Abstract
The presence of heavy metals and naturally occurring radioactive materials (NORMs) in high concentrations in soils can be hazardous to exposed humans. This study is aimed at measuring the concentrations of heavy metals (Cu, Pb, Ni, Zn, Cd, Co, and Cr) and activity concentration of 232Th, 238U, and 40K in soils affected by and around a solid waste dumpsite in Osogbo metropolis, Nigeria. Atomic absorption spectrometry and gamma-ray spectrometric techniques were used to determine the concentrations of metals and NORMs, respectively. Possible environmental impact of the heavy metal content and the probable radiological hazard by the NORMs to the general public were assessed. The calculated pollution indices reported in this work for Co, Cr, Pb, and Ni show low pollution status. Geoaccumulation indices for Cu, Zn, and Cd indicated that the area under study is strongly contaminated by these metals. Evaluated ecological risk index narrowed down Cd as the poisonous metal with high concentration. The measured radionuclides’ mean activity concentrations and the evaluated mean of radium equivalent and absorbed dose rate values are higher than the recommended safe limit, an indication of possible radiological hazard. The principal factor analysis results explained 76% of the collection of data and described chips of galvanized/chrome metals, scrap metals, waste from electronics, Cr, and Cd-containing waste as sources of the heavy metals. The practice of land cultivation around the dumpsite should be deterred to prevent the transportation of these vicious heavy metals into the food chain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid wastes contain untreated residential and business waste generated by a region inside a given territory (Nda-Umar et al., 2012). In recent times, population increase and the rapid pace of industrialization have adversely influenced the quality and amount of solid waste produced. The decay of aggregated waste over a significant stretch of time crumbles the nature of the soil. The ecological consequences of solid wastes are governed, to a reasonable extent, by their heavy metal susceptibility, which as a result of urbanization and industrialization keep increasing day after day (Getachew and Habtamu, 2015; Turan et al., 2017). Significant quantities of harmful metals generated by humans have been established to be accumulated in the soil (Agirtas & Kilicel, 1999; Amusan et al., 2005; Cheng et al., 2007; Esakku et al., 2005; Ideriah et al., 2010; Turan, 2019a, b). Lack of proper strong waste administration frameworks is evident in many developing countries, while the current framework was constrained by improper administrative design, institutional system, and fiscal restriction (Peter et al., 2008). Open waste disposal and failure to appropriately manage waste pose several health challenges to the populace, especially those living close to or working nearby the dumpsite (Ogundele et al., 2020).
The presence of heavy metals in little amount in undisturbed soils is fundamental for plants to remain healthy (Esakku et al., 2005; Ideriah et al., 2010). However, the copious concentrations of heavy metals in the soil system have been proved to be a global problem for living organisms (Amini et al., 2012; Shahbaz et al., 2018a, b; Turan, 2021). In addition, heavy metals can influence the ecosystem of host soil to bring about huge losses of soil quality (Esmaeili 2014; Shahbaz et al., 2019; Zubair et al., 2021). Since heavy metals are non-biodegradable (Turan et al. 2018), they are not easily detoxicated and ousted from the soil by metabolic activities once present in the ecosystem and consequentially threaten human health via their entry into the food chain (Balkhair & Ashraf, 2016; Turan et al., 2018).
Heavy metals can accumulate in the body of human through dermal assimilation of polluted dust stick to uncovered skin, consumption of food uncovered from dust that is contaminated with heavy metal, and the inhalation of suspended particles (Du et al., 2013; Ogundele et al., 2019a; Ogunsola et al., 1993; Taiwo et al., 2016). In addition, the cultivation of plant on heavy metal–contaminated soil can adversely affect the plant quality which may cause critical health issues to humans and animals that feed on such plants (Turan et al., 2018; Turan, 2019a, b). The consumption of food derived from plants contaminated by heavy metals can directly impair human health by inhibiting the functionalities of cells in inter-linked human body organs and thereby cause cardiovascular and renal instability, poor development of organs, gastrointestinal disturbance, intellectual disabilities in children, mental retardation, and infertility (Naeem et al., 2021; Ogundele et al., 2017; Rasool et al., 2021; Sofuoglu & Sofuoglu, 2017; Turan et al., 2018; Turan, 2019a, b; Van der Kuijp et al., 2013; Zubair et al., 2021).
The conceivable outcomes of a significant level of radiation in the dumpsite as a result of natural radionuclides present in the soil should not be underestimated. The possibility of exposure to a high level of radiation from radionuclide-contaminated soil through ingestion or inhalation has been confirmed (Ademola et al., 2015; Sayyed et al., 2018). These radionuclides can be conceivably harmful to humans’ health, even at low concentrations. Results of some research works have linked the source of vestiges of radionuclides in some staple foods consumed in Nigeria to dumpsites (Akinloye & Olomo, 2005; Jibiri et al., 2007). The vacant land around the dumpsite is noted for farming, and this can aid conveyance of possible high level of naturally occurring radioactive materials domicile in the soil from the study area by root-uptake and then through ingestion to the populace.
There has been an appraisal of the impact of solid waste on the quality of surface and shoal water within Osogbo metropolis using reliable methods (Oyelami et al., 2013; Ugwu et al., 2016), but evaluations of heavy metals and naturally occurring radionuclides in soils contaminated by solid waste from the study area along with possible pollution and radiation hazards they may present are still essential. In this research, the concentrations of Cu, Pb, Ni, Zn, Cd, Co, and Cr accumulated in the soil from Onibu-Eja, solid waste dumpsite, Osogbo, will be determined along with activity concentration of 232Th, 238U, and 40K in the soil from the same area. Associated health risks posed by the measured elements will also be assessed.
Methodology
Study area
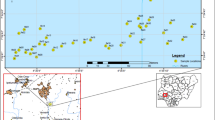
This study was carried out in Onibu-Eja solid waste dumpsite, which is located in Egbedore Local Government area, Osogbo, Southwest Nigeria. The study area coordinates are 7°47′41″N and 4°29′23″E. Geologically, the research area lies within the Southwestern Nigeria Precambrian basement complex, which forms part of Nigeria’s basement complex (Oyelami et al., 2013; Rahaman, 1988, 1989; Ugwu et al., 2016). Onibu-Eja dumpsite receives all sorts of wastes from different sources, such as kitchens, animal farms, hospitals, workshops (electronics and mechanical), saw-mill, and different industries (small, medium, and large scales) using various chemical substances. In the vicinity of the dumpsite under consideration, farming is the common practice where different arable crops are cultivated and animals grazing. There are also residential buildings and some small-scale shops for retail purposes.
Sample collection
Thirty-four (34) grids of 1 by 1 m, with a minimum of 10 m apart, were made in the dumpsite. The surface of each sampling point was cleared of solid material using a shovel until the soil beneath is reached. Soil samples taken from the edges and randomly in-between each grid were thoroughly mixed for homogeneity before a representative sample is collected into a well-labeled polythene bag. Another ten samples were collected 10 km in different directions from the dumpsite as controls. The samples were all obtained the same day. Then, they were conveyed to the environmental research laboratory in the Physics Department, Osun State University, Osogbo, for further preparation. In the laboratory, extraneous earth materials were removed from the samples after which they were air-dried to consistent weight at ambient temperature.
Sample preparation for heavy metal analysis
The samples were then sifted using a 2-mm mesh, after which 2.0 g of each sample was measured and souse with 15 mL mixture of perchloric (HClO4) and nitric acids in ratio 3:1 inside a Foss Tecator digestion vessel. The temperature of the content was raised to and maintained at 400 °C until the solution was clear and allowed to cool. After cooling, the impurities in the solution were filtered out and the filtrate was diluted with distilled water to 50 mL in a volumetric flask. Elemental concentrations of Co, Cr, Pb, Cu, Zn, Cd, and Ni were determined using the Atomic Absorption Spectrometer (Buck Scientific VGP 210 Model). The detection limits of the measured metals ranged from 0.001 to 0.005 μg/g (Ogundele et al., 2019a, b).
Pollution indices
Indices of pollution, like pollution and contamination load factors, have successfully been used to appraise comparatively the contamination levels in the soil (Chen et al., 2015; Ogundele et al., 2020). The contamination factor was determined in this study using Eq. (1).
ci and cb are the quantified concentrations of ith heavy metal and that of the background, respectively. Background concentration for soil used for agricultural purposes recommended by the Canadian Council of Ministers for the Environment (CCME) (2014) is utilized in this research. The classification for the range of contamination factor is presented in columns 1 and 5 of Table 1.
The degree of contamination (\({{\varvec{C}}}_{{\varvec{d}}{\varvec{e}}{\varvec{g}}}\)) is an indication of the total and order of magnitude of metal contamination in the soil (Islam et al., 2015). In this study, \({{\varvec{C}}}_{{\varvec{d}}{\varvec{e}}{\varvec{g}}}\) is quantified using Eq. (2).
The pollution status of the degree of contamination is categorized based on the ordered series given in columns 2 and 5 of Table 1.
The pollution load index (PLI) is used for the assessment of the total level of pollution of soil due to its heavy metal contents. It integrated the components of contamination in Eq. (1) for individual heavy metal that is being considered (Chen et al., 2015; Islam et al., 2015). Equation (3) is used to evaluate PLI
where n is the number of all the hazardous metals considered. The results of PLI can indicate the toxicity status of the soil from free of pollution to highly polluted in the range of zero to ten (0 → 10) (Chen et al., 2015; Dung et al., 2013; Zhang et al., 2011).
Geoaccumulation index
Geoaccumulation index (Igeo) for the quantified concentrations of heavy metal n (Cn)in mg kg−1 and geochemical background concentration (Bn) was calculated using Eq. (4). Igeo is a geochemical tool that has proved useful in appraising the pollution status of heavy metals’ elemental concentrations in the sediment, water, soil, and dust (Dung et al., 2013; Muller, 1969; Ogundele et al., 2020).
The constant 1.5 is added as suggested by Chen et al. (2015) as the correction factor for the background matrix to account for potential variation as a result of lithogenic effects in background values. In this study, the values suggested in the continental crust by Reimann and de Caritat (1998) for the background content of element n (Bn) are adopted. Igeo pollution status is rated as featured in Table 2.
Ecological risk factor
This study also uses the possible environmental risk factor (ERF), which has been widely used to assess the nature of sediments and soils concerning heavy metal contamination, to assess heavy metal accumulation in soils, and to relate ecological effects to their toxicology (Hakanson, 1980; Qingjie et al., 2008).
ERF of the quantified heavy metals in the soil sample impacted by solid waste is calculated using Eq. (5), where the number of quantified heavy metals is n. The classification of ERF pollution status is featured in columns 3 and 5 of Table 1. Equation (6) is utilized to evaluate the possible ecological risk index \({({\varvec{E}}}_{{\varvec{r}}}^{{\varvec{i}}})\) for each quantified heavy metal i (Islam et al., 2015). The toxicity factor \({({\varvec{T}}}_{{\varvec{r}}}^{{\varvec{i}}})\) used in this study can be found in columns 1 and 11 of Table 3. The ranking systems for the pollution status of the ecological risk index are presented in columns 4 and 5 of Table 1.
Sample preparation for gamma-ray spectrometry analysis
Thoroughly washed and dried cylindrical high-strength thermoplastic materials were filled with 100 g of fine sample each, sealed hermetically, and held for a minimum of 28 days for the attainment of secular equilibrium between the parent and the daughter nuclides (Gbenu et al., 2020a). The sealed samples were then taken to Biological Trace Element Laboratory, Department of Physics and Engineering Physics, Obafemi Awolowo University, Ile-Ife, Nigeria. In this laboratory, the measurement of the activity concentrations of the naturally occurring radioactive materials (NORMs) was carried out using gamma-ray spectrometry technique. Each sample was counted for 36,000 s using a well-calibrated Cesium Iodide (CsI) scintillation detector, while Universal Radiation Spectrum Analyzer (URSA II) was used to process the spectra. Energy calibration is done through the measurement of the spectrum of a source which emits precisely known gamma ray energy, and the position of the measured peak was compared with the energy. Coverage of full spectrum of energies expected in this study was assured in the detector energy calibration, which was done using 3 point sources of gamma emitter samples (Ba-133, Cs-137, and Co-60) of known energies that permitted the determination of a linear equation relating gamma energy to channel number. Evaluation of activity in the samples was done using Rocketdyne reference soil sample (ENV 94,084) as comparator standard for efficiency calibration of the detector. The peaks corresponding to 352 keV (Pb-214) for U-238, 1460 keV for K-40, and 240 keV (Ra-224) for Th-232 were employed for the estimation of natural radionuclides in all the samples. The integrated counts recorded under the energy peaks 1460, 352, and 240 keV were noted for each spectrum (Inuyomi et al., 2019).
Estimation of radiation hazard of soil samples
Radium equivalent activities (Raeq) were calculated using Eq. (7). This was done to appraise the gamma radiation exposure from the study area (Ademola et al., 2015; Gbenu et al., 2015, 2016; Oladejo et al., 2020).
\({{\varvec{A}}}_{{\varvec{T}}{\varvec{h}}}\), \({{\varvec{A}}}_{{\varvec{R}}{\varvec{a}}}\), and \({{\varvec{A}}}_{{\varvec{K}}}\) are the activity concentrations of 232Th, 238U, and 40K in Bq kg−1 respectively. 226Ra being one of the decay products in 238U decay series (Awwad et al., 2012), 238U is substituted for 226Ra in this work.
Also evaluated based on accepted data and recommended formulas was the absorbed dose rate (DR) of gamma radiation from the key primordial radioactive elements in the sampled soil from the study area using Eq. (8) (European Commission (EC), 1999); Gbenu et al., 2015; Oladejo et al., 2020; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 1993).
Statistical analysis
Multivariate statistical tools that utilize the approach of data dimensionality reduction were deployed to locate a couple of elements that gives explicit variance within the data set of the quantified concentrations of the element (Gbenu et al., 2020b; Hair et al., 2014; Ogundele et al., 2020). Correlation analysis between the measured concentrations of the element in the sampled soil was carried out to ascertain the connectivity between the elements. To identify basic factors that reflect what the variables (measured concentrations of the elements) have in common, principal component factor analysis was carried out (Ogundele et al., 2020). The target for using multivariate statistical analyses was to determine the meaning of the specific new calculated dimensions and to detect possible variable associations with a certain signification. The detection of possible key variances in the data collection through the statistical analysis performed was also an objective.
For optimization of valuating in the vicinage of either 0 or 1 in the loading factor without tampering with the variability in totality or as an individual element, varimax rotation was employed (Ogundele et al., 2020). With the approach of varimax rotation, high loadings (i.e., > 50% association) indicate an association between the variable and the factor, while loadings near zero indicate a clear lack of association (Ogundele et al., 2020). Subsequent upon rotation, elements emerging from similar sources are factored together (Hair et al., 2014). Statistical analysis in this study was performed using computer software Statistical Package for Social Sciences (SPSS Statistics 26).
Results and discussion
Concentrations of heavy metals
The results of Cu, Zn, Co, Cr, Pb, Cd, and Ni quantified from soil samples collected from Onibu-Eja dumpsite are presented in the descriptive statistical format in Table 3. The results show a great variance of dispersion of the appraised heavy metals in the area under study. This may be consequent upon the composition of different solid waste materials that are disposed in the dumpsite and had decomposed over time. The measured mean concentrations of Zn and Cu are 893.67 and 342.60 mg kg−1, respectively, much more prominent than the background values suggested by Reimann and de Caritat (1998), Radojevic and Bashkin (2006), and Canadian Council of Ministers of the Environment (CCME), (2014). Although Zn and Cu are essential elements for plants, their elevated concentrations are phytotoxic and directly affect crop yield and soil fertility (Balkhair & Ashraf, 2016; Caussy et al., 2003; Turan et al., 2018). The mean concentration of Pb is 20.29 mg kg−1. This mean concentration is within the stipulated background concentration for Pb (Canadian Council of Ministers of the Environment 2014; Radojevic & Bashkin, 2006; Reimann & de Caritat, 1998), but higher than the threshold range of 5 mg kg−1 set by World Health Organization. Food and Agriculture Organization WHO/FAO (2007), a clear indication that the examined soil poses toxic effects on plants, humans, and animals (Naeem et al., 2021). Furthermore, the mean concentration of Pb in soil affected by solid waste is higher than its concentration in other samples from the control area, a denotation of possible enhanced concentration as a result of dumping activity. The mean values for Co, Cr, Ni, and Cd concentrations are 9.36, 7.10, 6.91, and 2.70 mg kg−1, respectively. Co, Cr, and Ni mean concentrations in the dumpsite are lower than the stipulated background reported by Radojevic and Bashkin (2006) and Reimann and de Caritat (1998), but their transcendence of the mean values for the samples from the control area connotes an aggregation of these heavy metals in the dumpsite. The values of Cd concentration in the dumpsite are notably higher than those from the suggested background and the control samples. Elevated concentration of Cd has been implicated in causing metabolic irregularities and oxidative stress in plant which distort their respective physiological and morphological attributes (Bagheri et al., 2014; Jali et al., 2016; Kim et al., 2020; Yang et al., 2016; Zubair et al., 2021).
Descriptive statistics of elemental concentrations of heavy metals
The pattern and concentration variation of the appraised heavy metals is presented in column 3 of Table 3. The evaluated values for the standard deviation of Cu, Zn, and Cd are observed to be very high, which may be due to the large variation in the distribution of the elements in the area (Islam et al., 2015; Ogundele et al., 2020). Skewness in statistics connotes a lopsidedness and asymmetry from the mean of data distribution (Ogundele et al., 2020; Raghu et al., 2017). Column 4 of Table 3 features skewness calculated for the heavy metal distribution in the area. Cr and Pb concentrations were slightly skewed positively (< 1), a connotation of moderate distortion in distribution. While Zn, Cu, Co, Ni, and Cd concentrations with skewness greater than 1 (> 1) indicate that their distributions are highly distorted. Likewise, kurtosis of the distribution was evaluated to determine the peakness or flatness of the data collection. Results of evaluated kurtosis are presented in column 5 of Table 3. A subzero kurtosis is an indication of the flattened distribution of data and is called a platykurtic distribution. If the kurtosis is greater than zero, then the distribution is peaked and is called a leptokurtic distribution. In this work, all the distributions have kurtosis greater than 0, suggesting peaked distributions (Ogundele et al., 2020; Raghu et al., 2017).
Results of pollution indices
The evaluated average contamination factor values are presented in Table 3, column 8. The average contamination factors for Co, Cr, Pb, and Ni have values less than 1 (\({{\varvec{C}}}_{{\varvec{f}}}^{{\varvec{i}}}\)<1), indicating that these heavy metals contribute trifling or no pollution to the study area. Cd contamination factor is between 1 and 3 (\({1\le {\varvec{C}}}_{{\varvec{f}}}^{{\varvec{i}}}<3\),), which implies moderate contamination status. Cu and Zn contamination factors ranged between 3 and 6 \((3\le {{\varvec{C}}}_{{\varvec{f}}}^{{\varvec{i}}}<6)\), signifying considerate pollution status. The value of Cdeg evaluated in this study is 11.73, denoting a considerate level of pollution. The index of pollution load (PLI) evaluated in this study is 0.64, signaling an unpolluted status.
The results of the geoaccumulation index (Igeo) are presented in column 10 of Table 3. Geoaccumulation indices of Co, Cr, Pb, and Ni have values below 1 (Igeo < 1), thereby classified as unpolluted. Cu, Zn, and Cd have geoaccumulation index values ranging between 3 and 4 \({(3<{\varvec{I}}}_{{\varvec{g}}{\varvec{e}}{\varvec{o}}}\le 4\)), stipulating a strongly polluted status. This could be ascribed to the accumulation and decay of solid wastes that are rich in these heavy metals. This proffers that the sampled soil could be mainly contaminated by accretion of Cd, Zn, and Cu amidst the appraised heavy metals.
Potential ecological risk index (\({E}_{r}^{i}\)) results are presented in Table 3, column 11. All heavy metals quantified, Cd exempted, have \({E}_{r}^{i}\) values below forty (< 40), suggesting a low-risk classification. Cd has an ecological risk index value of 57.87, which suggests moderate risk. Regulation of Cd-prone wastes is very important to mitigate the possible health risks it posed to the populace (Ogundele et al., 2020; Zubair et al., 2021). Farming around the dumpsite is unhealthy, as a plausible channel of heavy metals into humans’ food chain, and consuming farm product from this region is hazardous (Ogundele et al., 2020). The average possible environmental risk factor (ERF) is 92.24, an indicator of a low degree of ecological pollution.
Radionuclide concentration results
The distribution of natural radionuclides in the sampled soil from the study area and the evaluated absorbed dose rate and radium equivalent are described statistically in Table 4. Negative skewness observed for the distributions of 232Th and 238U in Table 4 denotes a dispersion with asymmetric tail broadening toward more negative values. The negative kurtosis values for all the NORM distributions in Table 4 indicate a flattened distribution.
The presence of radionuclides 238U, 40K, and 232Th is evident in all the samples. The computed mean activity levels of 40K, 238U, and 232Th are 718.16 ± 67.05, 264.28 ± 27.54, and 53.65 ± 5.96 Bq kg−1, respectively. The mean values of the three natural radionuclide concentrations from the dumpsite’s soil are observed to be comparable with values from the control environment, suggesting that dumping activity does not influence the appraised concentrations of these radionuclides. Activity levels of 232Th, 40K, and 238U in the sampled soils are observed to have values greater than the worldwide average activity concentration of 30, 400, and 35 Bq kg−1, respectively (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), 2000).
Radium equivalent (Raeq) activity mean values in the dumpsite and the control area are 396.30 and 387.19 Bq kg−1, respectively. These mean values compare well with the suggested allowable limit of 370 Bq kg−1(Organization for Economic Cooperation and Development (OECD), 1979). Absorbed dose rate (DR) evaluated mean values are 187.86 and 183.58 nGyh−1 in soil samples from the dumpsite and control areas, respectively. The evaluated absorbed dose rate (DR) values are of the order of 2.23 and 2.18 greater than the worldwide population-weighted average of 84 nGyh−1 suggesting an enhanced ionizing radiation doses than what is acceptable internationally (OECD, Organization for Economic Cooperation and Development, 1979).
Correlation analysis result
The results of correlation analysis between all the measured elements are presented as correlation matrix in Table 5. The results show a considerable degree of interrelationship. For example, Cr and Pb are observed to be perfectly and directly correlated (correlation coefficient, R = 1.000), indicating the existence of a perfect relationship between the metals and suggesting a common source. Cu and Ni are also seen to be positively correlated with each other (R > 0.798), a reflection of good association as well. Zn is moderately correlated with Co, Cr, and Pb, and Ni with Cr and Pb with R slightly greater than 0.5. Cd lacks correlation with all other metals (R ≈ 0) except for Co that it is negatively and weakly correlated with, suggesting an independent source. Cu is weakly correlated with Zn, Cr, and Pb \(3<R<5\). These correlation coefficient results between the heavy metals suggest 2 different possible sources. Natural radionuclides are observed to be strongly and positively correlated (R > 0.840) with one another, predicting a common source.
Factor analysis results
The results of factor analysis for measured heavy metals and natural radionuclides are presented in Table 6. Premises on the initial eigenvalue’s results (total > 1), three factors with total cumulative variance > 76% were carefully considered. Factor loading is the statistical relationship of the variable (measured concentrations of the elements) and the factor (likely sources), while the squared loading is the amount of the variable’s total variance accounted for by the factor (Hair et al., 2014). Factor loadings greater than ± 0.5 are considered practically significant and are used for interpretation of the factor analysis. An intersection is noticed for Co in the component matrix for principal component 1 (PC 1) and PC 3, but after varimax rotation, it was reduced to PC 3. PC 1, which is responsible for 36.054% of the total variance, is loaded with Cr (0.88), Pb (0.88), Ni (0.83), Zn (0.65), and Cu (0.761). Indiscriminate disposal of waste from printing processes, construction materials, metals (iron, steel, and brass coated with Zn), a large quantity of used dry cells, used Ni–Cd and Pb-acid batteries, plumbing material, glassware, petrol, gasoline, Zn-containing pesticides (e.g., Zineb, Mancozeb, and Ziram), and large proportions of product packaging, waste metal cans, and containers are the main sources that could contribute to this factor (Mousavi & Seyedi, 2011; Naeem et al., 2021; Radojevic & Bashkin, 2006; Shahbaz et al., 2018a, b, 2019; Turan, 2019a). Besides, Pb concentrations can be traced to waste from day-to-day life products containing Pb (i.e., glazed ceramic, solder, and paints), metal plating, and leather tanning coupled with exhausts from automobiles along the main road (Kushwaha et al., 2018; Turan, 2019b).
Potential sources of Cu include components of electronic waste: laptops, toys, transistors, television, computers, wires, and cable (Ogundele et al., 2020). Consequently, the discovered sources are as follows: waste of chrome metals, chips of galvanized iron, used dry and wet cells, and electronics waste. PC 2 is populated with naturally occurring radioactive materials, 238U (0.98), 232Th (0.97), and 40K (0.94), which explained 29.474% of the sum of variation. The presence of these radionuclides can be traced to the geological formation of the study area. PC 3 is loaded with Co (0.69) and Cd (0.75). These metals explained 10.831% of the sum of variation. Probable sources leading to this constituent include wastes from electroplating processes, scrabs of alloys of the metals, Co-containing wastes, spent Ni–Cd batteries, used kitchen utensils, and soldering components. Figure 1 presents graphically the components that describe the relationship among the appraised metals.
Conclusion
Concentrations of selected heavy metals in soils affected by solid waste from Onibu-Eja dumpsite were appraised using Atomic Absorption Spectrophotometer. Indices of pollution were evaluated to adequately appraise the degree to which the appraised heavy metals pollute the study area. The results stipulate that the values of mean concentrations of Zn, Cu, Co, Cr, Cd, and Ni are greater than the stipulated background values in the soil. The calculated pollution indices reported in this work for Co, Cr, Pb, and Ni \({C}_{f}^{i}<{1, I}_{geo}<0\)) reflect low pollution status. Geoaccumulation index of the metals Cu, Zn, and Cd, with \(3<{I}_{geo}\le 4\) suggests a strong pollution status. Evaluated ecological risk index singles Cd out as the only heavy metal with poisonous concentration \({(E}_{r}^{i}>40)\). Activity levels of 232Th, 40 K, and 238U in the sampled soils were measured using the gamma-ray spectroscopy technique. Possible radiological risk and level of radiation exposure in the soil from the area were also evaluated. The calculated radium equivalent (Raeq) mean value is 387.19 Bq kg−1, and the absorbed dose rate (DR) is evaluated to be 187.86 nGyh−1. These are substantial values compared with the recommended safety ones. Hence, populace living, farming, and selling in the vicinity may be endangered by nocent radiation arising from this area. Factor analysis results explained 76% of the data collection and identified heavy metals sources as metals chips, liquidated electronic components, Cr and Cd-containing wastes. It is thereby recommended that governments discourage human activity in the dumpsite area. The practice of land cultivation around the dumpsite should be deterred to prevent the transportation of these vicious heavy metals into the food chain. Waste management agencies should be trained and equipped to identify and quantify the potentials of solid waste and to sensitize the general public of the appropriate means of recycling or disposal, as the case may be of solid waste.
Availability of data and material
Substantial data are embedded in the manuscript.
References
Ademola, A. K., Olaoye, M. A., Peter, O., & Abodunrin, . (2015). Radiological safety assessment and determination of heavy metals in soil samples from some waste dumpsites in Lagos and Ogun state, south-western, Nigeria. Journal of Radiation Research and Applied Sciences, 8, 148–153.
Agirtas, M. S., & Kilicel, F. (1999). Determination of Cu, Ni, Mn. and Zn pollution in soil at the shore of Van Lake with Flame Atomic Spectrophotmetry. Bulletin of Pure and Applied Science, 18, 45-47.
Akinloye, M. K., & Olomo, J. B. (2005). The radioactivity in some grasses in the environment of nuclear research facilities located within the OAU, Ile-Ife, Nigeria. Nigeria Journal of Physics, 17S, 219–225.
Amini, F., Jafari, A., Amini, P., & Sepasi, S. (2012). Metal ion release from fixed orthodontic appliances–An in vivo study. European Journal of Orthodontics, 34(1), 126–130. https://doi.org/10.1093/ejo/cjq181
Amusan, A. A., Ige, D. V., & Olawale, R. (2005). Characteristics of soils and crops’ uptake of metals in municipal waste dump sites in Nigeria. Journal of Human Ecology, 17(3), 167-171.
Awwad, N. S., Attallah, M. F., Ibrahim, H. A., & El Afifi, E. M. (2012). Natural gas - Extraction to end use (pp. 75–98). Publishing Company.
Bagheri, R., Bashir, H., Ahmad, J., Baig, A., & Qureshi, M. I. (2014). Effects of cadmium stress on plants. Environmental Sustainability: Concepts, Principles, Evidences and Innovations, 271-277.
Balkhair, K. S., Ashraf, M. A. (2016). Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi Journal of Biological Sciences, Volume 23, Issue 1, Supplement, S32-S44.
Canadian Council of Ministers of the Environment (2014). Canadian soil quality guidelines for the protection of environmental and human health. National Guidelines and Standards Office, Quebec. Summary table.
Caussy, D., Gochfeld, M., Gurzau, E., Neagu, C., & Ruedel, H. (2003). Lessons from case studies of metals: Investigating exposure, bioavailability, and risk. Journal of Ecotoxicology and Environmental Safety, 56, 45–51.
Chen, H., YanguoTeng, S. L., Wang, Y., & Wang, J. (2015). Contamination features and health risk of soil heavy metals in China. Journal of Science of the Total Environment, 512–513(2015), 143–153.
Cheng, J. L., Zhou, S. H. I., & Zhu, Y. W. (2007). Assessment and mapping of environmental quality in agricultural soils of Zhejiang Province, China. Journal of Environmental Sciences, 19(1), 50-54.
Du, Y., Gao, B., Zhou, H., Ju, X., Hao, H., & Yin, S. (2013). Health risk assessment of heavy metals in road dust in urban parks of Beijing, China. Procedia Environmental Sciences, 18, 299–309.
Dung, T. T. T., Cappuyns, V., Swennen, R., & KyPhung, N. (2013). From geochemical background determination to pollution assessment of heavy metals in sediments and soils. Reviews in Environmental Science and Biotechnology, 12, 335–353.
Esakku, S., Selvam, A., Joseph, K., & Palanivelu, K. (2005). Assessment of heavy metal species in decomposed municipal solid waste. Chemical Speciation & Bioavailability, 17(3), 95-102.
Esmaeili, A., Moore, F., Keshavarzi, B., Jaafarzadeh, N., & Kermani, M. (2014). A geochemical survey of heavy metals in agricultural and background soils of the Isfahan industrial zone, Iran. Catena, 121, 88-98.
European Commission (1999). Radiological protection principles concerning the natural radioactivity of building materials. Radiation protection unit. Finland: European Commission. Directorate-General Environment, Nuclear Safety, and Civil Protection.
Gbenu, S. T., Makinde, O. W., Ajenifuja, E., Olukotun, S. F., Fasasi, M. K., Balogun, F. A., & Ogundele, K. T. (2020a). Ground radiometric mapping of Ondo State Nigeria using gamma ray spectrometry and geographic information system. Ife Journal of Science, 22, 139–147.
Gbenu, S. T., Oladejo, O. F., Alayande, O., Olukotun, S. F., Fasasi, M. K., & Balogun, F. A. (2015). Assessment of radiological hazard of quarry products from southwest Nigeria. Journalof Radiation Research and Applied Sciences, 9, 20–25.
Gbenu, S. T., Oladejo, O. F., Olukotun, S. F., Makinde, O. W., Fasasi, M. K., & Balogun, F. A. (2016). Assessment of radioactivity and radiological hazards in commercial ceramic tiles used in Ife-Central, local government area of Osun State, Nigeria. Egyptian Journal of Basic and Applied Sciences., 3, 377–382.
Gbenu, S. T., Olukotun, S. F., Oladejo, O. F., Onumejor, C. A., Balogun, F. O., Ibitoye, F. I., & Fasasi, M. K. (2020). Assessment of the Elemental Concentration of Potassium, Uranium and Thorium with their Associated Radiogenic Heat Production in Granite Rocks of Southwestern Nigeria.
Getachew Demie Gebre and Habtamu Degefa Debelie (2015). Heavy metal pollution of soil around solid waste dumping sites and its impact on adjacent community: The case of Shashemane open landfill, Ethiopia. Journal of Environment and Earth Science, 5(15), 169–178.
Hair, J. F., Black, W. C., Babin, B. J., Anderson, R. E. (2014). Multivariate data analysis, Seventh Edition. Pearson Education Limited.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control: A sedimentological approach. Journal of Water Research, 14, 975.
Ideriah, T. J. K., Harry, F. O., Stanley, H. O., & Igbara, J. K. (2010). Heavy metal contamination of soils and vegetation around solid waste dumps in Port Harcourt, Nigeria. Journal of Applied Science and Environmental Management, 14(1), 101–109.
Inuyomi, S. O., Ore, O. T., & Abiodun, O. P. (2019). Evaluation of the natural radioactivity levels and radiological assessment of nuts commonly consumed in Ile-Ife, South West, Nigeria. Journal of Radiation Science and Technology, 5(2), 15–19. https://doi.org/10.11648/j.rst.20190502.12
Islam, M. S., Ahmed, M. K., Al-Mamun, M. H., & Masunaga, S. (2015). Potential ecological risk of hazardous elements in different land-use urban soils of Bangladesh. Science of Total Environment, 512–513, 94–102.
Jali, P., Pradhan, C., & Das, A. B. (2016). Effects of cadmium toxicity in plants: a review article. Journal of Biosciences, 4(12), 1074-1081.
Jibiri, N. N., Farai, I. P., & Alausa, S. K. (2007). Activity concentration of Ra-226, Th-228, and K-40 in different food crops from a high background radiation area in Bisichi Jos Plateau State, Nigeria. Radiation and Environmental Biophysics, 46, 53–59.
Kim, H. S., Seo, B. H., Owens, G., Kim, Y. N., Lee, J. H., Lee, M., & Kim, K. R. (2020). Phytoavailability-based threshold values for cadmium in soil for safer crop production. Ecotoxicology and Environmental Safety, 201, 110866.
Kushwaha, A., Hans, N., Kumar, S., & Rani, R. (2018). A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicology and Environmental Safety, 147, 1035–1045.
Luo, W., Yonglong, Lu., Giesy, J. P., Wang, T., Shi, Y., Wang, G., & Xing, Y. (2007). Effects of land use on concentrations of metals in surface soils and ecological risk around Guanting Reservoir, China. Environmental Geochemistry and Health, 29, 459–471.
Mousavi H. Zavvar & Seyedi S. R. (2011). Nettle ash as a low cost adsorbent for the removal of nickel and cadmium from wastewater. International Journal of Environmental Science & Technology, 8, 195–202
Muller, G. (1969). Index of Geoaccumulation in sediments of Rhine River. GeoJournal, 2, 108–118.
Naeem, I., Masood, N., Turan, V., & Iqbal, M. (2021). Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Journal of Ecotoxicology and Environmental Safety, 208, 111723. https://doi.org/10.1016/j.ecoenv.2020.111723
Nda-Umar, U. I., Nathaniel, G. S., Mann, A., & Yisa, J. (2012). Assessment of heavy metal species in some decomposed municipal solid wastes in Bida, Niger State. Nigeria. Advances in Analytical Chemistry, 2(1), 6–9.
Ogundele, L. T., Adejoro, I. A., & Ayeku, P. O. (2019a). Health risk assessment of heavy metals in soil samples from an abandoned industrial waste dumpsite in Ibadan, Nigeria. Environmental Monitoring and Assessment, 191(5), 1-10.
Ogundele, L. T., Ayeku, P. O., Adebayo, A. S., Olufemi, A. P., & Adejoro, I. A. (2020). Pollution Indices and Potential Ecological Risks of Heavy Metals in the Soil: A Case Study of Municipal Wastes Site in Ondo State, Southwestern, Nigeria. Polytechnica, 1-9.
Ogundele, L. T., Owoade, O. K., Hopke, P. K., & Olise, F. S. (2017). Heavy metals in industrially emitted particulate matter in Ile-Ife, Nigeria. Environmental Research, 156, 320–325.
Ogundele, L. Tunde, Oladejo, O. Felix & Akinola, A. Caleb (2019b). Concentrations, source identification, and human health risk of heavy metals in the road dust collected from busy junctions in Osogbo Southwest, Nigeria. EQA - International Journal of Environmental Quality, 38, 24–36. https://doi.org/10.6092/issn.2281-4485/9953
Ogunkunle, C. O., & Fatoba, P. O. (2013). Pollution loads and the ecological risk assessment of soil heavy metals around a mega cement factory in Southwest Nigeria. Polish Journal of Environmental Studies, 22(2), 487–493.
Ogunsola, O. J., Oluwole, A. F., Obioh, I. B., Akeredolu, F. A., Akante, O. A., & Spyrou, N. M. (1993). Analysis of suspended air particulates along some motorways in Nigeria by PIXE and EDXRF. Nuclear Instruments and Method in Physics Research B, 79, 404–407.
Oladejo, O. F., Olukotun, S. F., Ogundele, L. T., Gbenu, S. T., & Fakunle, M. A. (2020). Radiological risk assessment of naturally occurring radioactive materials (NORMS) from selected quarry sites in Edo State, South-south. Nigeria. Environmental Earth Sciences, 79, 93.
Olujimi, O., Steiner, O., & Goessler, W. (2014). Pollution indexing and health risk assessment of trace elements in indoor dust from classroom, living rooms and offices in Ogun state, Nigeria. Journal of African Earth Scinces, 101, 396–404.
Organization for Economic Cooperation and Development (1979). Exposure to radiation from the natural radioactivity in building materials. In: Report by a Group of Expert of the OECD Nuclear Energy Agency, Paris
Oyelami, A. C., Aladejana, J. A., & Agbede, O. O. (2013). Assessment of the impact of open waste dumpsites on groundwater quality: a case study of the Onibu-Eja dumpsite, southwestern Nigeria. Procedia Earth and planetary science, 7, 648-651.
Peter, O., Emmanuel, D., & Anthony, M. (2008). Assessment of institutional structures for solid waste management in Kumasi. Management of Environmental Quality an International Journal, 20(2), 106–120.
Qingjie, G., Jun, D., Yunchuan, X., Qingfei, W., & Liqiang, Y. (2008). Calculating pollution indices by heavy metals in ecological geochemisty assessment and a case study in parks of Beijing. Journal of China University of Geosciences, 19(3), 230.
Radojevic, M., Bashkin, V., & Bashkin, V. N. (2006). Practical environmental analysis: Second edition. The Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 0WF, UK.
Rahaman, M. A. (1989). Review of the basement geology of southwestern Nigeria. In C. A. Kogbe (Ed.), Geology of Nigeria (pp. 41–58). Elizabeth Publishing Co.
Rahaman, M. A. (1988). Recent advances in the study of the basement complex of Nigeria. In Precambrian Geology of Nigeria. Geological Survey of Nigeria, Kaduna South, 11–43.
Raghu, Y., Ravisankar, R., Chandrasekaran, A., Vijayagopal, P., & Venkatraman, B. (2017). Assessment of natural radioactivity and radiological hazards in building materials used in the Tiruvannamalai District, Tamilnadu, India, using a statistical approach. Journal of Taibah University for Science, 11(4), 523-533.
Rasool, B., Ramzani, P. M. A., Zubair, M., Khan, M. A., Lewińska, K., Turan, V., & Iqbal, M. (2021). Impacts of oxalic acid-activated phosphate rock and root-induced changes on Pb bioavailability in the rhizosphere and its distribution in mung bean plant. Environmental Pollution, 280, 116903.
Reimann, C., & De Caritat, P. (1998). Chemical element in the environment Springer, Berlin, Heidelberg.
Sayyed, M. I., Akman, F., Turan, V., & Araz, A. (2018). Evaluation of radiation absorption capacity of some soil samples. Radiochimica Acta. https://doi.org/10.1515/ract-2018-2996
Shahbaz, A. K., Iqbal, M., Jabbar, A., Hussain, S., & Ibrahim, M. (2018a). Assessment of nickel bioavailability through chemical extractants and red clover (Trifolium pratense L.) in an amended soil: Related changes in various parameters of red clover. Ecotoxicology and Environmental Safety, 149, 116–127.
Shahbaz, A. K., Ramzani, P. M. A., Saeed, R., Turan, V., Iqbal, M., Lewińska, K., Abbas, F., Saqib, M., Tauqeer, H. M., Iqbal, M., Fatima, M., & Rahman, M.-u. (2019). Effects of biochar and zeolite soil amendments with foliar proline spray on nickel immobilization, nutritional quality and nickel concentrations in wheat. Journal of Ecotoxicology and Environmental Safety, 173, 182–191.
Shahbaz, A. K., Lewińska, K., Iqbal, J., Ali, Q., Iqbal, M., Abbas, F., et al. (2018b). Improvement in productivity, nutritional quality, and antioxidative defense mechanisms of sunflower (Helianthus annuus L.) and maize (Zeamays L.) in nickel contaminated soil amended with different biochar and zeolite ratios. Journal of Environmental Management, 218, 256–270.
Sofuoglu, S. C., Sofuoglu, A. (2017). An exposure-risk assessment for potentially toxic elements in rice and bulgur. Environmental Geochemical Health. https://doi.org/10.1007/s10653-017-9954-1.
Taiwo, A. M., Awomeso, J. A., Taiwo, O. T., Oremodu, B. D., Akintunde, O. O., Ojo, N. O., Elegbede, O. O., Olanrewaju, H. H., & Arowolo, T. A. (2016). Assessment of health risks associated with road dusts in major traffic hotspots in Abeokuta metropolis, Ogun state, southwestern Nigeria. Stochastic Environmental Research and Risk Assessment. https://doi.org/10.1007/s00477-016-1302-y
Turan, V. (2019a). Confident performance of chitosan and pistachio shell biochar on reducing Ni bioavailability in soil and plant plus improved the soil enzymatic activities, antioxidant defense system and nutritional quality of lettuce. Journal of Ecotoxicology and Environmental Safety, 183, 109594. https://doi.org/10.1016/j.ecoenv.2019.109594
Turan, V. (2019b). Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Journal of Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.125611
Turan, V. (2021). Calcite in combination with olive pulp biochar reduces Ni mobility in soil and its distribution in chili plant. International Journal of Phytoremediation. https://doi.org/10.1080/15226514.2021.1929826
Turan, V., Khan, S. A., Mahmood-ur-Rahman, M. I., Ramzanid, P. M. A., & Fatima, M. (2018). Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Journal of Ecotoxicology and Environmental Safety, 161, 409–419.
Turan, V., Ramzani, P. M. A., Ali, Q., Abbas, F., Iqbal, M., Irum, A., & Khan, W.-u-D. (2017). Alleviation of nickel toxicity and an improvement in zinc bioavailability in sunflower seed with chitosan and biochar application in pH adjusted nickel contaminated soil. Journal of Archives of Agronomy and Soil Science. https://doi.org/10.1080/03650340.2017.1410542
Ugwu, N. U., Ranganai, R. T., Simon, R. E., & Ogubazghi, G. (2016). Geoelectric Evaluation of Groundwater Potential and Vulnerability of Overburden Aquifers at Onibu-Eja Active Open Dumpsite, Osogbo, Southwestern Nigeria. Journal of Water Resource and Protection, 8(03), 311.
United Nations Scientific Committee on the Effects of Atomic Radiation (1993). Report to General Assembly. New York: United Nations, 2, Ref 12 - 15
United Nations Scientific Committee on the Effects of Atomic Radiation (2000). Sources and effects of ionizing radiation. Report to general assembly with scientific annexes. United Nations, New York. ISBN 92–1–142238–8
WHO/FAO (World Health Organization/Food and Agriculture Organization). (2007). Joint FAO/WHO food standard programme codex alimentarius commission 13th session. Report of the Thirty Eight Session of the Codex Committee on Food Hygiene.
Van der Kuijp, T. J., Huang, L., & Cherry, C. R. (2013). Health hazards of China lead-acid battery industry; A review of its market drivers, production, processes, and health impacts. International Journal of Environmental Health Research, 12(1), 61.
Yang, Y., Li, Y., & Zhang, J. (2016). Chemical speciation of cadmium and lead and their bio availability to Cole (Brassica Campestris L) from multi-metals contaminated soil in Northwestern china. Chemical Speciation and Bioavailability, 28(1–4), 33–41.
Zhang, C., Qiao, Q., Piper, J. D., & Huang, B. (2011). Assessment of heavy metal pollution from a Fe- smelting plant in urban river sediment using environment magnetic and geochemical methods. Environmental Pollution, 159, 3057–3070.
Zheng-qi, Xu., Shi-jun, Ni., Xian-guo, T., & Cheng-jiang, Z. (2008). Calculation of Heavy Metals’ Toxicity Coefficient in the Evaluation of Potential Ecological Risk Index. Environmental Science and Technology, 31, 112–115.
Zubair, M., Ramzani, P. M. A., Rasool, B., Khan, M. A., Akhtar, I., Turan, V., & Iqbal, M. (2021). Efficacy of chitosan-coated textile waste biochar applied to Cd-polluted soil for reducing Cd mobility in soil and its distribution in moringa (Moringa oleifera L.). Journal of Environmental Management, 284, 112047.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. The preparation of materials, processing, and storage of samples were executed by Olubusayo F. Oladejo, Lasun T. Ogundele, and Mutiu A. Fakunle; gamma spectroscopic analysis was performed by Samuel O. Inuyomi and Stephen F. Olukotun. SPSS analysis was performed by Olubusayo F. Oladejo. The manuscript was first drafted by Olubusayo F. Oladejo, edited and reviewed by all the authors with constructive contributions. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oladejo, O.F., Ogundele, L.T., Inuyomi, S.O. et al. Heavy metals concentrations and naturally occurring radionuclides in soils affected by and around a solid waste dumpsite in Osogbo metropolis, Nigeria. Environ Monit Assess 193, 730 (2021). https://doi.org/10.1007/s10661-021-09480-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09480-6