Abstract
Interspecific hybrids were produced from reciprocal crosses between Brassica napus (2n = 38, AACC) and B. oleracea var. alboglabra (2n = 18, CC) to introgress the zero-erucic acid alleles from B. napus into B. oleracea. The ovule culture embryo rescue technique was applied for production of F1 plants. The effects of silique age, as measured by days after pollination (DAP), and growth condition (temperature) on the efficiency of this technique was investigated. The greatest numbers of hybrids per pollination were produced under 20°/15°C (day/night) at 16 DAP for B. oleracea (♀) × B. napus crosses, while under 15°/10°C at 14 DAP for B. napus (♀) × B. oleracea crosses. Application of the ovule culture technique also increased the efficiency of BC1 (F1 × B. oleracea) hybrid production by 10-fold over in vivo seed set. The segregation of erucic acid alleles in the self-pollinated backcross generation, i.e. in BC1S1 seeds, revealed that the gametes of the F1 and BC1 plants carrying a greater number of A-genome chromosomes were more viable. This resulted in a significantly greater number of intermediate and a smaller number of high-erucic acid BC1S1 seeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been well established that Brassica napus (AACC genome, 2n = 38) is an amphidiploid species resulting from hybridizations in nature between its diploid progenitor species, B. rapa (AA genome, 2n = 20) and B. oleracea (CC genome, 2n = 18) (Frandsen 1947; U 1935). The A- and C-genome diploid progenitor species are known to be genetically distinct from the natural amphidiploid B. napus (Song et al. 1988; Thormann et al. 1994), and carry important economic traits not normally found in B. napus. Several important traits have been introgressed from the diploid to the amphidiploid species. For example, self-incompatibility (Rahman 2005; Ripley and Beversdorf 2003) and cabbage aphid (Brevicoryne brassicae) resistance (Quazi 1988) from B. oleracea to B. napus; and resistance to clubroot (Plasmodiophora brassicae) from B. rapa to B. napus (Gowers 1982; Johnston 1974). Some important traits have also been transferred from the amphidiploid to the diploid species. For example, triazine resistance (Ayotte et al. 1988) from B. napus to B. oleracea and resistance to white rust (Albugo candida) race-7 from B. napus to B. rapa (Scarth et al. 1992).
Interspecific hybrid plants are relatively easy to obtain from crosses between B. napus and B. rapa, and does not require application of cell and tissue culture technique (Bing et al. 1996; Jørgensen and Andersen 1994). The cross between B. napus and B. oleracea, however, is known to be quite difficult (Downey et al. 1980). Self-pollinated progeny from the cross between amphidiploid and diploid species often stabilize into the amphidiploid type. However, backcrossing of the hybrids with the diploid parent often yields diploid type plants in the segregating population (Rahman 2001; Zaman 1988). It is therefore difficult to introgress a trait from B. napus into B. oleracea; as an extensive effort is needed to obtain viable F1 and BC1 hybrids. The development of an efficient method for producing F1 and BC1 hybrids from these two species would be advantageous for transfer of traits from B. napus to B. oleracea.
Ovary, ovule, and embryo culture are commonly used as embryo rescue techniques in interspecific crosses in Brassica (Inomata 1993). Several investigators compared the efficiency of these techniques for the production of various Brassica interspecific hybrids, and reported that ovule culture was superior to other techniques, especially when B. oleracea was used as the female (Takeshita et al. 1980). Cultured ovules develop better on liquid medium than solid medium as long as they are not immersed (Kameya and Hinata 1970, cited by Takeshita et al. 1980).
Erucic acid is the characteristic fatty acid of Brassica oilseed crops. Each of the two B. napus genomes is known to possess one locus responsible for production of erucic acid (C22:1) in seed oil (Chen and Heneen 1989; Dorrell and Downey 1964). Several alleles controlling the synthesis of erucic acid in seed oil have been reported in the literature, viz. e (0%), E a (10%), E b (12–15%), E c (30%), and E d (3.5%) (Getinet et al. 1997). These alleles act in an additive (Harvey and Downey 1964) or partly dominant (Rahman et al. 1994) manner depending on the strength of the allele. The zero-erucic acid phenotype occurs when the loci are homozygous for the zero-erucic alleles. The inheritance of erucic acid in Brassica species is well understood from several studies based on crosses between high and low erucic acid genotypes within the same species (Dorrell and Downey 1964; Getinet et al. 1997; Harvey and Downey 1964; Kondra and Stefansson 1965; Krzymanski and Downey 1969; Rahman et al. 1994). Inheritance of this fatty acid in interspecific hybrids is, however, difficult to predict as aneuploid gametes are produced in high frequency in the hybrid plants in their early generations.
The present study is a part of research that is being carried out in our laboratory to transfer the zero erucic acid trait from B. napus to B. oleracea. Such transfer would require knowledge on segregation of the erucic acid alleles in the interspecific hybrids. The development of low erucic B. oleracea germplasm would be valuable for broadening genetic diversity in B. napus, through interspecific crossings while maintaining its oil quality, for improvement of B. napus cultivars. Thus, the objectives of this study were two-fold: (i) to identify the optimal growth condition and time of rescue of hybrid embryos for in vitro ovule culture for the production of B. napus × B. oleracea interspecific F1 hybrids, as well as to investigate the efficiency of BC1 hybrid production with or without application of ovule culture technique; and (ii) to study the inheritance of erucic acid in a BC1S1 population derived from self-pollination of F1 × B. oleracea BC1 hybrids.
Materials and methods
Parent material
Two yellow-flowered, canola quality (zero-erucic, low glucosinolate) Brassica napus L. (AACC, 2n = 38) doubled haploid lines, Hi-Q and A01-104NA, and one white-flowered, high erucic (40.1% erucic acid) self-compatible inbred (F7) Brassica oleracea var. alboglabra Bailey (CC, 2n = 18) line were used. Brassica oleracea var. alboglabra, (Chinese kale), was domesticated in China (Prakash and Hinata 1980) and is considered to be a form of B. oleracea.
Experimental design
Parental plants were grown in six-inch pots in two growth chambers set at 20°/15°C and 15°/10°C day/night temperatures with 16 h photoperiod. Photosynthetic flux density in both cabinets was 450 μE (mV) m−2 s−1 at plant level. B. oleracea was seeded 14 days prior to the B. napus parents for synchrony of flowering time. Under each growth condition, there were two replications consisting of eight B. oleracea plants and four plants of each of the two B. napus parents. Reciprocal crosses were made where individual female plants were pollinated with bulk pollen from four male plants. Developing siliques at the age of 6 to 16 DAP were harvested at two-day intervals, and were used for rescue of hybrid embryos.
Ovule culture
The ovule culture technique was applied for rescue of the hybrid embryos, and was performed in a laminar flow hood under aseptic conditions. Excised siliques were surface sterilized with a 7% (w/v) calcium hypochlorite [Ca(OCl)2] solution for 10 min in sterile 50 ml conical tubes, and subsequently rinsed twice with distilled water. The siliques were longitudinally dissected using a sterile surgical blade and developing (fertilized) ovules were excised and counted. A small incision was made on the non-micropylar end of the developing ovules. These were floated on approximately 5 ml of liquid culture medium (Ripley and Beversdorf 2003) in a tissue culture petri dish (60 × 15 mm). The liquid medium was composed of Nitsch and Nitsch (1967) medium supplemented with 300 mg l−1 casein hydrolysate, 200 mg l−1 glutamine, and 13% sucrose. The medium was adjusted to pH 6.0, and filter-sterilized. Tissue culture petri dishes were sealed and placed on a shaker set at 60 rpm. After 2–3 weeks on the shaker, the number of developed embryos having an elongated root and shoot axis and conspicuous cotyledons (cotyledon stage) was recorded.
The embryos (cotyledon stage) were transferred from liquid culture medium to solid B5 medium containing 0.1 mg l−1 GA3, 20 g l−1 sucrose and 8 g l−1 agar (Coventry et al. 1988). Embryos were placed lightly on the solid medium in a petri dish (100 × 15 mm) and sealed. The petri dishes were initially placed at 4°C under lights (8 h photoperiod) for 2–4 days and were then moved to room temperature (22–25°C) and placed under lights (30 μE (mV) m−2 s−1 photosynthetic flux density at plant level; 12 h photoperiod). Embryos were kept on the solid medium for 3–4 weeks until fully germinated and roots were developed. These seedlings were transplanted to six-inch pots containing soil-free growth medium (Stringam 1971) and placed in a growth chamber (15°/10°C day/night temperature; 16 h photoperiod). The newly transplanted seedlings were covered with transparent plastic tubes for three to four days until hardened.
Confirmation of hybrids
A simple sequence repeat (SSR, microsatellite) molecular marker was used to confirm the hybrid nature of F1 plants. The details of this method are described elsewhere (Rahman et al. 2007).
Production of BC1 plants and BC1S1 seeds
The F1 hybrid plants were backcrossed with B. oleracea, and the ovule culture technique was applied to generate backcross (BC1) hybrids. The BC1 plants were self-pollinated manually as well as under bag isolation to generate BC1S1 seeds.
Fatty acid analysis
The half-seed technique of gas chromatographic fatty acid analysis was applied to determine the content of erucic acid in self-pollinated backcross (BC1S1) and in parental seeds. Seeds were germinated on filter paper in 100 × 15 mm petri dishes under room temperature (22–25°C). One cotyledon of each of the newly germinated seeds were dissected and transferred to a 10 × 75 mm glass test tube for fatty acid analysis. The remainder of the seed was grown into a plant.
The dissected cotyledons were dried at 80°C for 1 h, immersed in 1.2 ml Na+ methylating solution and 0.25 ml hexane solvent and crushed with a glass rod for extraction of oil and conversion to methyl esters. The tubes were capped and placed in the dark for 30 min to allow the methylation reaction to take place. A 20% NaCl solution (1–2 ml) was added to maximize the recovery of the short chain fatty acids (i.e. C12, C14 and C16). The hexane solvent (containing methyl esters) was transferred to the gas chromatography vials containing 250 μl glass BMI/spring inserts, and was evaporated to approximately 100 μl to increase the concentration of fatty acid methyl esters for ease of fatty acid analysis/detection. Gas chromatography was performed using a Hewlett-Packard chromatograph (model 6890 N) equipped with a flame ionization detector. A DB-WAX (crosslinked polyethyleneglycol) column was used to obtain greater peak resolution and adequate separation, ensuring measurement of each individual fatty acid.
Results
F1 hybrid embryo production
Hybrid embryo yield of these interspecific crosses, viz. B. napus (Hi-Q) × B. oleracea, B. oleracea × Hi-Q, B. napus (A01-104NA) × B. oleracea, and B. oleracea × A01-104NA was very poor which restricted the use of individual cross data in statistical analysis. Therefore, data for the two crosses of B. napus × B. oleracea and B. oleracea × B. napus were pooled and subjected to chi-square analysis using proc CATMOD in the SAS system (Statistical Analysis System, Inc. 1999), where H0 = no difference in the number of hybrid embryos per pollination between the six different ages of the siliques (6–16 DAP) under two temperature (20°/15°C and 15°/10°C) conditions.
Embryo yield was extremely low or almost zero at 6–8 DAP in all crosses. Using B. napus as female in the cross, the greatest efficiency of ovule culture technique was obtained when siliques were developed under 15°/10°C temperature and harvested at 14 DAP. Under this condition, 0.15 embryos per pollination were rescued (Fig. 1). On the other hand, under 20°/15°C, maximum efficiency of 0.11 rescued embryos per pollination was obtained at 10 DAP (Fig. 1). In the case of the reciprocal cross, where B. oleracea was used as female, maximum efficiency of the ovule culture technique was obtained at 16 DAP under 20°/15°C, where 0.31 embryos per pollination were rescued (Fig. 1). For this cross, a significantly greater number of embryos were rescued from the siliques developed under higher temperature compared to the siliques developed under lower temperature (Table 1). Only 0.7% of the fertilized ovules developed under the lower temperature yielded rescuable embryos, while 5.2% of the ovules from higher temperature condition yielded rescuable embryos (data not presented).
Confirmation of the F1 hybrids
Nineteen C-genome specific SSR primer pairs, distributed in the nine linkage groups, were used to screen the B. napus and B. oleracea parents for polymorphism. From these, the marker sS2129 was chosen to confirm hybridity of the F1 plants. This marker is located on the C-genome linkage group 15, and generates approximately 198 bp fragment in B. napus and 168 bp fragment in B. oleracea. A total of 62 embryos were obtained from reciprocal interspecific crosses, of which 46 (74.2%) produced plants. Testing of these plants by sS2129 confirmed 34 plants to be hybrids, eight from B. napus × B. oleracea crosses and 26 from B. oleracea × B. napus crosses. The non-hybrid plants (n = 12) originated from inadvertent self-pollination of B. napus and B. oleracea, and all were discarded.
Production of BC1 plants
Backcrossing was done on 31 F1 plants using B. oleracea as the male parent. Application of in vitro ovule culture technique was approximately 10-fold more effective compared to in vivo seed set for the production of BC1 hybrids (Table 2).
Inheritance of erucic acid in BC1S1 seeds
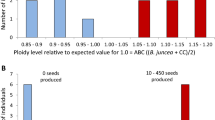
One hundred nine BC1S1 seeds were analyzed of which 19 (17.4%) seeds had less than 2.7% erucic acid (zero-erucic acid class). Only three seeds had >30% erucic acid (high erucic acid class), i.e. 2.8% of the total number of seeds (Fig. 2). In the remaining 87 (79.8% of total) seeds, the content of erucic acid ranged from 7 to 30% (Fig. 2). This 3:87:19 distribution deviated significantly (χ2 = 195.4, P < 0.01) from the 5:2:1 segregation that would be expected in BC1S1 seeds based on segregation of only the C-genome erucic acid alleles (Fig. 3). The greatest deviation was observed for high and intermediate erucic acid classes. The observed number of seeds in the high erucic acid class (33–40% erucic acid) was significantly lower, and the intermediate erucic acid class (7–30% erucic acid) significantly higher than expected (Fig. 4).
Observed number of BC1S1 seeds for three different erucic acid classes compared to the number of seeds to be expected from normal disomic segregation based on only the C-genome erucic acid alleles. BC1S1 seeds derived from self-pollination of (B. napus × B. oleracea var. alboglabra) × B. oleracea var. alboglabra BC1 plants
Low erucic BC1S1 plants
Pollen viability among BC1S1 plants was extremely low. Manual self-pollination was done on all BC1S1 plants. Of the 16 low erucic plants that grew to maturity, five were completely sterile, and only three produced seed. Almost all of these plants (14) had leaf morphology either intermediate to both parents or similar in appearance to the B. oleracea parent. Other morphological characteristics were quite variable, i.e. days to flowering ranged from 61 to 115 days after seeding; plant height ranged from 61 to 159 cm.
Discussion
Embryo rescue
Several investigations have focused on improving the interspecific hybridization efficiency within the family Brassicaceae (Ayotte et al. 1987; Bajaj et al. 1986; Inomata 1993; Rahman 2004; Takeshita et al. 1980; Zhang et al. 2004). These studies primarily focused on the method of embryo rescue, time of harvest of hybrid embryos (DAP), and type of culture media. To the best of our knowledge, no study so far has been done to examine the effect of growing temperature and age of siliques on the efficiency of embryo rescue for the production of Brassica interspecific hybrids. The data presented in this paper suggest that the efficiency of embryo rescue in B. napus × B. oleracea var. alboglabra interspecific crosses depends greatly on the interaction between maternal genotype and growth condition (temperature). The slower growing species B. oleracea yielded the greatest number of hybrid embryos under higher temperature and at 16 DAP. On the other hand, the relatively rapid growing species B. napus yielded the greatest number of hybrid embryos under lower temperature and at 14 DAP. This interspecific cross seems to be quite difficult to achieve, as reported by several authors. For example, U (1935) obtained 0.0033 hybrids per pollination and Chiang et al. (1977) obtained 0.00049 hybrids per pollination from B. napus × B. oleracea interspecific crosses under in vivo condition. This was also apparent from our study where no in vivo hybrid was obtained from 288 cross-pollinations (data not presented). Thus, application of the ovule culture technique greatly enhanced the rate of F1 hybrid production compared to in vivo hybrid seed set. Similarly, application of ovule culture technique was also highly effective for production of backcross hybrid plants in the present study. To our knowledge this is the first report on the comparison of the application of in vitro and in vivo techniques for the production of BC1 hybrids of (B. napus × B. oleracea) × B. oleracea crosses.
Erucic acid inheritance
The allelic composition of the B. napus × B. oleracea F1 hybrid plants (digenomic triploids) with respect to erucic acid alleles would be C+C0A0 (C+ = high erucic acid allele from B. oleracea; C0 and A0 = zero-erucic acid alleles from B. napus). Theoretically, the diploid set of the C-genome alleles in F1 and BC1 plants should follow a normal segregation; and this is modelled in Fig. 3. However, it is difficult to predict any definite segregation pattern of the A-genome chromosomes in gametes of F1 and BC1 plants due to their unbalanced genome composition. In F1 plants, the haploid set of the 10 A-genome chromosomes would segregate randomly and be included in the gametes in variable numbers ranging from 0 to 10. Backcrossing of the F1 plants with B. oleracea (CC) could therefore result in a BC1 population composed of variable genotypes ranging from 2n (CC), to 2n + 1 to 10 A-genome chromosomes. Meiosis in the BC1 population would produce gametes with variable numbers of A-genome chromosomes (0–10), and self-pollination of the BC1 population would result in BC1S1 seeds with genetic constitution ranging from 2n (CC), to 2n + 1 to 20 A-genome chromosomes.
Inclusion of the A-genome chromosome carrying the zero-erucic acid allele (A0) in the gametes would affect the content of erucic acid to be produced by the C-genome alleles (C+C+ and C+C0) in the seeds (Table 3). For example, Rahman (2002) investigated the effect of the zero-erucic acid allele of the A-genome (B. rapa ssp. trilocularis var. ‘yellow sarson’) when combined with the high erucic acid alleles of the BC-genome of B. carinata (41%), and found a significantly lower level of erucic acid in the seeds of trigenomic allohexaploid interspecific hybrids (AABBCC) compared to its B. carinata parent (mean 33.4% vs. 41.3%). The erucic acid content may further be affected by the doses of the A-genome allele, e.g. C+C+A0 and C+C+A0A0 genotypes would be expected to have different contents of this fatty acid. Taking the dose effect of the erucic acid alleles into account, nine seed genotypes would be possible in BC1S1 with respect to erucic acid alleles (Table 3). The phenotypes of these genotypes could be inferred based on the reports of earlier researchers (Chen et al. 1988; Rahman 2002; Table 3).
In the present study, occurrence of a significantly lower number of high erucic acid seeds but higher number of intermediate erucic acid seeds suggests that a large number of the BC1S1 seeds with a C+C+ genotype must have inherited either one or two A-genome erucic acid alleles (A0). Furthermore, within the intermediate class, the greater number of seeds fell within the phenotypic classes presumed to be produced by genotypes with two A-genome erucic acid alleles (Table 3): 36 seeds with 7.8–15.3% erucic acid would be C+C0A0A0 genotype and 33 seeds with 20.0–28.9% erucic acid would be either C+C0 or C+C+A0A0 genotype. On the other hand, a smaller number of seeds were found within the phenotypic classes expected to be produced by the genotypes having only one dose of the A-genome erucic acid allele (Table 3): 11 (C+C0A0, 16.0 to 19.3% erucic acid) and 7 (C+C+A0, 29.1–33.2% erucic acid) seeds fell into these classes. This suggests that the aneuploid gametes produced by the F1 and BC1 plants and carrying greater numbers of A-genome chromosomes may have been more viable than those with a lower number of A-genome chromosomes. Similarly, Fernandez-Escobar et al. (1988) speculated that female aneuploid gametes containing higher numbers of chromosomes were more viable in the F2 and BC1 generations of interspecific crosses between B. napus (AACC, 2n = 38) and B. carinata (BBCC, 2n = 34). In our study, only one seed approached the phenotype of the B. oleracea parent for erucic acid content (39.9% erucic acid). Chen and Heneen (1989) found that in the F2 generation of a cross between zero and high (56.5%) erucic acid B. rapa cultivars, no seeds with a level of erucic acid similar to the high parent could be recovered. They hypothesized that the high erucic alleles may function more effectively in the genetic background of its parent rather than the cultivar with which it was crossed. This, as well as possible aneuploid makeup of the seeds (+A0, +A0A0), might be the reason for the extremely low number of seeds that approached the erucic acid levels of the B. oleracea parent in our study.
The findings from this study can be applied for efficient introgression of trait(s) from B. napus to B. oleracea. It has been shown that in vitro ovule culture technique greatly increased the rate of F1 and BC1 hybrid production over that of in vivo seed set. Investigation on the silique age beyond 16 DAP will further extend our knowledge for efficient application of in vitro ovule culture technique in this interspecific cross. Zero-erucic acid seeds were obtained in BC1S1 at a rate of approximately 17%, suggesting that it is feasible to introgress the zero-erucic acid trait from B. napus into a B. oleracea background.
References
Ayotte R, Harney PM, Souza Machado V (1987) The transfer of triazine resistance from Brassica napus L. to B. oleracea L. I. Production of F1 hybrids through embryo rescue. Euphytica 36:615–624. doi:10.1007/BF00041511
Ayotte R, Harney PM, Souza Machado V (1988) The transfer of triazine resistance from Brassica napus L. to B. oleracea L. III. First backcross to parental species. Euphytica 37:189–197. doi:10.1007/BF00036857
Bajaj YPS, Mahajan SK, Labana KS (1986) Interspecific hybridization of Brassica napus and B. juncea through ovary, ovule, and embryo culture. Euphytica 35:103–109. doi:10.1007/BF00028547
Bing DJ, Downey RK, Rakow GFW (1996) Hybridizations among Brassica napus, B. rapa and B. juncea and their two weedy relatives B. nigra and Sinapis arvensis under open pollination conditions in the field. Plant Breed 115:470–473. doi:10.1111/j.1439-0523.1996.tb00959.x
Chen BY, Heneen WK (1989) Fatty acid composition of resynthesized Brassica napus L., B. campestris L., and B. alboglabra Bailey with special reference to the inheritance of erucic acid content. Heredity 63:309–314. doi:10.1038/hdy.1989.103
Chen BY, Heneen WK, Jönsson R (1988) Resynthesis of Brassica napus L. through interspecific hybridization between B. alboglabra Bailey and B. campestris L. with special emphasis on seed colour. Plant Breed 101:52–59. doi:10.1111/j.1439-0523.1988.tb00266.x
Chiang MS, Chiang BY, Grant WF (1977) Transfer of resistance to race 2 of Plasmodiophora brassicae from Brassica napus to cabbage (B. oleracea var. capitata). I. Interspecific hybridization between B. napus and B. oleracea var. capitata. Euphytica 26:319–326. doi:10.1007/BF00026993
Coventry J, Kott L, Beversdorf WD (1988) Manual for microspore culture technique for Brassica napus. OAC Publication 0489, University of Guelph, Canada
Dorrell DG, Downey RK (1964) The inheritance of erucic acid in rapeseed (Brassica campestris). Can J Plant Sci 44:499–504
Downey RK, Klassen AJ, Stringam GR (1980) Rapeseed and mustard. In: Fehr WR, Hadley HH (eds) Hybridization of crop plants. ASA, CSSA, Madison
Fernandez-Escobar J, Dominguez J, Martin A, Fernandez-Martinez JM (1988) Genetics of the erucic acid content in interspecific hybrids of Ethiopian mustard (Brassica carinata Braun) and rapeseed (B. napus L.). Plant Breed 100:310–315. doi:10.1111/j.1439-0523.1988.tb00257.x
Frandsen KJ (1947) The experimental formation of Brassica napus L. var. oleifera D.C. and Brassica carinata Braun. Dansk Bot Arkiv 12:1–16
Getinet A, Rakow G, Raney JP, Downey RK (1997) The inheritance of erucic acid content in Ethiopian mustard. Can J Plant Sci 77:33–41
Gowers S (1982) The transfer of characters from Brassica campestris L. to Brassica napus L.: production of clubroot-resistant oil-seed rape (B. napus ssp. oleifera). Euphytica 31:971–976. doi:10.1007/BF00039237
Harvey BL, Downey RK (1964) The inheritance of erucic acid content in rapeseed (Brassica napus). Can J Plant Sci 44:104–111
Inomata N (1993) Embryo rescue techniques for wide hybridization. In: Labana KS, Banga SS, Banga SK (eds) Breeding oilseed Brassicas. Springer-Verlag, Berlin
Johnston TD (1974) Transfer of disease resistance from Brassica campestris L. to rape (B. napus L.). Euphytica 23:681–683. doi:10.1007/BF00022490
Jørgensen RB, Andersen B (1994) Spontaneous hybridization between oilseed rape (Brassica napus) and weedy B. campestris (Brassicaceae): a risk of growing genetically modified oilseed rape. Am J Bot 81:1620–1626. doi:10.2307/2445340
Kondra ZP, Stefansson BR (1965) Inheritance of erucic and eicosenoic acid content of rapeseed oil (Brassica napus). Can J Genet Cytol 7:500–510
Krzymanski J, Downey RK (1969) Inheritance of fatty acid composition in winter forms of rapeseed, Brassica napus. Can J Plant Sci 49:313–319
Nitsch C, Nitsch JP (1967) The induction of flowering in vitro in stem segments of Plumbago indica L. I. The production of vegetative buds. Planta 72:355–370. doi:10.1007/BF00390146
Prakash S, Hinata K (1980) Taxonomy, cytogenetics and origin of crop Brassicas, a review. Opera Bot 55:1–57
Quazi MH (1988) Interspecific hybrids between Brassica napus L. and B. oleracea L. developed by embryo culture. Theor Appl Genet 75:309–318. doi:10.1007/BF00303970
Rahman MH (2001) Production of yellow-seeded Brassica napus through interspecific crosses. Plant Breed 120:463–472. doi:10.1046/j.1439-0523.2001.00640.x
Rahman MH (2002) Fatty acid composition of resynthesized Brassica napus and trigenomic Brassica void of genes for erucic acid in their A genomes. Plant Breed 121:357–359. doi:10.1046/j.1439-0523.2002.00711.x
Rahman MH (2004) Optimum age of siliques for rescue of hybrid embryos from crosses between Brassica oleracea, B. rapa and B. carinata. Can J Plant Sci 84:965–969
Rahman MH (2005) Resynthesis of Brassica napus L. for self-incompatibility: self-incompatibility reaction, inheritance and breeding potential. Plant Breed 124:13–19. doi:10.1111/j.1439-0523.2004.01045.x
Rahman MH, Rahman L, Stølen O, Sørensen H (1994) Inheritance of erucic acid content in yellow- and white-flowered yellow sarson × Canadian Brassica campestris L. Acta Agric Scand 44:94–97
Rahman MH, Hawkins G, Avery M, Thiagarajah MR, Sharpe AG, Lange R et al (2007) Introgression of blackleg (Leptosphaeria maculans) resistance into Brassica napus from B. carinata and identification of microsatellite (SSR) markers. Proceedings of the 12th international rapeseed congress, vol 4, pp 47–50
Ripley VL, Beversdorf WD (2003) Development of self-incompatible Brassica napus: (I) introgression of S-alleles from Brassica oleracea through interspecific hybridization. Plant Breed 122:1–5. doi:10.1046/j.1439-0523.2003.00780.x
SAS Institute, Inc (1999) SAS/STAT user’s guide, version 8. SAS Institute, Inc, Cary
Scarth R, Rimmer SR, McVetty PBE (1992) Reward summer turnip rape. Can J Plant Sci 72:839–840
Song KM, Osborn TC, Williams PH (1988) Brassica taxonomy based on nuclear restriction fragment length polymorphisms (RFLPs). Theor Appl Genet 75:784–794. doi:10.1007/BF00265606
Stringam GR (1971) Genetics of four hypocotyl mutants in Brassica campestris L. Heredity 62:248–250
Takeshita M, Kato M, Tokumasu S (1980) Application of ovule culture to the production of intergeneric or interspecific hybrids in Brassica and Raphanus. Jpn J Genet 55:373–387. doi:10.1266/jjg.55.373
Thormann CE, Ferreira ME, Camargo LEA, Tivang JG, Osborn TC (1994) Comparison of RFLP and RAPD markers to estimating genetic relationships within and among cruciferous species. Theor Appl Genet 88:973–980. doi:10.1007/BF00220804
U N (1935) Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilisation. Jpn J Bot 7:389–452
Zaman MW (1988) Limitations for introgression of yellow seed coat colour in Brassica napus. J Swed Seed Assoc 98:157–161
Zhang GQ, Tang GX, Song WJ, Zhou WJ (2004) Resynthesizing Brassica napus from interspecific hybridization between Brassica rapa and B. oleracea through ovary culture. Euphytica 140:181–187. doi:10.1007/s10681-004-3034-1
Acknowledgements
Funding for this project by Natural Sciences and Engineering Research Council of Canada (NSERC) and Alberta Canola Producers Commission (ACPC) to the last author is gratefully acknowledged. The authors are also thankful for the technical assistance of An Vo of the Canola Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bennett, R.A., Thiagarajah, M.R., King, J.R. et al. Interspecific cross of Brassica oleracea var. alboglabra and B. napus: effects of growth condition and silique age on the efficiency of hybrid production, and inheritance of erucic acid in the self-pollinated backcross generation. Euphytica 164, 593–601 (2008). https://doi.org/10.1007/s10681-008-9788-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-008-9788-0