work in SSR marker evaluation and map construction, Kunbo Wang and Fang Liu (Institute of Cotton Research, Chinese Academy of Agricultural Sciences) for providing part of SSR markers, Dr. Zhengsheng Zhang (Southwest University) for providing SWU and ICR SSR primers. This research was supported by a grant from the National Key R & D Program for Crop Breeding (2016YFD0100203).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, LL., Ijaz, B., Wang, YM. et al. Dynamic QTL analysis and validation for plant height using maternal and paternal backcrossing populations in Upland cotton. Euphytica 214, 167 (2018). https://doi.org/10.1007/s10681-018-2243-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-018-2243-y