Abstract
Embryogenic callus of Phalaenopsis amabilis derived from leaf tissue was cocultivated with Agrobacterium tumefaciens strain LBA4404 harboring a plant cloning vector. The vector carried the lipid transfer protein (LTP) encoding gene cloned from cold tolerant Brazilian upland rice cv. IAPAR 9. The highest transformation efficiency (12.16%) was obtained when 1–2 mm calli were infected and cocultivated with 0.4 (OD600) A. tumefaciens for 20 min. Transgene integration of kan-resistant plants was confirmed through polymerase chain reaction analysis and Southern hybridization. Four hundred seventy transgenic plants, each derived from an independent protocorm-like body, were obtained. The expression of rice cold-inducible LTP gene in transgenic P. amabilis improved its adaptive responses to cold stress. The examination of transgenic plants revealed that enhanced cold tolerance was most likely due to the increased accumulation of several compatible solutes such as total soluble sugars, proline, antioxidant superoxide dismutase, decreased accumulation of malondialdehyde, and maintained electrolytes within the membrane compared with controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phalaenopsis amabilis, a widely cultivated epiphytic monopodial orchid, has been exploited commercially as a cut flower and potted plant. The breeding of P. amabilis for more favorable traits, such as low temperature tolerance, new attractive colors, size and form modification, and disease and pest resistance using sexual hybridization has been limited due to the long duration of each generation and the lack of useful genetic variability. However, the establishment of more potentially alternative gene delivery systems, such as Agrobacterium-mediated transformation or micro-projectile bombardment avenue, greatly facilitated efforts for improvement of the plant’s properties via genetic engineering (Estruch et al. 1997). Several studies on successful Agrobacterium-mediated transformation of Phalaenopsis have been reported (Belarmino and Mii 2000; Chai et al. 2002; Chan et al. 2005; Mishiba et al. 2005; Semiarti et al. 2007), among which an effective Agrobacterium-mediated transformation system for P. amabilis was carried out as well. However, very few reports on valuable genes transferred into Phalaenopsis are available. Su and Hsu (2003) isolated a cDNA clone of a putative flavonoid-3′,5′-hydroxylase gene from Phalaenopsis. Transient transformation was achieved through particle bombardment of Phalaenopsis petals. Transgenic petals changed from pink to magenta, indicating that the product of the putative flavonoid-3′,5′-hydroxylase gene influences anthocyanin pigment synthesis. To enhance the resistance of Phalaenopsis to Cymbidium mosaic virus (CymMV) and Erwinia carotovora, Chan et al. (2005) transferred CymMV coat protein cDNA (CP) and sweet pepper ferredoxin-like protein cDNA (Pflp) to enable the expression of disease-resistant traits.
Phalaenopsis amabilis is a type of tropical flower with suitable growth temperature of above 18°C, resulting in poor growth or high expenditure for cultivation during the winter season. Therefore, breeding novel cultivars with cold resistance is necessary to widen its planting region. Plant lipid transfer proteins (LTPs) are a homogeneous class of small (9–10 kDa), abundant, ubiquitous, and mostly basic proteins. The proteins contain eight cysteine residues with four conserved disulfide bridges that exert different functions in defending itself against abiotic stresses, including drought, high salinity, low temperature, and wounding (Hughes et al. 1992; Choi et al. 2008), as well as biotic stresses such as bacterial and fungal pathogens (Kirubakaran et al. 2008).
In this study, we reported a method for genetic transformation mediated by A. tumefaciens using P. amabilis leaf-derived embryogenic calli as plant material source. Adventitious stem buds proliferation and callus formation conditions on induction mediums containing cytokinin [6-benzylaminopurine (6-BA) and thidiazuron (TDZ)], auxin (NAA), and coconut water were examined. Transformation efficiency was investigated under three factors, including the size of calli, the concentration of A. tumefaciens suspension culture, and the duration of transformation. In addition, the level of five potential contributors in transgenic plants in response to cold was examined. The objectives of this study are: (1) to establish an A. tumefaciens-mediated transformation system of Phalaenopsis and transfer LTP gene into Phalaenopsis, and (2) to improve cold tolerance by introducing the LTP gene to widen the Phalaenopsis planting region.
Materials and methods
Plasmid vector and bacterial strain
A cDNA LTP clone (Accession No. AK062463) identified from Brazilian upland rice (Oryza sativa cv. IAPAR 9) via subtractive suppression hybridization (SSH) was amplified by polymerase chain reaction (PCR) (Li 2004). The amplified fragments were introduced into multiple cloning sites of the binary vector. The cassette containing the entire coding region of the LTP cDNA and pUbi promoter was cloned in a pCAMBIA2300 binary vector to generate CaMV35S∷nptII-pUbi∷LTP (Fig. 1). This construct was introduced into the disarmed octopine type A. tumefaciens strain LBA4404.
Schematic representation of T-DNA of pCAM2300 containing LTP and nptII plant expression cassettes. RB: right border, LB: left border, 35S: CaMV 35S promoter, pUbi: maize promoter, nptII: neomycin phosphotransferase II, LTP: Oryza sativa lipid transfer proteins. Arrows indicate direction of transcription. The location of restriction site BamHI is also indicated
Embryogenic callus formation in P. amabilis
Phalaenopsis amabilis “Queen Beer, No.1227” cultured by Yangping Horticulture Co. Ltd. was used as experimental material in this study. Adult P. amabilis grown under 28/25°C (16/8 h, light/dark) white light (50 μmol m−2 s−1) was transferred to grow in a new environment under 20/15°C (16/8 h, light/dark) white light (50 μmol m−2 s−1). After 30–50 days, peduncle buds formed from the bottom of the stem and were then cut into 2 cm long pieces. These were soaked and gently shaken for 20 s in 75% (v/v) ethyl alcohol, surface-sterilized in 1% (v/v) NaOCl and 0.1% (v/v) Tween 20 for 30 min, and then rinsed three times with sterile distilled water (SDW). Finally, the peduncle buds were transferred into the stem bud germination medium (Table 1) and grown under 16/8 h (light/dark) white light (50 μmol m−2 s−1) at 25°C. A month later, when the stem buds were approximately 1 cm in length, they were transferred into the adventitious stem bud proliferation medium (Table 1) for 2 months. Young leaves growing from the top of adventitious stem buds were diced into 0.6 cm2 pieces. The leaf pieces were then transferred into the callus induction medium (Table 1) by placing the leaf dice upper epidermis up, grown for 2 weeks in the dark and then grown under 16/8 h (light/dark) white light (50 μmol m−2 s−1) at 25°C. A month later, green, compact 1–2 mm diameter embryogenic callus tissues were used as plant explants for transformation. Each treatment was repeated three times.
Transformation and regeneration of transformants
Overnight cultures of Agrobacterium grown in LB medium supplemented with 50 mg l−1 kanamycin (Shenggong Ltd., Shanghai, China) at 28°C were harvested by centrifuging at 6000 rpm for 10 min and suspended in a liquid inoculation medium (Table 1). The 1–2 mm diameter embryogenic calli were then immersed in the culture of 0.4 (OD600) Agrobacterium for 20 min. The calli were transferred onto sterile filter paper to dry and then placed into the callus induction medium without antibiotics. After 3 days, the explants were washed with SDW containing 500 mg l−1 cefotaxime (Shenggong Ltd., Shanghai, China) and transferred into the transformant selection medium (Table 1) containing 500 mg l−1 cefotaxime and 20 mg l−1 kanamycin, which inhibited the growth of Agrobacterium, and cultured for 5 days in the dark. Calli were then transferred onto a protocorm-like body (PLB) induction medium (Table 1). Developing protocorms were transferred into a new PLB induction medium every 3 weeks for further selection of transformants. PLB-regenerated putative transgenic shoots were separated and transferred into the root induction medium (Table 1). The rooted plantlets were transplanted into dried water milfoil (Myriophyllum spicatum) and grown in a glasshouse at 25°C.
Molecular characterization of transformants
Genomic DNAs were extracted from the leaf tissue of putative CaMV35S∷nptII-pUbi∷LTP transformants (Offringa and Lee 1995) and screened by PCR using primers nptII (Shenggong Ltd., Shanghai, China), which are specific for the nptII gene: 5′-ATCGGGAGCGGCGATACCGAT-3′; 5′-GAGGCTATTCGGCTATGACTG-3′, and primers LTP, which are specific for the LTP gene: 5′-GTTTCTTAATTTCGATCGCGAAGG-3′;5′-GGAGTATTATGGTTTAGTTTTAGCAGG-3′. PCR was performed for 35 cycles of 95°C for 1 min, 50°C for 30 s, and 72°C for 30 s. PCR products were separated in 0.8% agarose gel, stained with ethidium bromide, and visualized under UV-transillumination. For Southern hybridization, about 2.5 μg DNA was fragmented with BamHI and electrophoretically separated on 1% agarose gel. The DNA was transferred to positively charged nylon membranes through capillary blot, while the genomic fragments were detected using the DIG label and chemiluminescent detection system (CDP-Star) according to the instructions of the manufacturer (Roche, Mannheim, Germany). To prepare the hybridization probe, 250-bp fragment of the LTP gene was labeled with the PCR DIG probe synthesis kit (Roche). After hybridization at 40°C overnight, chemiluminescent signals were detected using Lumi-film (Roche).
Cold stress treatment and recovery
For the phenotypic analysis in response to cold, 12-month-old transgenic and control plants grown in the 25°C glasshouse were acclimatized in a 15/12°C (16/8 h, day/light) growth chamber for 5 days, and then transferred into a 10/7°C (16/8 h, day/light) growth chamber for another 10 days. For SOD, MDA, total soluble sugars, proline, and electrolyte leakage assay in cold-treated and cold-recovered P. amabilis, 12-month-old transgenic and control plants grown in the 25°C glasshouse were acclimatized in a 20/17°C, 15/12°C, 10/7°C, and 5/2°C (16/8 h, day/light) growth chamber for 3 days, and then transferred in a 15/12°C and 25/22°C (16/8 h, day/light) recovery chamber for 3 days, respectively. Each sampling was conducted in triplicate.
Tissue sampling
Leaf tissue of transgenic and control cold-treated and cold-recovered plants totalling 500 mg were excised and ground in a mortar using a pestle with 1 ml ice-cold 50 mM phosphoric acid buffer (pH 7.8). The mixture was then transferred into a centrifuge tube. The mortar was rinsed twice with the phosphoric acid buffer, and the washing liquid also transferred into the centrifuge tube. The same buffer was further added until the mixture reached a total of 10 ml. The homogenate was centrifuged at 10,000 rpm for 15 min at 4°C. The supernatant was stored in a 4°C fridge for SOD and MDA assay.
SOD, MDA, total soluble sugars, proline and electrolyte leakage assay
SOD assay employing superoxide production from xanthine oxidase and detection of a colored formazan product formed from nitroblue tetrazolium (NBT) was conducted as described previously (Beauchamp and Fridovich 1971). The NBT reaction solution was composed of 100 μl tissue homogenate (or 50 mM phosphoric acid buffer as control), 1.5 ml 50 mM phosphoric acid buffer (pH 7.8), 300 μl 130 mM methionine (MET), 300 μl 750 μM NBT, 300 μl 100 μM ethylene diaminetetraacetic acid (EDTA), 300 μl 100 μM VB2, and 500 μl SDW. The reaction was activated in a 30°C light incubator for 20 min and stopped in the dark. Absorbance of the solution was immediately measured at 560 nm.
The amount of lipid peroxide in plant tissues was determined similar to that of MDA using the method of Ohkawa et al. (1979) and Kurokawa et al. (2006) with some modifications. The thiobarbituric acid (TBA) solution was composed of 2.6 mM TBA, 918 mM trichloroacetic acid, 0.3 mM HCl, and 1.8 mM 2,6-di-tert-butyl-4-metylphenol in 22% ethanol. The reaction mixture contained 1.5 ml tissue homogenate, 1.5 ml 8.1% SDS, 2.5 ml 20% (v/v) acetic acid solution (pH 3.5), and 2.5 ml 0.6% (v/v) aqueous solution of TBA. The mixture was heated at 95°C for 15 min. After cooling with tap water, 1.0 ml SDW and 5.0 ml n-butanol were added and the mixture shaken vigorously. After centrifugation at 10,000 rpm for 1 min at 4°C, absorbance of the organic layer (upper layer) was measured at 450, 532, and 600 nm with 1,1,3,3-tetraethoxypropane as a standard.
Lyophilized leaf material totaling 500 mg was extracted with 70% (v/v) ethanol and the extracts evaporated to dryness in a vacuum. They were then placed in warm water and cleared with aluminium hydroxide. Anthrone reagent was prepared by dissolving 200 mg anthrone in 100 ml H2SO4, which was created by adding 500 ml concentrated acid to 200 ml water. The reagent was allowed to stand for 30 min with occasional shaking until it was perfectly clear. Next, 3 ml of the fresh prepared anthrone reagent was pipetted into thick-walled Pyrex tubes (150 × 25 mm) and chilled in ice water. A total 250 μl of the alcoholic extract solution was layered on the acid, cooled for a further 5 min, and then thoroughly mixed while still immersed in ice water. The tubes were loosely fitted with corks, heated as required in a boiling water bath for 10 min, and then cooled in water for 5 min. Measurements of the test solutions thiobarbituric acid and reagent blanks were conducted using water as reference. The relation between scale readings and amounts of sugars was not strictly linear, and it was necessary to use calibration curves for different sugars. Absorption spectra were determined in a spectrophotometer at 620 nm.
Extraction of 500 mg lyophilized leaf material was done using 5 ml 3% sulfosalicylic acid at 100°C for 15 min. Samples were shaken for approximately 1 h at room temperature and then allowed to stand to cool down. Afterwards, 2 ml of the upper layer of extracts was analyzed for proline content using the acid ninhydrin method. Briefly, 2 ml of the upper layer aqueous extract was mixed with 2 ml glacial acetic acid and 2 ml acid ninhydrin reagent (50 mg ninhydrin, 1.2 ml glacial acetic acid, and 0.8 ml 18 M orthophosphoric acid) and heated at 100°C for 15 min. After cooling, the reaction mix was partitioned against 5 ml toluene and the absorbance of the organic phase was determined at 520 nm. Resulting values were compared with a standard curve constructed using known amounts of proline.
Fresh leaves from transgenic and control cold-treated and cold-recovered plants totalling 20 g were collected, rinsed with deionized water three times, and dried on filter paper. Electrolyte leakage (EL) assay was carried out according to the method of Xuan et al. (2009). Relative EL was calculated as EL1/El2 × 100. EL1 and EL2 values were measured under cooled and boiled treatment, respectively.
Statistical analyses
Data were log-transformed to achieve normality and subjected to analysis of variance (ANOVA) with mean separation (P < 0.05) using Duncan’s New Multiple Range test (DMRT), SAS® vers. 9.1.3 (SAS Institute Inc. 2001).
Results
Conditions for embryogenic callus formation
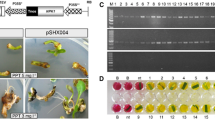
The conditions for callus formation of P. amabilis from flower peduncles were first analyzed. Flower peduncles were cultured on a stem bud germination medium (Table 1) and started to expand from its base 1 week later (Fig. 2a). Adventitious buds were formed (Fig. 2b) after 2 months. The addition of cytokinin and auxin to adventitious bud proliferation and callus induction medium increased the number of adventitious budding from stem buds and callus formation from leaf dice. The highest number of adventitious budding (11) from each stem bud was observed with 15 mg l−1 6-BA treatment. However, under this concentration, the adventitious stem buds were too thin to grow reasonably sized new leaves for inducing callus. Hence, 10 mg l−1 6-BA was used for proliferating adventitious stem buds. The highest number of callus formation (0.75) from each leaf dice was observed with 1 mg l−1 TDZ and 20% (v/v) coconut water. The number of callus formation from each leaf dice could also be strongly increased by placing the leaf dice upper epidermis up (14 calli per dice) in the callus induction medium (Figs. 2c, d).
Calli inducement of P. amabilis and Agrobacterium-mediated transformation. a Stem buds induced from peduncle bud dices after 1 month grown on stem bud germination medium. b Adventitious stem buds induced after 2 month grown on stem bud proliferation medium. c The 0.6 cm2 leaf dice (upper epidermis up) transferred onto callus induction medium. d Green, compact and 1–2 mm diameter embryogenic calli induced from the leaf dice after 1 month grown on callus induction medium. e Kanamycin-resistant plantlets regenerated from calli that were transformed with the pCaMV35S∷ nptII-pUbi∷LTP vector
Efficiency of transformation
We used a medium containing 20 mg l−1 kan and 500 mg l−1 cef, which appeared to be optimal (data not shown) for the selection of transformants. To determine the optimal conditions for A. tumefaciens mediated leaf-derived embryogenic callus transformation, three sizes of calli (<1, 1–2, and >2 mm) were cocultivated with three concentrations of A. tumefaciens (OD600: 02, 0.4, and 0.6) harboring CaMV35S∷npt II -pUbi∷LTP for 15, 20, and 25 min, respectively. The higher average transformation efficiency was achieved in 1–2 mm (9.39%) calli compared with <1 mm (7.46%) and >2 mm (7.91%) calli. In 1–2 mm calli, the higher average transformation efficiency was achieved by that cocultivated with 0.4 (OD600) A. tumefaciens (10.71%) compared with the 0.2 (OD600) (8.59%) and 0.6 (OD600) (8.88%) A. tumefaciens. The duration of cocultivation was also a key to improving the transformation frequency when optimal callus size and A. tumefaciens concentration were determined. Elongation of cocultivation duration from 15 to 20 min boosted the transformation frequency from 9.89 to 12.16%, but the frequency declined with 25 min (10.07%) cocultivation. Resistant calli were then subjected to the regeneration process (Fig. 2e). The highest transformation efficiency (12.16%) was obtained when 1–2 mm calli were infected and cocultivated with 0.4 (OD600) A. tumefaciens for 20 min. All plantlets developed good root system in the selective rooting medium in vitro and were transferred to pots in a greenhouse.
Molecular characterization of transformants
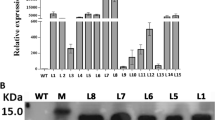
The presence of kan resistance gene in the genome of all plantlets regenerated on plates containing kan using PCR were examined first. The 0.7 kb fragment was amplified in all the plantlets examined (data not shown). The presence of the gene LTP was then examined by PCR amplification of a 250 bp fragment in the coding region of the LTP gene (Fig. 3). In total, 470 PCR-positive plantlets were obtained. These data suggested that the CaMV35S∷nptII-pUbi∷LTP gene was inserted into the genome of transformants. Southern hybridization confirmed that T-DNA was integrated and revealed that different copies of the LTP gene were present in the independent transformants tested (Fig. 4).
Phenotypes in transgenic P. amabilis that express the LTP gene
Among all CaMV35S∷nptII-pUbi∷LTP transgenic plantlets, 89 well-grown plants were selected to conduct the test of cold resistance (Fig. 5). During the first phase of cold treatment at 15/12°C, the young tender leaves of the control plants became droopy and finally fell off (Fig. 5c). However, those of the transgenic plants remained green, alive, and vivid. During the second phase of harsher cold treatment at 10/7°C, the leaves of control plants fell off completely, while those of transgenic plants remained strong and rarely fell off (Fig. 5d).
Phenotypes in transgenic P. amabilis plants harbouring the pUbi∷LTP gene. a Untreated 12-month-old transgenic transformants. b Untreated 12-month-old untransformed control plants. c Three transformants (upper row) and two control plants (lower row) cold-treated in 15/12°C (16/8 h, day/light) growth chamber for 5 days. d Sequentially, three transformants (upper row) and two controls (lower row) cold-treated in 10/7°C (16/8 h, day/light) growth chamber for another 10 days
In control plants, the SOD level increased about three times while the temperature was descending from 25 to 10°C. However, it then strongly decreased while the temperature was descending from 10 to 5°C, and continued to decrease during the recovery period (Fig. 6a). In transgenic plants, the SOD level increased about four times while the temperature was descending from 25 to 5°C, kept rising during the recovery period until it reached 15°C, and then decreased to a similar pre-treatment level (Fig. 6a). In control plants, MDA levels were increased five times throughout the cold treatment and recovery period (Fig. 6b). In transgenic plants, however, MDA levels dropped to a similar pre-treatment level during the recovery period after an initial mild increase during the cold treatment (Fig. 6b). In both control and transgenic plants, the total soluble leaf sugar level increased about two times while the temperature was descending from 25 to 15°C, and then strongly decreased while the temperature was descending from 15 to 10°C (Fig. 6c). Afterwards, in control plants, the total soluble leaf sugar level kept falling throughout the rest of the cold treatment and recovery period (Fig. 6c). However, in transgenic plants, the total soluble leaf sugar level kept rising throughout the rest of the cold treatment and recovery period until it reached 15°C, and then decreased to a similar pre-treatment level (Fig. 6c). In both control and transgenic plants, proline level increased about 2.5 and 4.5 times, respectively, while the temperature was descending from 25 to 5°C, and decreased throughout the recovery period (Fig. 6d). In transgenic plants, proline level decreased to a similar pre-treatment level after recovery (Fig. 6d). The relative electrolyte leakage (EL) of control plants is always increasing along with the temperature decreasing from 25 to 5°C and then increasing to 25°C for recovery. In comparison to control plants, under low temperature treatment (from 25 to 5°C), the ELs of LTP transgenic plants increased while the ELs increased and resumed its pre-treated level at the recovery stage (from 5 to 25°C) (Fig. 6e).
SOD, MDA, total soluble sugars, proline and relative electrolyte leakage levels in leaves of P. amabilis plants enduring cold treatment at different temperature and recovery period. Solid line and dotted line represent the outcomes of control and transgenic plants, respectively. The values denote means ± SEM from three samples
Discussion
Genetic transformation of plants through Agrobacterium has been successfully applied to various plants belonging to widely separated clades. Nevertheless, it is still difficult to apply this method to certain horticultural plants that cannot easily be propagated clonally. In this study, an Agrobacterium-mediated transformation method using leaf-derived embryogenic callus of P. amabilis was reported. The protocol described in this article is simple and reproducible. Improvements over previously published methods can be summarized as follows: (1) Compact embryogenic callus tissues were used for transformation. This is a simpler approach than methods using chopped and subcultured protocorms, or PLBs as described elsewhere, because of its high transformation efficiency (Liau et al. 2003; Men et al. 2003; Mishiba et al. 2005); (2) A kan resistance gene can be used as a selective marker; and (3) The CaMV35S∷ nptII-pUbi∷LTP construct is useful as a visible marker for selecting PLB-regenerated putative transgenic plants after transformation because it alters leaf tolerance in response to cold (Fig. 5).
Transgenic pepper plants transformed with CALTPI, a pepper LTP gene isolated from a pepper (Capsicum annuum) cDNA library from hypersensitive response (HR) lesions of leaves infected with pathogens, showed an induced expression of CALTPI in low temperature (Jung et al. 2003). In a previous report, five SiLTP1-SiLTP5 isoforms were isolated and named from developing sesame seeds. Northern blot analysis revealed these five SiLTP isoforms were most abundantly expressed in developing seeds and leaves, but were also detected in flower tissues (Choi et al. 2008). In addition, SiLTPs transcripts were significantly induced in 6-day-old sesame seedlings in response to low temperature (Choi et al. 2008). The abundant LTPs in developing sesame seeds were involved in lipid transfer into the extracellular matrix. LTP functions in the transfer of wax or cutin monomers, the stabilization of plasma membrane, and in the defense response against pathogen attack and environmental stress (Hughes et al. 1992; Pearce et al. 1996; Choi et al. 2008). In the present study, transgenic P. amabilis transformed with LTP, a cDNA clone identified from Brazilian upland rice (Oryza sativa cv. IAPAR 9), exhibited strong cold stress tolerance at 10/7°C with green, healthy, and vivid leaves (Fig. 5).
In this study, higher SOD activity and lower MDA (an index of lipid peroxidation) activity corresponded with higher protection from cold injury in transgenic P. amabilis (Figs. 6a, b). SODs are metalloenzymes that catalyze the dismutation of superoxide radical into hydrogen peroxide (H2O2) and molecular oxygen (O2), and consequently provide an important defense mechanism against superoxide radical toxicity. The potential role of antioxidant enzymes in protecting plants from cold injury is well established (Christie et al. 1994). With decreasing temperature, solubility of gas increases, leading to a higher concentration of oxygen and thus enhancing the risk of oxidative stress at low temperature (Polle 1997). Plant injury caused by freeze stress may be mediated by reactive oxygen species such as superoxide radicals, singlet oxygen, hydrogen peroxide, and hydroxyl radicals (Wise and Naylor 1987). Other than dehydration and mechanical injury, activated oxygen species participate in freezing stress, causing lipid peroxidation and a collapse of antioxidative systems in unhardened tissues (Sagisaka 1985; Kurodu et al. 1992; Walker and Mckersie 1993). Antioxidant systems can maintain a cellular homeostasis that governs the level of stress tolerance in the plant at low temperatures. In the present experiment, SOD activities of the control plants fell abruptly at 10°C, while those of transgenic plants continued to increase until about 15°C (Fig. 6a). Thus, the increasing activity of SOD illustrates the preferable cold resistance of the plants. As to the MDA content in this experiment, there were apparent differences between the transgenic LTP and the control plants. For transgenic LTP plants, the decrease in temperature caused the MDA content to increase; it decreased during recovery stage and returned to normal levels, while the control plants remained at an increasing trend even during the recovery stage (Fig. 6b). Proline protects the membranes and proteins against the adverse effects of temperature extremes (Santarius 1992; Santoro et al. 1992). It may also function as a protein-compatible hydrotrope (Srinivas and Balasubramanian 1995) and as a hydroxyl radical scavenger (Smirnoff and Cumbes 1989). Proline accumulated at a much higher level in the leaves of transgenic P. amabilis exposed to low temperatures compared with controls, although the level of proline in the leaves of both P. amabilis significantly increased with the decrease in temperature. Transgenic and control plants exhibited a similar trend; however, proline content of transgenic plants was higher than that of the control plants and returned to normal levels after recovery stage, in contrast with the control plants (Fig. 6d). The extent of freezing stress promoted enhancement of proline and MDA levels in P. amabilis, which lead us to believe that freezing stress-induced proline accumulation protects plants against freezing stress-promoted peroxidative processes.
In this study, the much higher soluble sucrose corresponded with better recovery from cold injury in transgenic P. amabilis. Leaf sugar content of both control and transgenic plants increased from 25 to 15°C, and then decreased from 15 to 10°C. After that, the sugar content of the transgenic plants increased and began to decrease at 10°C. However, the control plants presented a trend of decrease and failed to return to normal (Fig. 6c). Glucose, fructose, and oligosaccharides such as raffinose and stachyose are commonly found in soluble sugars that accumulate along with the development of freezing tolerance in plants in higher temperatures. It has been suggested that these sugars not only serve as osmoprotectants but also play a role in protecting cellular membranes from damage caused by dehydration and freezing through interaction with the lipid bilayer (Anchordoguy et al. 1987; Shalaev and Steponkus 2001; Shao et al. 2006). It was also proposed that the role of some oligosaccharides is to prevent the crystallization of sucrose, thus facilitating glass formation within the cell and leading to the protection of membrane phospholipids (Anchordoguy et al.1988; Crowe et al. 1988). Generally speaking, the accumulation of soluble sugars contributes to the increase in the cryostability of cellular membranes. Increased membrane cryostability is a prerequisite for freezing tolerance because freeze-induced destabilization of cellular membranes is the primary cause of injury in plants (Steponkus et al. 1993; Shao et al. 2008). An additional role of sugars during cold acclimation is that they may function as nutrients that allow plants to survive the lower temperatures and dryland conditions as well as recover from freezing stress (Eagles et al. 1993; Trunova 1982; Shao et al. 2008). Another important parameter to determine the effectiveness of the plasma membrane amounts is the relative electrolyte leakage, which reflects the degree of damage to the delicate and sensitive membrane (Campos et al. 2003). As shown in Fig. 6e, compared to the control plants, adaptability to the cell permeability of LTP transgenic plants was more controllable and convertible under low temperature stress, which reduced the possibility of causing damage to cells and plants. After measuring the above-mentioned physiological indexes in leaf samples via cold acclimation at 15, 10 and 5°C and its attendant recovery, the LTP gene transferred plant notably conferred tolerance to environmental stresses.
Abbreviations
- ANOVA:
-
Analysis of variance
- AS:
-
Acetosyringone
- BAP:
-
6-Benzylaminopurine
- Cef:
-
Cefotaxime
- EDTA:
-
Ethylene diaminetetraacetic acid
- EL:
-
Electrolyte leakage
- Kan:
-
Kanamycin
- LB:
-
Lauria bertani
- LTP:
-
Lipid transfer protein
- MDA:
-
Malondialdehyde
- MET:
-
Methionine
- MS:
-
Murashige and Skoog medium
- NAA:
-
Naphthalene acetic acid
- NBT:
-
Nitroblue tetrazolium
- npt II:
-
Neomycin phosphotransferase II
- PCR:
-
Polymerase chain reaction
- PLB:
-
Protocorm-like body
- pUbi:
-
Plant ubiquitin promoter
- SDW:
-
Sterile distilled water
- SDS:
-
Sodium dodecyl sulfate
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TDZ:
-
Thidiazuron
- TE:
-
Transformation efficiency
- VB6 :
-
Pyridoxine HCl
References
Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH (1987) Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology 24:324–331
Anchordoguy T, Carpenter JF, Loomis SH, Crowe JH (1988) Mechanisms of interaction of amino acids with phospholipid bilayers during freezing. Biochim Biophys Acta 946:299–306
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal Biochem 44:276–287
Belarmino MM, Mii M (2000) Agrobacterium-mediated genetic transformation of a Phalaenopsis orchid. Plant Cell Rep 19:435–442
Campos PS, Quartin V, Ramalho JC, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. Plants J Plant Physiol 160:283–292
Chai ML, Xu CJ, Senthil KK, Kim JY, Kim DH (2002) Stable transformation of protocorm-like bodies in Phalaenopsis orchid mediated by Agrobacterium tumefaciens. Sci Hortic 96:213–224
Chan YL, Lin KH, Liao LJ, Chen WH, Chan MT (2005) Gene stacking in Phalaenopsis orchid enhances dual tolerance to pathogen attack. Transgenic Res 14:279–288
Choi AM, Lee SB, Cho SH, Inhwan H, Cheol-Goo H, Suh MC (2008) Isolation and characterization of multiple abundant lipid transfer protein isoforms in developing sesame (Sesamum indicum L.) seeds. Plant Physiol Biochem 46:127–139
Christie PJ, Alfenito MR, Walbot V (1994) Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194:541–549
Crowe JH, Crowe LM, Carpenter JF, Rudolph AS, Wistrom CA, Spargo BJ, Anchordoguy TJ (1988) Interactions of sugars with membranes. Biochem Biophy Acta 947:367–384
Eagles CF, Williams J, Louis DV (1993) Recovery after freezing effect of sugar concentration on cold acclimation in Avena sativa L. Lolium perenne L. and L. multiorum Lam. New Phytol 123:477–483
Estruch JJ, Carozzi NB, Desai N, Duck NB, Warren GW, Koziel MG (1997) Transgenic plants: an emerging approach to pest control. Nat Biotechnol 15:137–141
Hughes MA, Dunn MA, Pearce RS, White AJ, Zhang L (1992) An abscisic-acid-responsive, low temperature barley gene has homology with a maize phospholipid transfer protein. Plant Cell Environ 15:861–865
Jung HW, Kim W, Hwang BK (2003) Three pathogen-inducible genes encoding lipid transfer protein from pepper are differentially activated by pathogens, abiotic, and environmental stresses. Plant Cell Environ 26:915–928
Kirubakaran SI, Begum SM, Ulaganathan K, Sakthivel N (2008) Characterization of a new antifungal lipid transfer protein from wheat. Plant Physiol Biochem 46:918–927
Kurodu H, Sagisaka S, Chiba K (1992) Collapse of peroxide-scavenging systems in apple flower-buds associated with freezing injury. Plant Cell Physiol 33:743–750
Kurokawa T, Itagaki S, Yamaji T, Nakata C, Noda T, Hirano T (2006) Antioxidant activity of a novel extract from bamboo grass (AHSS) against ischemia–reperfusion injury in rat small intestine. Biol Pharm Bull 29:2301–2303
Li CB (2004) Cloning of OsLTP1 gene from the Brazilian upland rice cv. IAPAR 9, its expression analysis and function identification. Dissertation of Institute of Genetics and Developmental Biology, Beijing, Chinese Academy of Sciences
Liau CH, You SJ, Prasad V, Hsiao HH, Lu JC, Yang NS, Chan MT (2003) Agrobacterium tumefaciens-mediated transformation of an Oncidium orchid. Plant Cell Rep 21:993–998
Men S, Ming X, Wang Y, Liu R, Wei C, Li Y (2003) Genetic transformation of two species of orchid by biolistic bombardment. Plant Cell Rep 21:592–598
Mishiba K, Chin DP, Mii M (2005) Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep 24:297–303
Offringa R, Lee F (1995) Isolation and characterization of plant genomic DNA sequences via (inverse) PCR amplification. In: Jones H (ed) Methods in molecular biology: plant gene transfer and expression protocols, vol 49. Humana Press, Totowa, pp 181–195
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pearce RS, Dunn MA, Rixon JE, Harrison P, Hughes MA (1996) Expression of cold-inducible genes and frost hardiness in the crown meristem of young barley (Hordeum vulgare L. cv. Igri) plants grown in different environments. Plant Cell Environ 19:275–290
Polle A (1997) Defense against photooxidative damage in plants. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbour Laboratory Press, Plainview, pp 623–666
Sagisaka S (1985) Injuries of cold acclimatized poplar twigs resulting from enzyme inactivation and substrate depression during frozen storage at ambient temperatures for a long period. Plant Cell Physiol 26:1135–1145
Santarius KA (1992) Freezing of isolated thylakoid membranes in complex media. VIII. Differential cryoprotection by sucrose, proline and glycerol. Physiol Plant 84:87–93
Santoro MM, Liu Y, Khan SMA, Hou L-X, Bolen DW (1992) Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochem 31:5278–5283
SAS Institute Inc (2001) SAS/START user’s guide version 8.2. SAS Institute, Cary
Semiarti E, Indrianto A, Purwantoro A, Isminingsih S, Suseno N, Ishikawa T, Yoshioka Y, Machida Y, Machida C (2007) Agrobacterium-mediated transformation of the wild orchid species Phalaenopsis amabilis. Plant Biotechnol 24:265–272
Shalaev EY, Steponkus PL (2001) Phase behavior and glass transition of 1, 2-dioleoylphosphaditylethanolamine (DOPE) dehydrated in the presence of sucrose. Biochem Biophys Acta Biomembr 1514:100–116
Shao HB, Liang ZS, Shao MA (2006) Osmotic regulation of 10 wheat (Triticum aestivum L.) genotypes at soil water deficits. Biointer 47:132–139
Shao HB, Chu LY, Lu ZH, Kang CM (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4:8–14
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochem 28:1057–1060
Srinivas V, Balasubramanian D (1995) Proline is a protein-compatible hydrotrope. Langmuir 11:2830–2833
Steponkus PL, Uemura M, Webb MS (1993) A contrast of the cryostability of the plasma membrane of winter rye and spring oat-two species that widely differ in their freezing tolerance and plasma membrane lipid composition. In: Steponkus PL (ed) Adv Low-Temp Biol, vol 2. Jai Press, London, pp 211–312
Su V, Hsu BD (2003) Cloning and expression of a putative cytochrome P450 gene that influences the colour of Phalaenopsis flowers. Biotechnol Lett 25:1933–1939
Trunova TI (1982) Mechanisms of winter wheat hardening at low temperature. In: Li PH, Sakai A (eds) Plant cold hardiness and freezing stress, vol 2. New York, Academic Press, pp 41–47
Walker MA, McKersie BD (1993) Role of ascorbate-glutathione antioxidant system in chilling resistance of tomato. J Plant Physiol 141:234–239
Wise R, Naylor A (1987) Chilling enhanced photooxidation. Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 83:278–282
Xuan J, Liu J, Gao H, Hu H, Cheng X (2009) Evaluation of low-temperature tolerance of zoysia grass. Trop Grasslands 43:118–124
Acknowledgments
This work was funded by Chinese National Major Program of Transgenic Research (2009ZX08004-002B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, X., Liu, Y., Mao, S. et al. Genetic transformation of lipid transfer protein encoding gene in Phalaenopsis amabilis to enhance cold resistance. Euphytica 177, 33–43 (2011). https://doi.org/10.1007/s10681-010-0246-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-010-0246-4