Abstract
Water elements pollution has attracted public attention globally. Wuxi is located in East China, and its water source, Taihu Lake, has been severely polluted since 2007. Studies of elemental pollution profiles have yet to be conducted in this area. In this study, 56 water samples were collected in 2018, and 33 elements were determined using inductively coupled plasma-mass spectrometry (ICP-MS). The results showed that the levels of 33 elements ranged from 1.35 × 10–3 μg/L(Tl) to 101 mg/L(Ca), with Sr, Al, Fe, B, Ti, Ba, and Zn levels being relatively higher. A comprehensive literature review showed spatial distribution of conspicuous elements in drinking water worldwide. Meanwhile, Monte Carlo simulations were applied to evaluate exposure health risks. The total hazard index(HI) for 14 non-carcinogens and the average incremental lifetime cancer risk (ILCR) of As and Pb exposure through drinking water were found acceptable. Sensitivity analyses suggested that Sb and As in the drinking water represent an increasing risk to human health. The results of this study provide key data on local metal pollution characteristics, help identify potential risk factors, and contribute to the development of effective environmental management policies for Taihu Lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world is currently facing many cases of environmental contamination and ecological damage, which have been at least partly attributed to a growing population and human activities, with aquatic ecosystems being the most adversely affected (Mostofa et al., 2013). Water pollution reduces the water availability and increases pressure on demand, as the result of which some populations face major challenges in addressing pollution and experience water scarcity (Han & Currell, 2017; Ma et al., 2020). Such issues are particularly challenging in China as more than 40% of the countries’ rivers are polluted, 80% of its lakes suffer from eutrophication, and approximately 300 million rural residents lack access to safe drinking water (An et al., 2017; Liu & Yang, 2012; Zhou et al., 2018). In recent decades, the presence of various waterborne elements, including heavy metals, has been widely reported in China (Peng et al., 2017; Zhang et al., 2011). Even the Qinghai and Tibet Plateau, regarded as the area of China least disturbed by human activity, have been found to contain several metal pollutants, such as Mg, Al, Fe, and Hg at relatively high concentrations (Wu et al., 2016).
Taihu Lake, the third largest freshwater lake in China, is located in one of China’s most developed areas (Liu et al., 2020) and has been suffered heavily pollution due to local industrial and urban development in recent decades. The lake’s condition has improved significantly since 2007, when an algal outbreak reduced the water quality and caused widespread concern in government and public circles (Huang et al., 2020; Li et al., 2020). However, the main focus has been on the routinely monitoring items and organic pollutants. Previous studies on inorganic elements have mostly focused on single elements or heavy metals. Cd, Cr, Cu, Ni, Pb and Zn were the most common elements in the past (Chen et al., 2019; Li et al., 2018;J Liu et al., 2017; Rajeshkumar et al., 2018).
Elements are critical to both humans and the environment. For example, Mg, Se, K, and Ca are vital to physiological functions. However, heavy metals, such as Cd, Pb, Cu, Hg, and Zn, are common pollutants in surface water, due to their environmental persistence, toxicity, and accumulation in food chains (Meneghel et al., 2020). Study reported that drinking 1 L/day water containing 50 μg/L As could result in the development of cancer in the liver, lung, kidney, or bladder and an increase in the cases of skin damage could occur with 0.0012 mg/kg/day As exposure through drinking water (Chowdhury et al., 2016). However, systematic research on the distribution of elements in Taihu Lake has been limited. Considering the potential effects they may have on humans, it is necessary to monitor levels of exposure to these elements in people’s daily lives, to identify potential health risks and facilitate improvements in public health.
In this study, we chose 14 sites in Wuxi which uses Taihu Lake as its water source for collecting quarterly water samples in 2018. Analysis of these samples revealed the presence of 33 elements. We subsequently reviewed the spatial distribution information with respect to these elements in drinking water, as reported in the literature for various countries and areas. Finally, we conducted a “Four steps” health risk assessment as per the US Environmental Protection Agency (US EPA), to establish the health risk to local adults and children posed by the substances identified in the Wuxi water supply.
Experimental
Water pollution profile in Wuxi
Chemicals and reagents
Thirty-three standard element solutions and internal standards (Sc, Y, In, Tb) were obtained from Inorganic Ventures (Lakewood, NJ, USA). Deionized water was purified using an ELGA Purelab Ultra system (Vivendi Water Systems, Buckinghamshire, UK). Individual stock solutions were prepared in pure water (100 mg/mL), for ICP-MS analysis.
Sample collection
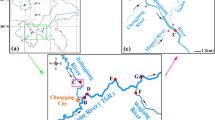
Wuxi city is located in the southeastern part of Jiangsu province and on the north shore of Taihu Lake, East China (31°07′–32°02′N, 119°33′–120°38′E). Samples were collected on a quarterly basis from 14 sites which were the routine monitoring sampling points in Wuxi city in 2018, as shown in Fig. 1. Water samples were collected in 250 mL polypropylene plastic bottles, transported to the laboratory under refrigeration, stored at 4 °C, and analyzed within 7 days.
Sample preparation
Water samples were prepared according to the National Environmental Protection Standards of the People’s Republic of China (Water quality—Determination of 65 elements—Inductively coupled plasma-mass spectrometry) (Department EP, 2014). Finished water and tap water samples were acidified at pH < 2 using nitric acid, while raw water samples were filtered through a 0.45 μm membrane before acidified. The internal standard stock solution (Sc, Y, In, Tb, Bi, 10 μg/L) was then added to each sample. The samples were transported to instrument for analysis.
To ensure the quality of the results, the 1 ppb tuning solution was injected to maintain optimum conditions across the entire mass range (4–290 amu), depending on the sensitivity of Li, Co, In, and U, oxide (CeO), and double charge (Ba2+) levels before sample analysis. Tuning requirements: at standard mode sensitivity 7Li ≥ 50 K cps/ppb, 59Co ≥ 100 K cps/ppb, 115In ≥ 200 K cps/ppb, and 238U ≥ 300 K cps/ppb. Standard mode double charge Ba2+/Ba < 3.0%, and standard mode oxide 156CeO/140Ce < 2.0%. The solvent blanks, sample blanks, and spiked samples were analyzed simultaneously throughout the sample preparation and analytical process. The recovery of spiked samples was between 91.0% ~ 102%. Additionally, the fluctuation of the internal standard values was in the range of 89–112%, indicating good stability and accuracy. The methodological data are summarized in Table S1.
Analyses
A Thermo Scientific iCAP Qc inductively coupled plasma mass spectrometer (Thermo Scientific, USA) was used for all analyses. Details of the instrumental operating conditions and optimized measurement parameters are listed in Table S2.
Literature review
Search strategy
The relevant biomedical sciences database (PubMed) was used to search for appropriate literature. Articles included were identified using a manual search, in which the keywords “element name” and “drinking water” were applied from inception through September 2017.
Statistical analysis
To clarify the regional distribution characteristics of the target element residuals, elemental data from previous studies were compared with our study. The data were analyzed statistically using software program (SPSS 13.0 for windows) The difference in elements levels between different areas based on the literature was analyzed by the t-test after log-transformation to obtain an approximate normal distribution with the significance criterion set at p value below to 0.05. To improve the statistical robustness, we conducted the comparison using a bootstrap method which was suitable for an unknown distribution in the comparison between the current study and the literature.
Health risk assessment
The recommended EPA guidelines specify that human health risk assessments should be conducted in four steps: hazard identification, exposure assessment, toxicity assessment, and risk characterization (Jiřík et al., 2016).
This process enabled the probability of adverse reactions by the exposed population to the exposure dose to be established. Risk was characterized using the hazard index (HI; applicable to non-carcinogens) and incremental lifetime cancer risk (ILCR; applicable to carcinogens) parameters.
Hazard identification
According to the latest evaluation results from the Risk Assessment Information System (RAIS; sponsored by the U.S. Department of Energy, Office of Environmental Management),14 elements detected in the present study were non-carcinogens and two were carcinogens. The toxicity values and classifications are listed in Table 1.
Exposure assessment
In this study, the primary exposure pathway considered for the element exposure assessment was through water ingestion by adults (21–70) and children (0–6). Exposure due to oral ingestion of drinking water was quantified using Eq. (1), after (YH Liu et al., 2017):
where CDI refers to the chronic daily intake (mg/kg/day), Ci is the element concentrations in tap water (μg/L), IR is the ingestion rate (L/day), EF is the exposure frequency (days/year), ED is exposure duration (years), BW is body weight (kg), and AT is the time over which exposure was averaged (days). The input values for these parameters were derived from the literature and are listed in Table S3.
Toxicity assessment
The carcinogen toxicity parameter was represented by the average oral cancer slope factor (CSF), which is the amount of carcinogen ingested per unit of time, per unit of body weight. As recommended by the US EPA, the ingestion reference dose (RfDi) through drinking water was used as the non-carcinogenic toxicity parameter in the present study (Jarabek et al., 1990). The CSF and RfDi details applicable to the target compounds herein are listed in Table 1.
Risk characterization
The non-carcinogen HI was defined as the ratio of CDI to RfDi for an individual substance, and when potentially hazardous compounds consisted of more than one type, the total HI was regarded as the sum of those developed for individual substances, as shown in Eq. (2) and (3):
Based on experience, if HI < 1, the non-carcinogenic health risk to the recipient is within acceptable limits, and if not, remediation and reassessment may be required (Tan et al., 2016).
The ILCR for oral ingestion was employed to assess carcinogenic health risk, as calculated using Eq. (4):
According to the US EPA, an ILCR within the range 10–6 to 10–4 is acceptable.
Results
Drinking water pollution profile
The concentrations of 33 elements found in water samples taken from 14 sites in Wuxi are summarized in Table 2. Element concentrations ranged from 0.01 μg/L (Tl) to 60.96 mg/L (Ca), with a detectable rate of 98.48%. The data showed that the average concentrations of Sr, Al, Fe, B, Ti, Ba, and Zn were comparatively higher than those of the other elements.
Literature review findings
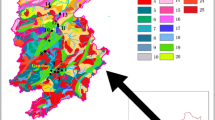
A review of 10,741 published papers identified 47 articles as relevant to our study. Details of the PubMed literature searched can be seen in Tables S4 and S5. Statistical comparisons between our data and those reported in the literature indicated that element distributions in tap water exhibited significant regional characteristics, as shown in Fig. 2. We also compared differences in the regional distribution of tap water elements in China. Sites representing its major river basins and regions include Beijing (N China, capital), Shanghai (E China, Yangtze River delta), Hainan (S China), Chongqing (midlands), Inner Mongolia (interior of Eurasia), Tibet (SW Tibetan Plateau), and five major cities in NW China. As shown in Fig. 3, elemental water pollution was more severe in N China and the midlands. In a district of Chongqing, Al, Ba, Mn, and Ni levels exceeded the recommended values of the World Health Organization (WHO) and the national hygienic standards for drinking water in some water samples (Liu et al., 2018; WHO, 2017).
The literature review found that drinking water elements pollution has significant regional characteristics across the world. Many countries have suffered from a shortage of safe drinking water. Industrial, mining, and agricultural activities in the surrounding areas were main sources of elemental pollution in developing areas. Besides, climate change, soil contamination, marine pollution can also exacerbates the problem (Barnett-Itzhaki et al., 2019).
Drinking water health risk assessment
Monte Carlo simulations have been widely used to compute difficult and multi-dimensional integrals in many disciplines, including physics and chemistry, for over 50 years (Belew et al., 2020). In the recent years, as this technique has become less computationally intensive than deterministic or point estimate methods, it has been applied to public health and environmental risk assessments, which has led to significant advances (Anchal et al., 2020; Djahed et al., 2020; Fakhri et al., 2020).
Among the 14 non-carcinogens, the CDI values were found to be in the order of Sr>B>Ba>Zn>other elements. Sr was identified as the highest daily intake, with average values of 1.53×10-3 and 3.47×10-4 mg/kg/day for adults and children, respectively, as shown in Table S6. Next, Monte Carlo simulations, conducted using Oracle Crystal ball software, were used to analyze the degree of health assessment uncertainty. Oracle Crystal Ball is the leading spreadsheet-based application for predictive modeling, forecasting, simulation, and optimization.
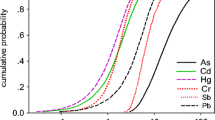
As can be seen in Table S6, the total HI for the 14 non-carcinogens ranged, from 1.23 × 10–5 to 6.64 × 10–1 for adults, with an average of 7.79 × 10–2, and from 2.16 × 10–9 to 1.21 × 10–4 for children, with an average of 1.17 × 10–5, which suggested that the health risk posed by these elements in the local drinking water was acceptable. Among these elements, HI of Sb and As were relatively higher than others, with values of 2.73 × 10–2 ± 2.49 × 10–2 and 2.60 × 10–2 ± 2.42 × 10–2.
After 10,000 simulations, the average ILCRs for As and Pb in the drinking water were 1.17 × 10–5 and 2.47 × 10–8 respectively, for adults, and 2.59 × 10–6 and 5.57 × 10–9 respectively, for children—which were within acceptable limits. The range of carcinogenic risks acceptable by the USEPA ranged from 10−6 to 10−4.The details are shown in Table S7.
Sensitivity analysis
To obtain a deeper understanding of the contribution of the parameters in the model to prediction, we performed sensitivity analyses on the HI and ILCR risk evaluations.
Considering the lower exposure levels of children, the model was only applied to adult exposure levels. We found that the exposure duration made the highest contribution to the total HI (55.09%), with Sb playing an important role in the prediction results for the 14 non-carcinogens.
Scatter plots were used to review correlations between the multiple variables and the total HI. The higher the value, the stronger the correlation. We found that the correlation coefficients for exposure duration, ingestion rate, and Sb levels were 0.7844, 0.5003, and 0.0924, respectively, and the body weight was -0.1796, as shown in Fig. 4.
The tornado tool and spider chart were used to verify the sensitivity analyses. The tornado tool represents the oscillation amplitude between the maximum and minimum predicted values for each variable. In this index, the variable with the largest oscillation amplitude has the highest rating and has the most effect on the prediction, whereas variables with smaller oscillation amplitudes have correspondingly less influence.
A spider chart was obtained by numerically testing all variables to show the difference between the minimum and maximum predicted values. The greater the slope of the variable curve, the greater the effect on the prediction. The slope angle represents a positive or negative correlation. The Tornado tool and spider chart analysis results (Fig. 5) were consistent with previous results. Exposure duration, ingestion rate, and body weight had a greater impact on health risk forecasting while body weight also present the positive effect followed by elements Sb, As, Hg, and V.
Discussion
All water sample element concentrations in Wuxi complied with the China Sanitary standards for drinking water requirements, with the exception of Zn, whose concentration exceeded the national standard in some tap water samples. We noted that the Fe and Zn concentrations were significantly higher in the tap water than in the initially finished water. This could be attributed to the materials used in fabricating water-supply pipelines, as galvanized pipes are widely used in water supply systems to improve corrosion resistance. Prolonged contact time during the transportation of water from the plant to consumers may lead to the accumulation of metals (Yao et al., 2021).
Common techniques used to remove elements from water include precipitation, coagulation-flocculation, ionic exchange, adsorption, and membrane separation (Abdullah et al., 2019). We also noted that the present water treatment process in Wuxi, including conventional technology (coagulation, sedimentation and sand-filtration), ozone-biological activated carbon process and ultrafiltration membrane treatment process, was effective in reducing the concentration of several elements including V, Mn, Fe, Cu, and As whose concentrations in finished water and tap water were much lower than in untreated raw water, which indicated a high removal efficiency. However, for the most elements, such as B, Na, Mg, K, Ca, Ti, Ni, Se, Rb, Sr, and Ba, current water treatment technology cannot remove them effectively.
In analyzing elements distribution differences in drinking water, developing countries or those often at war, such as India, Bangladesh, Pakistan, and Ghana, were found higher levels of heavy metal in drinking water, such as Cd, Cu, Hg, Ni, As, Pb compared with other countries. Various of industrial activities such as those involving minerals, steel, ores, milling, plating, and ancillaries which discharge their effluents to the main river basin along with trace metals affect the quality of water (Sahoo & Swain, 2020). In Kamrup of India, for example, 37 of the 44 sampling stations investigated had Cd levels much higher than the guideline value of 0.003 ppm set by the WHO(WHO, 2017). And 14 sampling stations contained Pb above the EPA guideline value of 0.015 ppm (Chakrabarty & Sarma, 2011). The high elemental pollution of drinking water in Saudi Arabia and Pakistan were attributed to the Gulf War and the Kuwaiti oil fires. In South America, drinking water in Venezuela and Chile has been found to be low in macroelements, such as Na, K, Ca and Mg, whereas Al, Cu, As, and Pb concentrations were relatively high, compared to those in other countries. Ca and Mg levels in water have been associated with the carbonate bedrock weathering. The components of which is mainly formed by coarse grained quartz sands and ferruginous materials instead of carbonate bearings. Therefore bio-essential elements were scarce (Mora et al., 2009). In developed countries, where suitable water quality standards have been implemented, drinking water quality is comparatively better. However, Zn content (674 ± 620 mg/L) was detected in the pipes with brass components in Finland (Inkinen et al., 2014). In North Texas, USA, Sr ranged from 66 ~ 18,195 µg/L was found significantly higher than other areas. The EPA currently recommends no more than 4,000 µg/L of Sr in drinking water (Fontenot et al., 2013).
It should also be noted that Pb pollution is seen in Tibet, the NW, and in Inner Mongolia in China, where industrial development has been slower than in other areas. The Tibetan Plateau, is the origin of many large rivers, such as the Yangtze River and Yellow River. Even less impacted by the anthropogenic activities due to its location, river water quality has also been negatively affected by rapid economic development in recent years. Surface soil evaluations of Qinghai-Tibet Plateau revealed higher Pb levels, which ranged from 9.43 ~ 122.51 mg/kg, than soil background values in China (26 mg/kg) (Yang et al., 2020). Pb levels in snow pit samples from three glaciers on the Tibet Plateau ranged from 0.82 ~ 5.72 ng/g (Chen et al., 2021).
Sr was found to be the element with the highest daily intake, the median concentration of Sr in tap water was 190μinL, making it the most abundant among the trace elements. The levels of Sr are of concern in other areas as well, especially where they were found to be 400 ± 280 μg/L, 376.46 ± 39.15 μg/L and 1610.00 ± 787.10 μg/L in Japan, Saudi Arabia, and the USA, respectively (Al-Saleh, 1996; Fontenot et al., 2013; Kikuchi et al., 1999). In Chongqing, China, high levels of Sr have also been found in drinking water samples (Liu et al., 2018). Sr is an alkaline earth metal that occurs in a wide range of compounds, such as celestite (SrSO4) and strontianite (SrCO3). The natural dissolution of these materials and conventional water treatment processes is not effective at removing Sr. As a result, Sr from surface water or ground water often enters into drinking water and is transported to customers (Zhang et al., 2018). Sr is an important mineral existing in human bones and teeth and is not currently regulated under hygienic standards. However, studies have showed that ingestion of Sr may pose a potential threat to human skeletal health leading to an increased risk of leukemia and other diseases.
As is intensely studied for its mutagenicity, carcinogenicity, and teratogenicity, the maximum ILCR value for As was 1.21 × 10–4, which was close to the USEPA maximum acceptable value. In view of the multiple exposure pathways, people can be exposed to As through the digestive tract, respiratory tract, and skin. Therefore the risk of exposure to As is high. As-contaminated drinking water is one of the major causes of arsenic toxicity in more than 30 countries, especially in developing countries, such as Bangladesh, India, and Vietnam (Kabir & Chowdhury, 2017). Even in developed areas, a considerable public health burden may result from exposure to As, like in the USA, where exposure lead to 500 ischemic heart deaths and 1,070 cases of cancer, 80 of which were nonmelanoma skin cancer, and the mean burden corresponded to $10.9 billion (2017 USD) (Greco et al., 2019). Removal of As is usually achieved by physicochemical methods, such as oxidation, precipitation, ion exchange, separation, and adsorption (Kumar et al., 2019). In the present study, the average content of As in raw water was 1.74 μg/L, ranged from 1.56 to 13.0 μg/L, which was well above finished water and tap water (0.687 μg/L and 0.745 μg/L respectively). This difference suggested that the current water treatment process was effective for arsenic removal. Among natural and anthropogenic sources of As, natural geological sources are dominant and can be accelerated by human activities such as mining. It is important to improve metallurgical processes to regulate As emissions in ore mining and dressing industries, restrict the export of As-containing primary materials, and regulate the use of As in growing applications such as semiconductors (Shi et al., 2017). By regulating anthropogenic activities, the threats posed by As to human and environmental health can be minimized.
In the sensitivity analysis, Sb was found to play an important role among the 14 elements. Sb is a heavy metal with applications in various industrial section including semiconductors, infrared detectors and diodes, and its usage increases annually. Sb detected in water samples was mainly released from sediments. Furthermore, recent studies of Taihu Lake found that microcystis from algal blooms can absorb Sb. The Sb values in water samples ranged from 1.79 to 6.99 µg/L in the algae-dominated zone and from 1.02 to 41.46 µg/L in the macrophyte-dominated zone. The levels of Sb in soil samples were 3.5 mg/kg and 3.2 mg/kg respectively (Ren et al., 2019). The data suggested that Sb pollution in this area exceeded the average global Sb concentration in rivers (1 μg/L). Long-term exposure to Sb can result in antimony spots on the skin, stomach pains, colic, nausea, and vomiting. An early study found that two-thirds of rats exposed to 209 mg Sb/m3 as SbO3 for 63 days failed to conceive and incidences of spontaneous abortions and disturbances in menstruation increased (Ren et al., 2019). In consideration of its higher contribution to the total HI and toxicity, it is necessary to intensify the monitoring of Sb.
Conclusions
The results of elemental analysis of Taihu Lake and drinking water in Wuxi confirmed that attention should be paid to potential long-term health impacts arising from the local water, considering the spatial variability of sources. Overall, the drinking water quality in this area complied with the Chinese National Sanitary Standards for drinking water, and non-carcinogenic risk levels were found to be within safe limits. However the health risk associated with local drinking water consumption was found to be significantly correlated with the Sb and As levels in the water. Therefore, we conclude that further work is required to understand the toxicity of Sb and As ingested through drinking water, and to evaluate the efficacy of the current water treatment process, in terms of its ability to remove or dilute hazardous metals and other potential pollutants associated with human health risks.
Overall, the work described here establishes a comprehensive understanding of the levels of various metal elements in local water and has helped highlight important knowledge gaps which should be addressed attention in future investigations.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdullah, N., Yusof, N., Lau, W. J., Jaafar, J., & Ismail, A. F. (2019). Recent trends of heavy metal removal from water/wastewater by membrane technologies. Journal of Industrial and Engineering Chemistry, 76, 17–38.
Al-Saleh, I. A. (1996). Trace elements in drinking water coolers collected from primary schools, riyadh, saudi arabia. The Science of the Total Environment, 181, 215–221.
An, J., Wang, J., Zhang, Y., & Zhu, B. (2017). Source apportionment of volatile organic compounds in an urban environment at the yangtze river delta, china. Archives of Environmental Contamination and Toxicology, 72, 335–348.
Anchal, P., Kumari, M., & Gupta, S. K. (2020). Human health risk estimation and predictive modeling of halogenated disinfection by- products (chloroform) in swimming pool waters: A case study of dhanbad, jharkhand, india. Journal of Environmental Health Science & Engineering, 18, 1595–1605.
Barnett-Itzhaki, Z., Eaton, J., Hen, I., & Berman, T. (2019). Heavy metal concentrations in drinking water in a country heavily reliant on desalination. Environmental Science and Pollution Research International, 26, 19991–19996.
Belew, S., Suleman, S., Wynendaele, E., Duchateau, L., & De Spiegeleer, B. (2020). Environmental risk assessment of the anthelmintic albendazole in eastern africa, based on a systematic review. Environmental pollution, 116106.
Chakrabarty, S., & Sarma, H. P. (2011). Heavy metal contamination of drinking water in kamrup district, assam, india. Environmental Monitoring and Assessment, 179, 479–486.
Chen, M., Wang, D., Ding, S., Fan, X., Jin, Z., Wu, Y., et al. (2019). Zinc pollution in zones dominated by algae and submerged macrophytes in lake taihu. Science of the Total Environment, 670, 361–368.
Chen, Y., Zou, J., Sun, H., Qin, J., & Yang, J. (2021). Metals in traditional chinese medicinal materials (tcmm): A systematic review. Ecotoxicology and Environmental Safety 207, 111311.
China MoHotPsRo. (2006). Standards for drinking water quality. In: the State Standard of the People's Republic of China, Vol. GB5749–2006.
Chowdhury, S., Mazumder, M. A. J., Al-Attas, O., & Husain, T. (2016). Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. The Science of the Total Environment, 569–570, 476–488.
Department EP. (2014). Water quality—determination of 65 elements—inductively coupled plasma-mass spectrometry. In: National Environmental Protection Standards of the People's Republic of China Vol. HJ 700–2014.
Djahed, B., Kermani, M., Farzadkia, M., Taghavi, M., & Norzaee, S. (2020). Exposure to heavy metal contamination and probabilistic health risk assessment using monte carlo simulation: A study in the southeast iran. Journal of Environmental Health Science & Engineering, 18, 1217–1226.
Fakhri, Y., Nematollahi, A., Abdi-Moghadam, Z., Daraei, H., Ghasemi, SM., & Thai, V.N. (2020). Concentration of potentially harmful elements (phes) in trout fillet (rainbow and brown) fish: A global systematic review and meta-analysis and health risk assessment. Biological Trace Element Research.
Fontenot, B. E., Hunt, L. R., Hildenbrand, Z. L., Carlton, D. D., Jr., Oka, H., Walton, J. L., et al. (2013). An evaluation of water quality in private drinking water wells near natural gas extraction sites in the barnett shale formation. Environmental Science & Technology, 47, 10032–10040.
Greco, S. L., Belova, A., Haskell, J., & Backer, L. (2019). Estimated burden of disease from arsenic in drinking water supplied by domestic wells in the united states. Journal of Water and Health, 17, 801–812.
Han, D., & Currell, M. J. (2017). Persistent organic pollutants in china’s surface water systems. The Science of the Total Environment, 580, 602–625.
Huang, X., Xu, X., Guan, B., Liu, S., Xie, H., Li, Q., et al. (2020). Transformation of aquatic plant diversity in an environmentally sensitive area, the lake taihu drainage basin. Frontiers in Plant Science, 11, 513788.
Inkinen, J., Kaunisto, T., Pursiainen, A., Miettinen, I. T., Kusnetsov, J., Riihinen, K., et al. (2014). Drinking water quality and formation of biofilms in an office building during its first year of operation, a full scale study. Water Research, 49, 83–91.
Jarabek, A. M., Menache, M. G., Overton, J. H., Jr., Dourson, M. L., & Miller, F. J. (1990). The u.S. Environmental protection agency’s inhalation rfd methodology: Risk assessment for air toxics. Toxicology and Industrial Health, 6, 279–301.
Jiřík, V., Machaczka, O., Miturová, H., Tomášek, I., Šlachtová, H., Janoutová, J., et al. (2016). Air pollution and potential health risk in ostrava region - a review. Central European Journal of Public Health, 24(Suppl), S4–S17.
Kabir, F., & Chowdhury, S. (2017). Arsenic removal methods for drinking water in the developing countries: Technological developments and research needs. Environmental Science and Pollution Research International, 24, 24102–24120.
Kikuchi, H., Iwane, S., Munakata, A., Tamura, K., Nakaji, S., & Sugawara, K. (1999). Trace element levels in drinking water and the incidence of colorectal cancer. The Tohoku Journal of Experimental Medicine, 188, 217–225.
Kumar, R., Patel, M., Singh, P., Bundschuh, J., Pittman, CU Jr., Trakal, L., et al. (2019). Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: Methods, experience from, and options for latin america. The Science of the total environment, 694, 133427.
Li, B. B., Peng, Z. Y., Zhi, L. L., Li, H. B., Zheng, K. K., & Li, J. (2020). Distribution and diversity of filamentous bacteria in wastewater treatment plants exhibiting foaming of taihu lake basin, china. Environmental Pollution, 267, 115644.
Li, Y., Zhou, S., Zhu, Q., Li, B., Wang, J., Wang, C., et al. (2018). One-century sedimentary record of heavy metal pollution in western taihu lake, china. Environmental Pollution, 240, 709–716.
Liu, J., & Yang, W. (2012). Water management. Water sustainability for china and beyond. Science, 337, 649–650.
Liu, J., Wang, P., Wang, C., Qian, J., & Hou, J. (2017). Heavy metal pollution status and ecological risks of sediments under the influence of water transfers in taihu lake, china. Environmental Science and Pollution Research International, 24, 2653–2666.
Liu, Y., Yuan, Y., & Luo, K. (2018). Regional distribution of longevity population and elements in drinking water in jiangjin district, chongqing city, china. Biological Trace Element Research, 184, 287–299.
Liu, Y. H., Zhang, S. H., Ji, G. X., Wu, S. M., Guo, R. X., Cheng, J., et al. (2017). Occurrence, distribution and risk assessment of suspected endocrine-disrupting chemicals in surface water and suspended particulate matter of yangtze river (nanjing section). Ecotoxicology and Environmental Safety, 135, 90–97.
Liu, Y. Y., Tian, T., Zeng, P., Zhang, X. Y., & Che, Y. (2020). [surface water change characteristics of taihu lake from 1984–2018 based on google earth engine]. Ying yong sheng tai xue bao = The Journal of Applied Ecology 31, 3163–3172.
Ma, T., Sun, S., Fu, G., Hall, J. W., Ni, Y., He, L., et al. (2020). Pollution exacerbates china’s water scarcity and its regional inequality. Nature Communications, 11, 650.
Meneghel, J., Kilbride, P., & Morris, G. J. (2020). Cryopreservation as a key element in the successful delivery of cell-based therapies-a review. Frontiers in Medicine, 7, 592242.
Mora, A., Mac-Quhae, C., Calzadilla, M., & Sanchez, L. (2009). Survey of trace metals in drinking water supplied to rural populations in the eastern llanos of venezuela. Journal of Environmental Management, 90, 752–759.
Mostofa, K. M., Liu, C. Q., Vione, D., Gao, K., & Ogawa, H. (2013). Sources, factors, mechanisms and possible solutions to pollutants in marine ecosystems. Environmental Pollution, 182, 461–478.
Peng, F. J., Pan, C. G., Zhang, M., Zhang, N. S., Windfeld, R., Salvito, D., et al. (2017). Occurrence and ecological risk assessment of emerging organic chemicals in urban rivers: Guangzhou as a case study in china. The Science of the Total Environment, 589, 46–55.
Rajeshkumar, S., Liu, Y., Zhang, X., Ravikumar, B., Bai, G., & Li, X. (2018). Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the meiliang bay of taihu lake in china. Chemosphere, 191, 626–638.
Ren, M., Wang, D., Ding, S., Yang, L., Xu, S., Yang, C., et al. (2019). Seasonal mobility of antimony in sediment-water systems in algae- and macrophyte-dominated zones of lake taihu (china). Chemosphere, 223, 108–116.
Sahoo, M. M., & Swain, J. B. (2020). Modified heavy metal pollution index (m-hpi) for surface water quality in river basins, india. Environmental Science and Pollution Research International, 27, 15350–15364.
Shi, Y. L., Chen, W. Q., Wu, S. L., & Zhu, Y. G. (2017). Anthropogenic cycles of arsenic in mainland china: 1990–2010. Environmental Science & Technology, 51, 1670–1678.
Tan, S. Y., Praveena, S. M., Abidin, E. Z., & Cheema, M. S. (2016). A review of heavy metals in indoor dust and its human health-risk implications. Reviews on Environmental Health, 31, 447–456.
WHO. (2017). Guidelines for drinkingwater quality.
Wu, J., Duan, D., Lu, J., Luo, Y., Wen, X., Guo, X., et al. (2016). Inorganic pollution around the qinghai-tibet plateau: An overview of the current observations. The Science of the Total Environment, 550, 628–636.
Yang, A., Wang, Y. H., Hu, J., Liu, X. L., & Li, J. (2020). [evaluation and source of heavy metal pollution in surface soil of qinghai-tibet plateau]. Huan jing ke xue= Huanjing kexue, 41, 886–894.
Yao, C., Che, F., Jiang, X., Wu, Z., Chen, J., & Wang, K. (2021). Study on antimony mobility in a contaminated shallow lake sediment using the diffusive gradients in thin films technique. Chemosphere, 267,128913.
Zhang, G., Chen, L., Liu, Y., Chon, T., Ren, Z., Wang, Z., et al. (2011). A new online monitoring and management system for accidental pollution events developed for the regional water basin in ningbo, china. Water Science and Technology : A Journal of the International Association on Water Pollution Research, 64, 1828–1834.
Zhang, H., Zhou, X., Wang, L., Wang, W., & Xu, J. (2018). Concentrations and potential health risks of strontium in drinking water from xi’an, northwest china. Ecotoxicology and Environmental Safety, 164, 181–188.
Zhou, C., Chen, J., Xie, H., Zhang, Y. N., Li, Y., Wang, Y., et al. (2018). Modeling photodegradation kinetics of organic micropollutants in water bodies: A case of the yellow river estuary. Journal of Hazardous Materials, 349, 60–67.
Acknowledgements
The authors are thankful to the colleagues from the Wuxi Center for Disease Control and Prevention (CDC) for their assistance with water sample collection.
Funding
This work was supported by Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (BJ2020095, HB2020098) and Public Health Research Center Project of Jiangnan University (JUPH201844).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, K., Meng, Y., Gong, Y. et al. Drinking water elements constituent profiles and health risk assessment in Wuxi, China. Environ Monit Assess 194, 106 (2022). https://doi.org/10.1007/s10661-022-09768-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-09768-1