Abstract
Low-cost activated carbons were prepared from poplar wood by chemical activation with KOH as a chemical activating agent. The thermal behavior of KOH-impregnated poplar wood was analyzed in detail via the Thermogravimetric analysis (TG, DTG and DSC). Moreover, the effects of impregnation ratio, activation temperature and activation time on the porous structure were investigated. Results showed that KOH has catalytic effect which could promote the pyrolysis of poplar wood at lower activation temperature. The activated carbons from poplar wood with KOH activation could be formed at 550–700 °C. The specific surface area and pore volume of activated carbon increased with increasing the impregnation ratio, activation temperature and activation time. Moreover, influence of increasing impregnation ratio on the porosity of activated carbon is sensitive to activation time. The maximum specific surface area (1551 m2/g) activated carbon with uniform micropores distribution was obtained at activation temperature of 700 °C, impregnation ratio of 3/1 and activation time of 30 min. Carbon skeleton could break seriously when activation temperature is higher than 750 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Activated carbons are particularly useful due to their highly developed porous texture and large adsorption capacity and they are widely used as absorbents in various fields, such as separation and purification of pollutants in both liquid and gas phases, catalyst support, methane and hydrogen storages and electrodes [1–11]. All of these varied applications are associated with their extraordinary porosity in terms of surface area, porous texture and pore size distribution [12]. The porous texture and chemical properties of activated carbons are strongly affected by the nature of raw materials and the activation process [13, 14]. In principle, the preparation methods for activated carbons can be classified into physical activation and chemical activation. During the physical activation process, raw material is first carbonized and then the carbonized material is activated by CO2 or steam. In the chemical activation, raw material is impregnated with a chemical agent, and the impregnated material is heated in an inert atmosphere. The porous structure of activated carbon is developed with the help of chemical agent due to its dehydration and degradation properties. Compared to physical activation, chemical activation presents several advantages, such as lower temperature for pyrolysis, much higher carbon yield, higher surface area and better-developed porosity [15, 16].
Lignocellulosic materials, such as forest products (hardwood and softwood), are currently the most appropriate candidates for cost effective preparation of activated carbons because they are renewable resource, cheap and easy to obtain commercially. Thus, there are many studies related to the preparation of activated carbons from lignocellulosic materials [17–22]. Díaz-Terán et al. [22] prepared activated carbon from esparto-grass with KOH, and obtained the high BET surface area of 1960 m2/g. They found that the porosity development was directly affected by the formation of K-compounds during the activation process. El-Hendawy et al. [19] reported that KOH ratio was the basic indicator of microporosity development in the chemical activation process of maize stalks. The formation of porous texture was inferred to due to the reactions between char, K2O, H2 and CO2, and the intercalation of K2CO3 and metallic potassium. Gao et al. [20] prepared activated carbons with high BET surface area of 2943 m2/g from papermaking black water lignin with KOH and they also found that the lignin/KOH ratio was the main factor affecting the BET surface area while the activation temperature and activation time were the subordinate factors. Therefore, KOH has been considered an effective chemical agent due to its excellence in producing high quality activated carbon with larger specific surface area and well developed micropores from lignocellulosic materials.
Poplar wood, which has been identified as a short rotation woody crop, is an ideal lignocellulosic material for preparing porous activated carbon due to its low price, easy renewal, widespread geographic distributions and high biomass yield [21, 23]. However, few studies [21] are available on the preparation of activated carbon from poplar wood. Moreover, the thermal behavior of KOH-impregnated wood during pyrolysis is seldom investigated, which is important when slow pyrolysis is applied for produce of activated carbon. In an effort to contribute to addressing this deficit, activated carbons were prepared from poplar wood by chemical activation with KOH in the present study. The influence of different preparation conditions including impregnation ratio, activation temperature and activation time on the porosity development of activated carbons were investigated in a TGA/SDT600 apparatus, in order to better understand the activation process of poplar wood.
2 Experimental method
Poplar wood used as a raw material was obtained from Tianjin, China. The elemental analysis and main constituents (DSC 204F1NETZSCH, Germany) of the poplar woods are listed in Tables 1 and 2, respectively. The raw materials were prepared with the diameter of 4 mm and height of 4 mm, and then naturally dried to use. The raw polar wood samples were impregnated with aqueous solutions of KOH (30 or 50 wt%) for a couple days at 60 °C, and then they were dried in an oven at 105 °C for 12 h, to obtain the experimental mixture at different ratios (1:1 or 3:1) by weight (KOH/poplar wood). The mixture was carbonized in a TGA/SDT600 apparatus (TA Instruments, U.S.) by using a heating rate of 10 °C/min up to the final activation temperature in an N2 flow (100 ml/min). Meanwhile, thermogravimetric analysis (TGA), derivative of TG (DTG) and differential scanning calorimetry (DSC) of the activation process were conducted. The final activation temperature was 550 or 700 °C. Samples were kept at the final temperature for different activation time period of 0 or 30 min before cooling down under N2 flow. The activated carbon samples under different conditions were designated as shown in Table 3. After activation process, the samples were washed with distilled water until the pH of solution reached about 7 to remove the chemicals left in the carbonized samples. After drying, several resultant activated carbon products under the same experimental conditions were collected in a whole for microstructure measurement. The pore structures of the activated samples were characterized by measuring N2 adsorption/desorption isotherms at 77 K (Belsorp-max, MicrotracBEL Corp., Japan). The specific surface area (S BET ) and total pore volume (V P ) of the activated samples were determined using the Brunauer–Emmett–Teller (BET) method and the Dubinin–Radushkevich (D–R) equation, respectively. The micropore and mesopore size distributions were calculated from N2 adsorption isotherm by the Micropore Analysis (MP) method and Berret–Joyner–Halenda (BJH) method, respectively. Carbon yield is defined as the weight ratio of activated carbon (the obtained material after washing and drying of the carbonized product) to the dried raw material [11].
3 Results and discussion
3.1 Thermal behavior of raw material (poplar wood) and its KOH-impregnated sample
Figure 1a, b, c shows the TG, DTG and DSC curves of raw poplar wood and its KOH-impregnated samples. For raw poplar wood, the weight loss from the TG curve can be divided into three stages. The first stage occurred at room temperature −220 °C, and slight weight loss (about 1 %) was observed. This weight loss could be ascribed to the elimination of water (such as free water and bound water) and light volatile materials [19, 24]. The second stage happened at 220–450 °C, where a rapid weight loss (around 70 %) occurred. Meanwhile, a single maxima happening at 380 °C between 220 and 450 °C was observed as shown in DTG curve in Fig. 1b. This indicates that the main decomposition process for the raw poplar wood should occur in this temperature region. The weight loss in second stage can be attributed to the decomposition of lignin, hemicelluloses and cellulose, accompanied by the evolution of tar and gas products (such as CO, CO2 and CH4) [25–27]. The third stage between 450 and 700 °C, and weight loss is about 5 % which can be owned to the recombination of structure and formation of fundamental carbon skeleton [20]. Besides this, the DSC curve in Fig. 1c shows that the whole pyrolysis process of raw poplar wood is almost endothermic. The endothermic heat flow decreased sharply with increasing pyrolysis temperature at 30–160 °C. The endothermic heat flow at this temperature range can be attributed to the water evaporation. The remaining water decreased with increasing pyrolysis temperature, and hence the endothermic heat flow of water evaporation decreased. Moreover, a sharp endothermic peak at 380 °C was observed, which is corresponding to the maximum rate of weight loss occurred as shown in the DTG curve.
In contrast to raw poplar wood, KOH-impregnated samples show the significant difference in the pyrolysis characteristics. For the KOH-impregnated samples with impregnation ratio of 1/1 and 3/1, the first weight loss stage happened at room temperature −130 °C, which are earlier than the raw poplar wood (room temperature −220 °C). The weight loss may be attributed to the evolution of water, which is similar to the raw poplar wood. Then, the second weight loss occurred between 130 and 510 °C, and the weight loss, for example, was around 30 % for KOH-impregnated samples (1/1), which is much less than that of the raw poplar wood. As shown in DTG curve in Fig. 1b, the maximum rate of weight loss of KOH-impregnated sample (1/1) happened at 230 °C, while the maximum rate of weight loss of the raw poplar wood occurred at 380 °C. This indicates that KOH has catalytic effect which could promote the decomposition of poplar wood at lower heat treatment temperature. Moreover, the peak rate of weight loss of KOH-impregnated sample (1/1) is much less than that of the raw poplar wood, which is in agreement with the trend of weight loss change shown in the TG curve. This can be explained by the fact that KOH impregnation would enhance the formation of cross-linked polymer via the reaction between poplar wood and the K-compounds, and suppress the tar evolution [19, 22, 28]. Consequently, the weight loss of KOH-impregnated sample was lower than that of the raw poplar wood. Moreover, this weight loss would further decrease with increasing impregnation ratio as shown in the TG curve in Fig. 1a, indicating that the reaction between poplar wood and the K-compounds would be promoted with increasing impregnation ratio, and fewer tar was formed. Consistent with this, the rate of weight loss decreased with increasing impregnation ratio as shown in the DTG curve in Fig. 1b. The third weight loss of KOH-impregnated samples occurred at 510–700 °C, and slight weight losses were observed, which can be ascribed to the decomposition of K2CO3 [29].
Different with the raw poplar wood, the endothermic heat flow of KOH-impregnated (1/1 and 3/1) slightly increased with increasing pyrolysis temperature at 30–160 °C. This fact can be explained that the endothermic heat flow was used to prepare for the KOH melting, and then the endothermic heat flow increased. It is interesting to note that the exothermic peaks around 230 °C were observed for KOH-impregnated samples. Moreover, the exothermic peak decreased with increasing impregnation ratio. The exothermal peak can be attributed to the charring process of hemicelluloses [27, 30]. As discussed above, the catalytic effect of KOH could promote the decomposition of poplar wood at lower heat treatment temperature. Thus, it may be explained that the hemicelluloses started into charring process earlier for KOH-impregnated samples and reached peak heat release at around 230 °C while it is just the entrance into second weight loss stage for the raw poplar wood. Besides this, increasing impregnation ratio would enhance the reaction between poplar wood and the K-compounds, thus the charring process of hemicelluloses would be suppressed, and consequently, the exothermic peak was decreased.
According to the discussion above, there were some important temperature points during the activation process of KOH-impregnated samples, for example, the peak rate of weight loss occurred at around 230 °C (as well as 310 °C), and main weight loss ended at about 510 °C. The carbonized products with impregnation ratio of 1/1 at these temperature points (240, 310, and 450 °C) were wet. The carbonized products at 240, 310, and 450 °C were partly soluble in water, respectively. Furthermore, the carbonized products at 240 and 310 °C had better solubility in water than that at 450 °C. The solutions of carbonized products with impregnation ratio of 1/1 at 240, 310 and 450 °C are shown in Fig. 2. The colors of solutions of carbonized products at 240 and 310 °C seemed much blacker than that at 450 °C. Moreover, the Tyndall effect was observed for each solution of carbonized product at 240, 310 and 450 °C, respectively, indicating that the solutions of these carbonized products were colloids. Due to organic acid and aromatics produced during pyrolysis process were soluble in water, it can be deduced that the carbon matrix in poplar wood was transformed into the small molecule carbon sources, which suspended in water. It should be noted that the carbonized products with impregnation ratio of 1/1 at 550 and 700 °C were black solid and not soluble in water. Hence, it can be inferred that the activated carbon from poplar wood with KOH activation could be formed when activation temperature is more than 550 °C (such as at 550 or 700 °C), whereas the physical properties of intermediate products before reaching this temperature threshold (e.g. 550 °C) should be considered carefully for smooth operation in practical batch processing without a high heating rate.
Additionally, the thermal behavior of KOH-impregnated sample above 700 °C was also investigated. Figure 3 shows the thermal analysis data (TG, DTG and DSC) of KOH-impregnated sample at impregnation ratio of 1/1 till finish temperature of 1000 °C. It shows that a rapid weight loss was occurred from 750 to 950 °C and the remaining weight (wt%) was less than 10 %. Moreover, the maximum rate of weight loss happened at around 900 °C. At the same time, the endothermic peak was observed. This can be ascribed to the effect of burn-off at high temperature, i.e. breaking the carbon skeleton seriously. This indicates that the activation temperature during the chemical activation process of poplar wood should not exceed 750 °C.
From these results, it was summarized that the activated carbons from poplar wood with KOH activation could be formed at 550–700 °C. To explore the microstructure development of activated carbons from KOH-impregnated poplar wood at 550 and 700 °C, the pore structure of these samples are comprehensively examined in the next section.
3.2 Pore structure of the prepared activated carbons
3.2.1 N2 adsorption isotherms at 77 K of the obtained activated carbons
Figure 4 shows the N2 adsorption–desorption isotherms of the activated carbons prepared by KOH at different impregnation ratios, activation temperatures and times. It can be seen that all the isotherms of activated carbons belong to type I isotherm [31], in which the knees of the isotherms at lower relative pressure (less than 0.05) are very sharp and the plateaus are fairly flat. This indicates that all the activated samples had well-developed micropores. As presented in Fig. 4a, the sample KY1-550 shows a small N2 adsorption capability, about one-third of the sample KY1-700, indicating the porosity of activated carbon increases significantly as activation temperature increases from 550 to 700 °C. The N2 adsorption capability of the sample KY3-550 is nearly the same as that of the sample KY1-550; the N2 adsorption capability of the sample KY3-700 is slightly larger than that of the sample KY1-700. This suggests that increasing impregnation from 1/1 to 3/1 has slight influence on the porosity of activated carbon at activation temperature of 550 °C (as well as 700 °C) with zero activation time. On the other hand, the isotherm of the sample KY1-700 exhibits a very narrow hysteresis loop as the relative pressure increases to 0.3, indicating that a small amount of mesopores were developed.
To investigate the effect of activation time on the pore characteristics of activated carbons, the properties of KOH-activated samples with zero activation time was compared with that of 30 min under the same other conditions. Generally, the N2 adsorption capabilities of activated samples increase steadily with prolonging activation time as shown in Fig. 4a, b. This indicates that prolonging activation time could promote the porosity of activated sample. Besides this, increasing activation temperature promotes the N2 adsorption capabilities of activated carbons are also observed in Fig. 4b, which is similar to those shown in Fig. 4a. From Fig. 4b, it is interesting to note that the N2 adsorption capability of the sample KY3-550-30 increase sharply as compared with the sample KY1-550-30; the N2 absorption capability of the sample KY3-700-30 is much larger than that of the sample KY1-700-30. This suggests that the porosity of activated carbon increases significantly with increasing impregnation ratio at activation time of 30 min. In contrast, there is slight effect on the porosity of activated carbon with increasing impregnation ratio at zero activation time, as mentioned above. This phenomenon demonstrated that the influence of increasing impregnation ratio on the porosity of activated carbon is sensitive to activation time, and this would be useful for preparing activated carbons.
In addition, it is interestingly found that the adsorption capability of the sample KY3-700 is similar to that of KY3-550-30, but there is a larger difference in adsorption between the samples at an impregnation ratio of 1/1 (KY1-700 vs. KY1-550-30). Therefore, advancing the activation through a heating process from 550 to 700 °C (taking 15 min) or maintaining 30 min at 550 °C results in different adsorption characteristics dependent on impregnation ratio.
3.2.2 The micropore and mesopore size distributions of the obtained activated carbons
Figures 5 and 6 show the micropore and mesopore size distributions of the activated carbons prepared by KOH at different impregnation ratios, activation temperatures and activation times, respectively. It can be seen that the micropores are dominant for all the activated carbons and the pore sizes around 0.6 nm, whereas the mesopores can be considered negligible. As shown in Fig. 5, the samples KY1-550 and KY3-550 have similar amount of micropores, and the similar trend is also observed for KY1-700 and KY3-700. This indicates that increasing impregnation ratio at zero activation time has slight effect on the development of micropores. In contrast, as shown in Fig. 5b, the sample KY3-550-30 is found to have much more better-developed micropores as compared with the sample KY1-550-30; the sample KY3-700-30 shows a larger amount of micropores than the sample KY1-700-30, indicating the micropores were much more developed with increasing the impregnation ratio at activation time of 30 min. This trend is in good accordance with the results of the N2 adsorption isotherms shown in Fig. 4. Therefore, the effect of increasing impregnation ratio on the development of micropores of activated carbon is sensitive to the activation time. On the other hand, the amount of micropores increases steadily with increasing activation temperature as shown in Fig. 5a, b, indicating increasing the activation temperature from 550 to 700 °C could enhance the development of micropores.
3.2.3 The BET surface areas and pore volumes of the obtained activated carbons
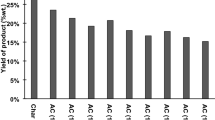
The specific surface areas (S BET ), total pore volumes (V P ), average micropore diameter and carbon yields of the activated carbons were calculated according to the adsorption isotherms and given in Table 4. It can be seen that the S BET and V P increase gradually with increasing activation temperature, time and impregnation ratio, indicating the well-developed porosity of activated carbons at higher values of these parameters. The mechanism of KOH activation can be generally considered as a reaction of KOH with carbon: 6KOH + 2C → 2 K + 2K2CO3 + 3H2 [5, 14, 32, 33]. During the activation process, the development of porosity and specific surface area can be attributed to formations of K2CO3 and metallic potassium, which produce the new pores and widening the existence pores in the carbon matrix [4, 18, 20, 34]. Activation temperature is an important factor that affects the chemical reaction and species formation during the KOH-activation process. For a given activation time and impregnation ratio, for example, when the activation temperature increases from 550 to 700 °C at impregnation ratio of 3/1 with activation time of 30 min, the S BET and V P increase by about 42 % (from 1092.43 to 1551.68 m2/g) and 45 % (from 0.3575 to 0.5186 cm3/g), respectively. These increases can be ascribed to the fact that, the K2CO3 would be formed at activation temperature of 550 °C, which would induce the pores in the raw materials [22, 35]. As the activation temperature rose up to 700 °C, metallic potassium would be produced and transported in the carbon matrix, which could lead to further development of pores [20, 35]. Moreover, K2O would be formed due to the reaction of KOH with carbon and it could take part in reaction with carbon [20, 35], which also could contribute to the further development of pores. In the work of Ehrburger et al. [35], activated carbon was prepared from coal by KOH chemical activation, and the activation temperature was up to 927 °C. They found that the K2CO3 was primarily formed after 327 °C and its formation increased with the temperature up to 597 °C and then decreased. Furthermore, the K2O formation was observed in the temperature range of 647–927 °C. Their findings can be reasonably employed in present study to explain the pore formation mechanism of activated carbon. Prolonging activation time would provide a sufficient space for the development process of pores. For a given activation temperature and impregnation ratio, for example, when the activation time prolongs from 0 to 30 min at activation temperature of 700 °C with impregnation ratio of 3/1, the S BET and V P increase by about 52 % (from 1019.08 to 1551.68 m2/g) and 57 % (from 0.3310 to 0.5186 cm3/g), respectively. Impregnation ratio plays a crucial parameter in KOH-activation process, which affects the pore formation mechanism and pore widening mechanism [4, 15, 29]. For a given activation temperature and time, for example, when the impregnation ratio increases from 1/1 to 3/1 at activation temperature of 700 °C with activation time of 30 min, the S BET and V P increase by about 31 % (from 1183.27 to 1551.68 m2/g) and 34 % (from 0.3874 to 0.5186 cm3/g), respectively. This increases can be attributed to the more new pores were produced and the existence pores were widened with increasing KOH amount, and consequently the specific surface area and pore volume were increased.
It is interesting to note that, when the impregnation ratio increases from 1/1 to 3/1 at activation temperature of 550 °C with zero activation time, the S BET and V P increase slightly by about 6 % (from 248.48 to 262.69 m2/g) and 11 % (from 0.1054 to 0.1174 cm3/g), respectively. In contrast, the S BET and V P increase significantly by about 196 % (from 369.01 to 1092.43 m2/g) and 145 % (from 0.1460 to 0.3575 cm3/g), respectively, as the impregnation ratio increases from 1/1 to 3/1 at activation temperature of 550 °C with activation time of 30 min. The similar trend was found for the activation temperature of 700 °C, where the S BET and V P of the sample KY3-700-30 are much larger than those of the sample KY1-700-30, whereas the S BET and V P of the sample KY3-700 is similar to those of KY1-700. This indicates that the increasing impregnation ratio has significant effect on the specific surface area and pore volume of the activated carbon at activation time of 30 min, whereas it has slight effect at zero activation time. This phenomenon is in good agreement with the results of the N2 adsorption isotherms and pore size distributions as shown in Figs. 4 and Fig. 5. This can be explained that, K2CO3 was formed in a short time during the activation process of the sample KY1-550. Though increasing the impregnation ratio to 3/1, less amount of K2CO3 were formed due to the non-sufficient residence time (zero activation time), and therefore the porosity was not well-developed for the sample KY3-550. In contrast, for the sample KY1-550-30, as the impregnation ratio increases to 3/1, much more K2CO3 would be produced due to the higher impregnation ratio and longer residence time, then resulting in a well-developed porosity for the sample KY3-550-30. Moreover, the S BET of KY3-700 (1019.08 m2/g) is similar to that of KY3-550-30 (1092.43 m2/g). In this study, the heating rate is 10 °C/min, thus it would take 15 min to increase temperature from 550 to 700 °C. Hence, the preparation of activated sample KY3-700 would save 15 min compared with activated sample KY3-550-30. Furthermore, the average micropore diameter of KY3-700 is smaller than that of KY3-550-30, while the carbon yield (18.1 %) is about 1.2 times larger than that of KY3-550-30 (8.3 %). This indicates that an activated carbon with well-developed porosity and high carbon yield could be obtained at zero activation time.
In addition, it should be noted that, though increasing impregnation ratio (an increase of K-compounds) and prolonging activation time are beneficial to the development of porosity, it is not reasonable to increase impregnation ratio and activation time immoderately. The much higher impregnation ratio would lead to merger and erosion of the existing pores by breaking the pore wall during the activation process, and then decreases the specific surface area [4, 15, 29]. And the non-sensible longer activation time would pay an expensive cost for energy consumption. Therefore, suitable impregnation ratio and activation time should be chosen for the KOH-activation process in the practical application. In present study, the maximum S BET (1551.68 m2/g) and V P (0.5186 cm3/g) were obtained at activation temperature of 700 °C with impregnation ratio of 3/1 and activation time of 30 min. On the other hand, the carbon yield decreases significantly with increasing impregnation ratio and activation time. During the activation process, KOH would be reacted with the char and volatile matter from raw material (poplar wood). As the impregnation ratio increases, the KOH would be further reacted with the surface carbon atoms of the raw material [14, 22], which could result in an increase in the weight loss (after washing treatment) and hence a low carbon yield. Moreover, the reaction of KOH-char or KOH-carbon atoms would be progressed with prolonging the activation time, and then reduces the carbon yield.
4 Conclusions
Poplar wood is a good raw material for the preparation of activated carbons. The activated carbons from poplar wood with KOH activation could be formed at 550–700 °C. Moreover, the specific surface area and pore volume increased with increasing impregnation ratio, activation temperature and activation time. The largest specific surface area (1551 m2/g) and highest pore volume (0.5 cm3/g) activated carbon was obtained at activation temperature of 700 °C, impregnation ratio of 3/1 and activation time of 30 min. Furthermore, the porosity of activated carbons increase significantly with increasing impregnation ratio at activation time of 30 min, and that effect is dependent on activation time. By balancing activation temperature and time at impregnation ratio of 3/1, the activated carbon with smaller average micropore diameter and higher carbon yield may be obtained without loss of adsorption capability. These findings would be useful for preparing the low-cost activated carbons from poplar wood in the industrial application.
References
X. Xin, K. Ito, Y. Kubo, Carbon 99, 167–173 (2016)
G. Sethia, A. Sayari, Carbon 99, 289–294 (2016)
M.J. Prauchner, F. Rodríguez-Reinoso, Microporous Mesoporous Mater. 109, 581–584 (2008)
D. Lozano-Castelló, M.A. Lillo-Ródenas, D. Cazorla-Amorós, A. Linares-Solano, Carbon 39, 741–749 (2001)
T. Otowa, Y. Nojima, T. Miyazaki, Carbon 35, 1315–1319 (1997)
Z.Y. Li, K.W. Wang, J.T. Song, Q. Xu, N. Kobayashi, J. Mater. Cycles Waste Manag. 16, 359–366 (2013)
K.Y. Foo, B.H. Hameed, Bioresour. Technol. 102, 9814–9817 (2011)
T. Maneerung, J. Liew, Y. Dai, S. Kawi, C. Chong, C.-H. Wang, Bioresour. Technol. 200, 350–359 (2015)
S. Yang, H. Hu, G. Chen, Carbon 40, 277–284 (2002)
T. Tsubota, M. Morita, S. Kamimura, T. Ohno, J. Porous Mater. 23, 349–355 (2016)
G. Gentscheva, P. Vassileva, P. Tzvetkova, L. Lakov, O. Peshev, E. Ivanova, J. Porous Mater. 15, 331–334 (2008)
M. Kubota, A. Hata, H. Matsuda, Carbon 47, 2805–2811 (2009)
A.C. Lua, F.Y. Lau, J. Guo, J. Anal. Appl. Pyrolysis 76, 96–102 (2006)
M.A. Lillo-Ródenas, D. Cazorla-Amorós, A. Linares-Solano, Carbon 41, 267–275 (2003)
J.A. Maciá-Agulló, B.C. Moore, D. Cazorla-Amorós, A. Linares-Solano, Carbon 42, 1367–1370 (2004)
M.S. Contreras, C.A. Páez, L. Zubizarreta, A. Léonard, S. Blacher, C.G. Olivera-Fuentes, Carbon 48, 3157–3168 (2010)
S. Yorgun, D. Yıldız, J. Taiwan Inst. Chem. Eng. 53, 122–131 (2015)
J. Díaz-Terán, Colloids Surf. A Physicochem. Eng. Asp 187–188, 167–175 (2001)
A.N.A. El-Hendawy, Appl. Surf. Sci. 255, 3723–3730 (2009)
Y. Gao, Q. Yue, B. Gao, Y. Sun, W. Wang, Q. Li, Chem. Eng. J. 217, 345–353 (2013)
H. Demiral, I. Uzun, Surf. Interface Anal. 42, 1338–1341 (2010)
J. Díaz-Terán, D.M. Nevskaia, J.L.G. Fierro, A.J. López-Peinado, A. Jerez, Microporous Mesoporous Mater. 60, 173–181 (2003)
P. Sannigrahi, A.J. Ragauskas, G.A. Tuskan, Biofuels Bioprod. Biorefining 4, 209–226 (2010)
G. Hasegawa, K. Kanamori, K. Nakanishi, T. Hanada, Carbon 48, 1757–1766 (2010)
M. Zhang, F.L.P. Resende, A. Moutsoglou, D.E. Raynie, J. Anal. Appl. Pyrolysis 98, 65–71 (2012)
J.C. Domínguez, M. Oliet, M.V. Alonso, M.A. Gilarranz, F. Rodríguez, Ind. Crops Prod. 27, 150–156 (2008)
H. Yang, R. Yan, H. Chen, D.H. Lee, C. Zheng, Fuel 86, 1781–1788 (2007)
G.H. Oh, C.H. Yun, C.R. Park, Carbon Sci. 4, 1–5 (2003)
D. Lozano-Castelló, J.M. Calo, D. Cazorla-Amorós, A. Linares-Solano, Carbon 45, 2529–2536 (2007)
R. Ball, A.C. Mcintosh, J. Brindley, Combust. Theory Model. 8, 281–291 (2004)
S.J. Gregg, K.S.W. Sing, Adsorption, Surface Area and Porosity, 2nd edn. (Academic Press, New York, 1982)
M.A. Lillo-Ródenas, J. Juan-Juan, D. Cazorla-Amorós, A. Linares-Solano, Carbon 42, 1371–1375 (2004)
Y. Lv, F. Zhang, Y. Dou, Y. Zhai, J. Wang, H. Liu, J. Mater. Chem. 22, 93–99 (2012)
Z. Hu, E.F. Vansant, Microporous Mater. 3, 603–612 (1995)
P. Ehrburger, A. Addoun, F. Addoun, J. Donnet, Fuel 65, 1447–1449 (1986)
Acknowledgments
The research is supported by International Joint Research and Development Project of Tianjin Talent Introduction and Science and Technology Cooperation Plan (14RCGFGX00850).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Gao, X., Wu, L. et al. Preparation of activated carbons from poplar wood by chemical activation with KOH. J Porous Mater 24, 193–202 (2017). https://doi.org/10.1007/s10934-016-0252-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-016-0252-6