Abstract
A survey of macro-invertebrates and their monthly variations occupying the Tons river in Doon Valley was conducted from August 2003–July 2004. Macro-invertebrate collections and water samples were taken from three sampling stations every month during the period of study. All the hydrological attributes were measured monthly for 1 year. The present study showed that the water velocity, hydromedian depth, turbidity and dissolved oxygen and nature and size of the bottom substrates do play a major role in determining the macro-invertebrate diversity of Tons river. The ecological relevance of the measured hydrological attributes was investigated by comparing their degree of correlation with invertebrate density and diversity. The Shannon–Wiener index (H′) of macro-invertebrates was found to be highest (3.60) during spring season (February and March) and lowest (2.59) during monsoon season (July and August). The high values of diversity index of macro-invertebrates at all the three sampling sites indicate diverse macro-invertebrate communities in the Tons river in Doon Valley, India.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The macro-invertebrates act as the secondary producers in the aquatic ecosystems. They are an important link in the food web of aquatic ecosystem. Insects are the dominant class among macro-invertebrates. The diversity of insects can only be described as amazing. Current estimates of known species of insects are well over 1 million, of which about 8,600 species of insects are associated with freshwater environments during some part of their lives. Aquatic insects play a role in the processing of organic matter (Cummins 1974; Cummins et al. 1984). Aquatic macro-invertebrates are important food for fish and waterfowls. They also play an important role in transferring energy from the first trophic level to second trophic level in freshwater ecosystems. Benthic aquatic macro-invertebrates are sensitive indicators of environmental changes in streams because they express long-term changes in water and habitat quality rather than instantaneous conditions (Johnson et al. 1993). Aquatic organisms can exhibit a great breadth of genetic diversity and species richness, maintenance of which is essential for the survival of any lotic or lentic ecosystem (Singh and Sharma 1998). Aquatic habitats are known to support an extraordinary array of species. Most of the aquatic fauna are small invertebrates. As compared to the fish fauna, the invertebrate species diversity in most parts of the world, particularly the tropics, is poorly known. The lack of knowledge is caused by a number of factors, such as the great diversity of invertebrates, and their numerical abundance has contributed to their neglect. In addition to this, invertebrates are small and difficult to identify. In spite of the dearth of studies on the invertebrate fauna, many of the aquatic invertebrates are being lost as their habitat deteriorates, some without ever been discovered and made known as species.
A considerable work on the aquatic macro-invertebrate has been done by several aquatic biologists (Collier and Smith 1995; Quinn et al. 1997; Clausen and Biggs 1997, 1998, 2000; Collier et al. 1997, 2000; Walsh et al. 2001; Ward and Tockner 2001; Jacobson et al. 2003). Some of the studies have also been made in the different parts of the Indian subcontinent (Das 1971; Bisht and Das 1979a, b; Badola 1979; Mitra and Nandi 1998; Sharma 1986; Sharma et al. 2004, 2008). However, no effort has been made so far to study the macro-invertebrates dwelling in the rivers of Doon Valley, India, inspite of the fact that the wide variety of microhabitats (rock surfaces, plant surfaces, leaf debris, logs, back waters, sandy sediments, crevices in gravel and pebbles) are available in the rivers of Doon Valley to aquatic macro-invertebrates. Therefore, a maiden attempt has been made to survey macro-invertebrate diversity of the Tons river, one of the important rivers of Doon Valley, India.
2 The study area
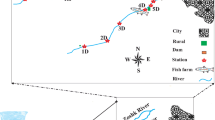
The Tons river is one of the most important tributaries of the Yamuna river. It originates from the southern slope of the Mussoorie range and flows south-southwest. It joins the northwest-flowing river Asan, which latter joins the Yamuna river. The study area is located in Doon Valley, a very important valley of the Garhwal Himalayas (Fig. 1). Three sampling sites were identified depending upon the accessibility and the anthropogenic interventions. The first site (S1) was identified at Guchchupani, near a small village mostly inhabitated by Nepalis. Anthropogenic activities like washing and bathing are common at this site. Composition of river bottom substrates at S1 was dominated by pebbles and cobbles and small numbers of boulders. The second sampling site (S2) was identified near the Tapkeshwar temple dedicated to Lord Shiva, where there is a huge disposal of worship materials (sacred tree leaves, flowers, fruits, sweets, milk and milk products, etc.) by the priests and devotees to Lord Shiva into the river. Bottom substrates at this site were dominated by boulders and cobbles along with some sand. The third sampling site (S3) was selected at Bilaspur Kandli, 2.5 km downstream from S2, which is devoid of any anthropogenic activity. Composition of river bottom substrates is dominated by big boulders at this site. This site has high macrophyte growth in the Tons river towards the left bank of the river, where the temple is located.
3 Methodology
Monthly samplings for analyzing the hydrological attributes and biotic components of macro-invertebrates were conducted for one annual cycle (August 2003 to July 2004). The temperature of air and water was recorded with the help of a Celsius (0–110°C) thermometer. The mean velocity of water was measured using the Electromagnetic Current Meter (Model-PVM-2A Montedoro-Whitney Corp., San Luis Obispo, CA). The vertical velocity profile was established by measuring the velocity at depths ranging from 0.10 to 1.5 m above the substratum. The pH of the water was determined by the Control Dynamics pH meter (Model-APX15/C), while turbidity was measured by the Elico Turbidity Meter (Model-5D1 M). Inorganic phosphate (H2PO4, HPO−2 and PO4 −3) were determined with the help of the Systronics Spectrophotometer (Model-Spectronic 20 D Series). The phosphate present in the water reacts with ammonium molybdate and forms complex molybdophosphoric acid, which gets reduced to a complex of blue color in the presence of SnCl2. Hence, the stannous chloride method was followed for estimating phosphate in the water sample. Two milliliters of ammonium molybdate solution followed by five drops of SnCl2 solution were added to 50 ml of the filtered clear sample in a clean Erlenmeyer’s flask, developing a blue color. The absorption of light by this blue color in a sample was measured at 690 nm on the spectrophotometer using a blank solution with a similar quantity of the chemicals. The readings were taken after 5 min, but before 12 min before the addition of the last reagent. The readings were noted at concentration mode, and direct values were obtained. The values were expressed as mg l−1.
The concentration of nitrate was also estimated spectrophotometrically at 410-nm wavelength with the help of a Systronic Spectrophotmeter (Spectronic 20 D Series). Fifty milliliters of water sample was taken in an Erlenmeyer flask, adding the same amount of silver sulphate solution to it so as to remove the chloride contents from the sample. It was then heated slightly on a hot plate, and the precipitate of AgCl was filtered out with the help of a filter paper. The filtrate was then dried in a porcelain basin. After cooling in a desicator, it was dissolved in 2 ml phenol disulphonic acid, and the contents were diluted to 50 ml with distilled water. To develop a yellow color, 6 ml of liquid ammonia was added to it. The samples were then analyzed spectrophotometrically. The readings were noted at the concentration mode, and direct values were obtained. The values were expressed in terms of mg l−1. Chloride, alkalinity and hardness were calculated by titration methods following the standard methods outlined in Wetzel and Likens (1991) and APHA (1998). Five replicates of each physico-chemical parameter from each sampling site (S1, S2 and S3) were taken. These were pooled, and the mean was calculated.
The percentage cover of different-sized substrata within each Surber quadrate was estimated visually using the substrate size classes of sand (0.06–2.0 mm), fine gravel (2–32 mm), coarse gravel (32–64 mm), cobbles (64–256 mm) and boulders (>256 mm).
Benthic macro-invertebrates colonizing the substrate were collected with the help of the Surber sampler (0.50-mm mesh net) to a depth of about 10 cm in a quadrat and by hand picking from microhabitats. Quantitative estimation of benthic macro-invertebrates was based on numerical counting, i.e., units per square meter (ind. m−2). The benthic macro-invertebrates were preserved in 4% formalin. These macro-invertebrates were identified to the possible lowest taxonomic level. The qualitative analyses of macro-invertebrate samples were made with the help of Fraser (1933); Needham and Needham (1962); Hickel (1977); Macan (1974); Disney (1975); Hynes (1977); Elliott and Humpesch (1983); Kumar and Khanna (1984); Elliott et al. (1988); Wallace et al. (1990); Ward and Whipple (1992); Rao (1933), Biswas et al. (1995); Edington and Hilldrew (1995); Mitra (1999); Mitra (2003); Ramkrishna and Mitra (2004); Sharma et al. (2004, 2008). The density of macro-inveretbrates was calculated from all the five replicates collected from each sampling site.
Alpha diversity among the macro-invertebrates was analyzed by counting the total number of taxa present in the sampling sites (S1, S2 and S3) under different environmental conditions.Alpha diversity \( \left( \alpha \right) = N \); where, N = total number of taxa present.
3.1 Statistical treatment of the data
For statistical treatment of the data, species diversity indices were calculated using the Shannon and Wiener (1964) diversity index: Shannon–Wiener Diversity index \( \left( {H^{\prime}} \right) = \sum\limits_{l - 1}^{s} {\left( {\frac{{n_{i} }}{N}} \right)\log_{2} \left( {\frac{{n_{i} }}{N}} \right)} \); where H′ = Shannon–Wiener index of diversity; n i = total number of individual of a taxon; N = total number of individuals of all taxa.
Karl Pearson’s correlation coefficient (r values) was used to quantify relationships between various hydrological attributes and the influence of hydrological attributes on biological communities. All the monthly samples of each taxon and physico-chemical attribute collected from all the three sites were pooled, and the mean of these attributes was computed and the inter-relationships (r-values) were calculated (Bailey 1995).
Regression analysis was also performed between the density of macro-invertebrates and hydrological attributes to determine the influence of hydrological attributes on biological communities. The total monthly density of all the macro-invertebrates from all the three sites was taken together, and means were used to perform regression. Regression analysis was performed with each physico-chemical attribute one by one, and the results (R value, R 2, standard error, F value, intercept, X variable, t-stat and p-value) were presented in Table 8.
4 Results
4.1 Physico-chemical attributes of water
The physico-chemical parameters (\( \overline{X} \pm {\text{SD}} \)) recorded at all the three sites of the Tons river are given in Table 1. Maximum air temperature was recorded in the month of July (33.67 ± 0.58°C) and minimum in the month of January (15.33 ± 1.53°C), while the mean water temperature was recorded with a maximum in the month of July (27.67 ± 2.31°C) and minimum in the month of February (14.33 ± 1.53°C). Water velocity was fairly good throughout the year with the highest value in the month of July (2.00 ± 0.00 ms−1) and lowest recorded in the month of May (0.50 ± 0.22 ms−1) and June (0.50 ± 0.22 ms−1). Hydro median depth (HMD) was also considerable round the year with the highest in the month of July (1.23 ± 1.10) and lowest in the month of June (0.24 ± 0.09 m). Turbidity was found to be highest in the month of July (335.43 ± 58.60 NTU), and the water was almost clear in the month of January and February. Dissolved oxygen was recorded highest in the month of February (14.00 ± 2.03 mg l−1) and minimum in the month of July (7.43 ± 0.59 mg l−1). Turbidity and total dissolved solids (TDS) showed a decreasing trend from August to February (Table 1). Other parameters like hardness, pH, alkalinity, chlorides, nitrates and phosphate, sodium and potassium showed an irregular trend in their concentrations in the Tons river throughout the study.
4.2 Macro-invertebrate density and diversity
The macro-invertebrates dwelling in the Tons river were mostly represented by the larvae (immature stages of aquatic insects). These larvae belong to the orders of Ephemeroptera, Plecoptera, Hemiptera, Coleoptera, Trichoptera, Diptera and Odonata. Most of the taxa found were scrappers and collector-gatherers. Few taxa of Mollusca and Annelida were also present in the Tons river. The monthly variations in the density of macro-invertebrates recorded for one annual cycle from all the three sites (S1, S2 and S3) of Tons river have been presented in Tables 2–4. The overall density of the macro-invertebrates was found to be maximum (2,775–3,000 ind. m−2) in the month of February and minimum (250–325 ind. m−2) in the month of July in the Tons river. Analysis of the monthly abundance of macro-invertebrates in the Tons river also revealed that the maximum abundance of macro-invertebrates was recorded at the sampling site S3 and minimum at S1. The sequence of abundance of macro-invertebrates in all the three sites was S3 > S2 > S1 with decreasing altitude. Thus, the density of macro-invertebrates of the Tons river increased along the decreasing altitude.
Annual percentage composition of macro-invertebrates contributed by various taxa has revealed that the major contribution was made by Ephemeroptera (43–45%) and minimum (1–3%) by Mollusca (Fig. 2). A considerable contribution was also made by Trichoptera (9–11%) and Diptera (10–13%) to the total annual percentage composition of macro-invertebrates.
Altogether 37 macro-invertebrate taxa were recorded from the Tons river in Doon Valley. A total of 36 taxa were present at S1 and S2. However, all the 37 taxa were present at S3. Philopotamus (member of Trichoptera) was not present at all at S1, while the Potamanthus (member of Ephemeroptera) did not show its presence at S2 (Tables 2–4). A qualitative analysis of the macro-invertebrates dwelling in the Tons river of the Doon Valley has revealed that the order Ephemeroptera was represented by 12 taxa, namely Baetis niger, Baetis muticus, Caenis robusta, Centroptilum luteolum, Cleon spp, Ephemera vulgata, Ephmerella notata, Habrophlebia, Leptophlebia marginata, Potamanthus and Rhithrogena semicolorata. However, the order Plecoptera was represented by only three genera (Perla, Isoperla and Taeniopteryx). The order Hemiptera was represented by two genera (Gerris and Hesperocorixa). Coleoptera was represented by three genera (Hydaticus, Limnius and Psephenus). Trichoptera and Diptera were found to be the second most abundant components among the macro-invertebrates dwelling in the Tons river. Trichoptera was represented by the taxa of Hydroptila, Hydropsyche, Philopotamus and Rhyacophila, while the Diptera was represented by four genera (Tabanus, Culex, Simulium and Chironomus). Odonata of the Tons river was represented by four genera (Agrion, Ceriagrion, Ishnura and Ophiogomphus). Other macro-invertebrates like Mollusca (Bithynia, Campeloma, Pleurocera) and Annelida (Hirudinaria and Tubifex) were represented by three and two taxa, respectively.
The mean monthly Shannon–Wiener index of macro-invertebrates was found to be maximum (3.60 ± 0.02) in the month of February and minimum (2.59 ± 0.11) in the month of July (Table 5). Overall, there was no significant difference in the annual mean (3.30, 3.32, 3.34) of the diversity index at all the three sampling sites (S1, S2 and S3).
4.3 Relationship between hydrological attributes
Pearson’s correlation (r values) calculated to quantify relationships between various hydrological attributes revealed that the air temperature, water temperature, transparency and turbidity were found to be highly inter-correlated (Table 6). Air temperature was highly positively correlated with water temperature (r = 0.95, p > 0.001). Air temperature was negatively correlated with dissolved oxygen (r = −0.90, p > 0.001). Water temperature was strongly negatively correlated with dissolved oxygen (r = −0.99, p > 0.001). Water velocity was found to be positively correlated with hydro median depth (r = 0.85, p > 0.001). Turbidity was found to be positively correlated with water velocity (r = 0.94, p > 0.001). Conductivity was found to be positively correlated with total dissolved solids (r = 0.81, p > 0.001). Turbidity was found to be positively correlated with conductivity (r = 0.80, p < 0.001). Turbidity was also found to be positively correlated with total dissolved solids (r = 0.83, p > 0.001). Transparency was found to be positively correlated with dissolved oxygen (r = 0.61, p < 0.02). Dissolved oxygen was found to be negatively correlated with free carbon dioxide (r = −0.70), p > 0.01). Total dissolved solids were found to be positively correlated with phosphate (r = 0.73, p > 0.01). pH was found to be positively correlated with alkalinity (r = 0.54, p < 0.05). No significant correlation was found between the concentration of potassium and chloride during the study period.
4.4 Relationship between hydrological attributes and the density of macro-invertebrates
Correlation coefficient (r-values) calculated between physico-chemical environmental variables and macro-invertebrates dwelling in the Tons river revealed that the air temperature, water temperature, turbidity, conductivity, dissolved oxygen, nitrate and sodium were found to be significantly correlated with density and diversity of macro-invertebrates dwelling in the Tons river (Table 7). The macro-invertebrate diversity showed a negative relation with air temperature (r = −0.78, p < 0.001), water temperature (r = −0.81, p > 0.001), water velocity (r = −0.85, p > 0.001), hydro median depth (r = −0.74, p > 0.01) and turbidity (r = −0.94, p > 0.001). Macro-invertebrate diversity showed a positive correlation with transparency (r = 0.50, p < 0.05, and dissolved oxygen (r = 0.79, p < 0.001). The macro-invertebrate diversity index was found to have a negative relationship with conductivity (r = −0.79, p > 0.01).
The results of regression analysis performed between the density of macro-invertebrates and the physico-chemical attributes one by one revealed that the air temperature, water temperature, HMD, conductivity, TDS, DO, phosphate and sodium concentrations were found to strongly influence the macro-invertebrate diversity in the Tons river of the Doon Valley, India (Table 8).
5 Discussion
Benthic aquatic insects are sensitive indicators of environmental changes in streams because they express long-term changes in water and habitat quality rather than instantaneous conditions (Johnson et al. 1993). Physicochemical variables, such as water temperature, dissolved oxygen, discharge, nutrients and substrate, influence community structure and function of aquatic insects (Resh and Rosenberg 1984; Ward 1992). Invertebrate communities are also good indicators of water quality conditions (Resh 1995; De Shon 1995). Furthermore, studies of temporal variation in the community structure of streams (Fisher et al. 1982; Scrimgeour and Winterbourn 1989) have indicated that physical disturbances can be important determinants of community structure in lotic systems (Sousa 1984; Resh et al. 1988). The physical disturbances in terms of water flow, temperature and substrates are the major factors determining the composition and abundance of benthic invertebrates (Ward and Stanford 1979).
Every species is restricted to a certain temperature range. Lehmkuhl (1972) studied the influence of water temperature variations on benthic communities. Ward and Stanford (1979) also suggested that temperature pattern influences the life cycle phenomenon of insects such as emergence, which leads to an increase in density. Relationship between the mean density of macro-invertebrates and water temperature was also found to be negatively correlated (r = −0.92, p > 0.001) in the present study on the Tons river (Table 7). Similar results were also found during the study on the river Chandrabhaga (Sharma et al. 2008). Maximum abundance of macro-invertebrates was found during spring season (February–March) in the Tons river, which may be due to increased growth efficiency of insects during this period in addition to hydrological attributes. However, the maximum abundance was also reported by Sharma et al. (2004) in the Dhauliganga in Nanda Devi Biosphere Reserve, India.
The abundance of benthic macro-invertebrates dwelling in the Tons river was found to be increasing from October to March and then gradually decreases from April. The increase during October to March may be due to low turbidity, increased transparency, low hydromedian depth, low water velocity and high dissolved oxygen. The abundance of macro-invertebrates in the Tons river was found to be at the minimum during the monsoon season (July–August). This can be explained by high turbidity, high total dissolved solids, high water velocity and low dissolved oxygen during the monsoon season. Emergence of insects from the Tons river may also be one of the possible reasons for the decrease in abundance during the monsoon season.
Diversity of macro-invertebrates was found to be decreasing from April to June. This may be due to a substantive increase in water temperature. Water temperature was inversely related with the diversity index (r = −0.81, p > 0.001) in the Tons river of the Doon Valley. The same kind of relationship was found by Sharma et al. (2004) in the fluvial system of Dhauliganga. Several studies have also found that insect taxa richness may be influenced within the same stream ecosystem by annual water temperature variations (Stanford and Ward 1982).
The diversity of macro-invertebrates has a negative correlation with water velocity (r = −0.85, p > 0.001) under the present study on the Tons river. Many aquatic populations living in harsh environments of unpredictable flow suffer high mortality directly through physical disturbance (Cushman 1985); Erman and Mahoney (1983) and Kamler (1967) also analyzed the results of the flow regime on macro-invertebrate communities. Hence, the present study confirms that high water velocity during the monsoon season in the Tons river influenced the macro-invertebrate density and diversity due to the unpredictable flow regime.
Dissolved oxygen was found to be positively correlated with diversity indices of macro-invertebrates of the Tons river in Doon Valley (r = 0.79, p < 0.001). Nelson et al. (2000) have also shown that dissolved oxygen has a strong influence on macro-invertebrate community structure. They opined that the higher dissolved oxygen level in open water habitats is necessary for substantive growth of macro-invertebrate populations.
The fluvial ecosystem of the Tons river consisted of bottom substrates ranging from sand to big boulders. Silt and clay were totally absent in the Tons river. The substrate composition was also different at different sampling sites (S1, S2 and S3). The physical substrate types (leaves, gravel, wood and macrophytes) generally support more diversity than structurally simple substrates such as sand and bedrock (Angradi 1996; Hawkins 1984). This can be a good explanation for the high abundance and diversity of macro-invertebrates at sampling site S3, which has high macrophyte growth in the Tons river. However, the river bed is dominated by big boulders and cobbles.
The diversity of macro-invertebrates in the river Tons at different sites was found to be in the order S3 > S2 > S1 along the river gradient. The annual mean macro-invertebrate diversity index was found to be highest (3.34) at S3 and minimum (3.30) at S1. The abundance of macro-invertebrates was also found to be highest at S3 in the present study on the Tons river in Doon Valley. This variation may be due to the variations in substrate combination as well the hydrological attributes prevailing at all the sites. A host of anthropogenic activities of the villagers of Guchchupani at S1 may be the cause for depletion of density and diversity in macro-invertebrates of the Tons river at this sampling site. The river Tons is the only source of water for daily domestic activities like washing utensils and clothes for the people inhabiting the village and thus polluting the water. There are considerable anthropogenic activities (disposal of worship offerings—sacred tree leaves, flowers, fruits, sweets, milk and milk products) by the temple goers into the river at S2, which may be a good reason for the low density of macro-invertebrates. At the sampling site S3, the river side is dominated by macrophytes, thus providing a suitable niche for the macro-invertebrates. Inspite of the differences in substrate composition and other anthropogenic activities, the macro-invertebrate diversity at all the three sites remains fairly high, suggesting that the water quality of the Tons is fairly good and supports diverse and well-balanced macro-invertebrate communities in the Tons river of the Doon Valley. Metcalfe-Smith (1996) also observed that the higher the value of H′ (Shannon–Wiener index) is, the greater the diversity and supposedly the healthier the environment.
References
Angradi TR (1996) Inter-habitat variations in benthic community structure, function and organic matter storage in three Appalachian headwater streams. J N Am Benthol Soc USA 15:42–63. doi:10.2307/1467432
APHA (1998) Standard methods for the examination of water and waste water. American Public Health Association, New York
Badola SP (1979) Ecological studies on the Icthyofauna of some water sources of Garhwal region. Ph.D Thesis, Department of Zoology, H·N.B. Garhwal University, Srinagar, 280 pp
Bailey NTJ (1995) Statistical methods in biology, 3rd edn. Cambridge University Press, Cambridge
Bisht RS, Das SM (1979a) Aquatic entomofauna in relation to coldwater fisheries of Kumaun lakes. Environ India 2:49–50
Bisht RS, Das SM (1979b) Aquatic entomofauna in relation to coldwater fisheries of Kumaun lakes. In: Second All India Seminar on Ichthyology, Nainital, India, pp 37–38
Biswas S, Mukhopadhyay P, Saha SK (1995) Insecta: Coleoptera: Adephoga iv. fam. Gyrinidae, fam Haliplidae, vol 64. Zoological Survey of India, Kolkatta, State Fauna Series 30. Fauna W Bengal, pp 77–220
Clausen B, Biggs BJF (1997) Relationship between benthic biota and hydrobiological indices in New Zealand streams. Freshw Biol 38:327–342. doi:10.1046/j.1365-2427.1997.00230.x
Clausen B, Biggs BJF (1998) Stream flow variability indices for riverine environmental studies. In: Kirby WH (ed) A changing environment I. Wiley, New York, pp 357–364
Clausen B, Biggs BJF (2000) Flow variable for ecological temperate streams: groupings based on covariance. J Hydrol (Amsterdam) 237:184–197. doi:10.1016/S0022-1694(00)00306-1
Collier KJ, Smith BJF (1995) Sticky trapping of adult mayflies, stoneflies and caddisflies alongside three contrasting streams near Hamilton, New Zealand. N Z Nat Sci 22:1–9
Collier KJ, Smith BJ, Baillie BR (1997) Summer light catches of adult chiropteran in hill country catchments of contrasting land use, Walkathon. N Z J Mar Freshw Res 31:623–634
Collier KJ, Fowls C, Hogs I (2000) Management, education and conservation. In: Collier KJ, Winterbourne MJ (eds) New Zealand stream invertebrates: ecology and implications for management. The Limnological Society, Christchurch, New Zealand, pp 230–250
Cummins KW (1974) Structure and function of stream ecosystems. Bioscience 24:631–641. doi:10.2307/1296676
Cummins KW, Merritt RW, Burton TM (1984) The role of aquatic insects in the processing and cycling of nutrients. In: Resh VH, Rosenberg DM (eds) The ecology of aquatic insects. Praeger, New York, pp 134–163
Cushman RM (1985) Review of ecological effects of rapidly varying flows downstream from hydroelectric facilities. N Am J Fish Manage 5:330–339. doi :10.1577/1548-8659(1985)5<330:ROEEOR>2.0.CO;2
Das SM (1971) Teaching and research in limnology in India. In: Proceedings of the symposium UGC NCSE, Bangalore, pp 67–72
De Shon JE (1995) Development and application of the invertebrate community index (ICI). In: Davis WS, Simon TP (eds) Biological assessment and criteria. Lewis Publishers, England, pp 217–243
Disney RH (1975) A key to British dixidae. Scientific Publication No. 31. Freshwater Biological Association, The Ferry House, Ambleside, Cumbria, pp 1–78
Edington JM, Hilldrew AG (1995) Caseless caddis larvae of the British Isles: a key with ecological notes. Scientific Publication No. 53. Freshwater Biological Association, The Ferry House, Ambleside, Cumbria, UK, pp 1–134
Elliott JM, Humpesch UH (1983) A key to the adults of the British ephemeroptera. Freshwater Biological Association, Ambleside, Cumbria, UK, pp 1–101
Elliott JM, Humpesch UH, Macan TT (1988) Larvae of the British ephemeroptera: a key with ecological notes. Scientific Publication No. 49. Freshwater Biological Association, The Ferry House, Ambleside, Cumbria, pp 1–145
Erman DC, Mahoney SD (1983) Recovery after logging in streams with and without buffer stripes. California Water Research Center, University of California, Northern California, p 50
Fisher SG, Gray LJ, Grimm NB, Busch DE (1982) Temporal succession in a desert stream ecosystem following flash flooding. Ecol Monogr 52:93–110. doi:10.2307/2937346
Fraser FC (1933) The fauna of British India, including Ceylon and Burma, Odonata, vol I. Taylor and Fancis, London
Hawkins CP (1984) Substrate association and longitudinal distribution in species of ephemerellidae (ephemeroptera: Insecta) from Western Oregon. Freshw Invertebr Biol 3(94):18–88
Hickel B (1977) Limnological investigation in the lakes of the Pokhra Valley, Nepal. Int Rev Gesamten Hydrobiol 58:659–672
Hynes HBN (1977) Adults and nymphs of British stoneflies (Plecoptera): a key. Freshwater Biological Association, Ambleside, Cumbria, pp 1–101
Jacobson D, Rostgaard S, Vasconez JJ (2003) Are macroinvertebrates in high altitude streams affected by oxygen deficiency? Freshw Biol 48(11):2025–2032. doi:10.1046/j.1365-2427.2003.01140.x
Johnson RK, Wiederholm T, Rosenberg DM (1993) Freshwater biomonitoring using individual organisms, populations, and species assemblages of benthic macro-invertebrates. In: Rosenberg DM, Resh VH (eds) Freshwater biomonitoring and benthic macro-invertebrate. Chapman & Hall, New York, pp 40–158
Kamler E (1967) Distribution of plecoptera and ephemeroptera in relation to altitude above sea level and current speed in mountain waters. Pol Arch Hydrobiol 14(27):29–42
Kumar A, Khanna V (1984) A review of the taxonomy and ecology of Odonata larvae from India. Orient Ins 17:127–157
Lehmkuhl DM (1972) Change in thermal regime as a cause of reduction of benthic fauna downstream of a resources. J Fish Res Board Can 29:1322–1329
Macan TT (1974) Freshwater ecology. Longman Group Ltd., London
Metcalfe-Smith JL (1996) Biological water-quality assessment of rivers: use of macro-invertebrate communities. In: Petts G, Calow P (eds) River restoration. Blackwell Science, Oxford, pp 17–43
Mitra A (1999) Studies on the behavioural pattern and seasonal ecology of some species of dragon flies (odonata: Insecta) in Asan reservoir (Dehradun). D. Phil. Thesis, H·N.B. Garhwal University, Srinagar (Garhwal)
Mitra RT (2003) Ecology and biogeography of Odonata with special reference to Indian fauna, vol 202. Zoological Survey of India, Kolkatta, pp 1–41
Mitra PS, Nandi NC (1998) Impact of anthropogenic activities on Hoogli estuary with special reference to the distribution pattern of intertidal macrozoobenthos. In: Adityya AK, Haldar P (eds) Proceedings of the national seminar on environmental biology, Daya Publishing House, New Delhi, pp 94–101
Needham JG, Needham PR (1962) A guide to the study of freshwater biology, 5th edn. Holden Inc., San Francisco, USA
Nelson MS, Roline RA, Thullen JS, Sartoris JJ, Boutwell JE (2000) Invertebrate assemblage and trace element bioaccumulation associated with constructed wetlands. Wetlands 20:406–415. doi:10.1672/0277-5212(2000)020[0406:IAATEB]2.0.CO;2
Quinn JM, Cpper AB, Davies-Colley RJ, Rutherford JC, Williamson RB (1997) Land-use effects on habitat, water quality, periphyton and benthic invertebrates in Waikato, New Zealand, Hill Country Streams. N Z J Mar Freshw Res 31:579–598
Ramkrishna C, Mitra SC (2004) Pictoral handbook-Indian land snails (selected species). Zoological Survey of India, Kolkatta, pp 1–344
Resh VH, Rosenberg DM (1984) The ecology of aquatic insects. Praeger, New York
Resh VH (1995) Freshwater benthic macro-invertebrates and rapid assessment procedures for water quality monitoring in developing and newly induistrialized countries. In: Davis WS, Simon TP (eds) Biological assessment and criteria. Lewis, Chelsea, pp 167–177
Resh VH, Brown AV, Covich AP, Gurtz ME, Li HW, Minshall GW et al (1988) The role of disturbance in stream ecology. J N Am Benthol Soc 7:433–455. doi:10.2307/1467300
Scrimgeour GJ, Winterbourn MJ (1989) Effects of floods on epilithon and benthic macro-invertebrate populations in an unstable New Zealand river. Hydrobiologia 171:33–44. doi:10.1007/BF00005722
Shannon CE, Wiener W (1964) The mathematical theory of communication. University of Illinois Press, Urbana, USA
Sharma RC (1986) Effects of physicochemical factors on benthic fauna of Bhagirathi River, Garhwal Himalaya. Indian J Ecol 13:133–137
Sharma RC, Bhanot G, Singh D (2004) Aquatic macro-invertebrate diversity in Nanda Devi Biosphere Reserve, India. Environmentalist 24:211–221. doi:10.1007/s10669-005-0996-z
Sharma RC, Sharma A, Anthawal A (2008) Surveying of aquatic insect diversity of Chandragbhaga river, Garhwal Himalayas. Environmentalist. doi:10.1007/s10669-007-9155-z
Singh D, Sharma RC (1998) Biodiversity, ecological status and conservation priority of fish of the river Alaknanda, a parent stream of the river Ganges (India). Aquat Conserv: Mar Freshwat Ecosyst 8:761–772. doi :10.1002/(SICI)1099-0755(1998110)8:6<761::AID-AQC311>3.0.CO;2-3
Sousa WP (1984) The role of disturbance in natural communities. Annu Rev Ecol Syst 15:353–391. doi:10.1146/annurev.es.15.110184.002033
Stanford JA, Ward JV (1982) Insect species diversity as a function of environmental variability and disturbances in stream system. In: Barnes JR, Minshal W (eds) Stream ecology: application and testing of general ecological theory. Plenum, New York, pp 265–278
Wallace ID, Wallace B, Philipson GM (1990) A key to the case-bearing caddis larvae of British and Ireland. Freshwater Biological Association, Ambleside, Cumbria, UK, p 237
Walsh CJ, Sharp AK, Breen PF, Sonnerman JA (2001) Effects of urbanization on streams of the Melbourn region, Victoria, Australia. I. Benthic macroinvertebrate communities. Freshw Biol 46:535–551. doi:10.1046/j.1365-2427.2001.00690.x
Ward JV, Stanford JA (1979) Ecological factors controlling stream zoobenthos with emphasis on thermal modification of regulated streams. In: Ward JV, Stanford JA (eds) Ecology of regulated streams. Plenum, New York, pp 35–53
Ward JV, Tockner K (2001) Biodiversity: towards a unifying theme for river ecology. Freshw Biol 46(6):807–819. doi:10.1046/j.1365-2427.2001.00713.x
Ward HB, Whipple GC (1992) Freshwater biology, 2nd edn. Wiley, New York, pp 1–1248
Ward JV (1992) Aquatic insect ecology. 1. Biology and habitat. Wiley, New York
Wetzel RG, Likens GE (1991) Limnological analyses. Springer, New York, pp 1–175
Acknowledgement
The help in terms of computation of data extended by Dr. Deepak Singh is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, R.C., Arambam, R. & Sharma, R. Surveying macro-invertebrate diversity in the Tons river, Doon Valley, India. Environmentalist 29, 241–254 (2009). https://doi.org/10.1007/s10669-008-9187-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10669-008-9187-z