Abstract
Heavy metal-polluted wetlands could be remediated by harvesting metal accumulating plants, i.e., using phytoextraction. We studied a macrophyte Phragmites australis and assessed its potential to be utilized in the phytoremediation of heavy metal-polluted wetlands, specifically in wadis in the Arabian Peninsula. We sampled six polluted wadi sites and measured Mn, Fe, Ni, Cu, Zn, Cd, and Pb concentrations in the roots, rhizomes, stems, and leaves of P. australis, as well as in sediment and water. We analyzed the correlations between different plant organs, water, and sediment, and calculated the accumulation and translocation of the metals to the plant organs. We found indications for the accumulation of Cd, Zn, and Pb into P. australis and somewhat contradictory indications for the accumulation of Cu. We suggest that P. australis is a good candidate to be utilized in the phytoremediation of heavy metal-polluted wadis in the Arabian Peninsula where the few wadis offer many valuable ecosystem services for urban citizens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is an ever-growing problem in urban environments worldwide (Hanfi et al. 2020). In wetlands, the pollution hinders drinking water supply and irrigation, and reduces the biodiversity of the ecosystems, thus needing remediation actions. Remediation using traditional excavation techniques poses great challenges in wetlands; they may have adverse effects both on the wetland ecosystem itself and its receiving water ecosystems (Ali et al. 2013). Instead of the excavation and transportation of the polluted soil, ecologically sustainable and economic in situ bioremediation techniques are needed.
Phytoremediation techniques use plants to stabilize the pollutants or to take up the pollutants from their growing environment. The latter can be called phytoextraction where certain plants accumulate pollutants in their tissues that can be harvested (Lajayer et al. 2019). One species studied commonly in water ecosystems is water hyacinth (Newete and Byrne 2016). Another species often suggested to be used in phytoextraction is a macrophyte Phragmites australis (common reed) (Rezania et al. 2019). Phragmites australis is a sub-cosmopolitan wetland species that forms large beds in shallow water. The species has been used as a bioindicator for heavy metals, and it is also known to accumulate heavy metals at least to some extent (Bonanno 2011; Giuseppe Bonanno 2013; Morari et al. 2015; Phillips et al. 2015; Salem et al. 2014; Wang and Chen 2009). The species was shown to be the most efficient among six species in accumulating mercury recently (Mbanga et al. 2019). Previously, the species has been suggested to be useful in the phytoextraction of heavy metal-polluted wetlands (G Bonanno 2011; Giuseppe Bonanno 2013; Bragato et al. 2006; Klink 2017; Rai 2009; Vymazal et al. 2007). However, a recent review of Vymazal and Březinová (2016) reveals a great variation in the results, which seem to depend on the ecosystem and the metal. Therefore, the studies are not highly generalizable. Instead, local in situ studies are needed to assess the remediation potential of the plant in question.
The Arabian Peninsula is mostly dry lacking permanent rivers. Many cities are located near so called wadis. They are seasonal wetlands, i.e., riverbeds gathering water only during rainy seasons. The wadis serve inhabitants offering recreation and irrigation water. The wadis are also important resting areas for many migratory birds. Information about the pollution in the inlands of the Arabian Peninsula indicates heavy metal contamination (Abdel-Baki et al. 2011; Al-Homaidan et al. 2011; Aldjain et al. 2011; Bounessah et al. 2001). However, information about the pollution, especially in the wadis, in the Arabian Peninsula is scarce and fragmentary. It can be assumed that oil industry and traffic, as well as poorly developed sewage and waste treatments, have in general polluted many of the wadis with heavy metals and several other pollutants. The mixture of pollutants present in wadis was one reason to choose P. australis, a species shown for its potential to remediate several pollutants (Fahid et al. 2020). Wadi Hanifa in central Saudi Arabia is a valley running 120 km from northwest to southeast and cutting the Riyadh city, the capital of Saudi Arabia. The bioindicators call for attempts to remediate the wadi areas (Al-Homaidan et al. 2011), and there indeed are governmental plans for the preservation of the wetlands in the Wadi Hanifa. However, knowledge about in situ management practices is lacking.
Our aim was to assess the possibility to use a macrophyte Phragmites australis, growing naturally on the area, in the phytoremediation of heavy metal-polluted wadis in the Arabian Peninsula. We studied the accumulation of heavy metals in P. australis by measuring the concentrations in six wadis in Riyadh and Aldawadmi. We calculated factors describing the accumulation of the metals into different plant organs, and the translocation of the metals from the belowground plant organs to the aboveground organs.
Materials and methods
Study area and sampling
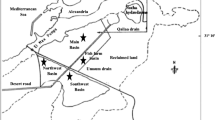
Samples were collected in March 2016 from the areas where P. australis was growing naturally. Six 100 m2 sampling sites were established. Four sites were located in the south and east of Riyadh city and two sites (Ashira valley and Sewage station) in Aldwadmi city, Saudi Arabia (Fig. 1). The sites were presumed to be polluted with heavy metals due to municipal waste and urban emissions.
The collection of plant, water, and sediment samples was performed according to Bonanno and Cirelli (2017). Three P. australis plants were randomly collected from each sampling site. The plants were divided into four parts (roots, rhizomes, stems, and leaves) and put into plastic bags separately and transferred into laboratory. The roots had been gently washed with the natural water of the site in the field, in the laboratory they were washed first with tap water and then with distilled water. Wetland water (500 ml) was sampled into three plastic bottles. Three sediment samples (500 ml) from each site were sampled. During the sampling, the climatic conditions were stable: sunny, not windy, no recent rain.
The samples were spread on filter papers and dried at 75 °C for 72 h. The dried samples were homogenized in an agate mortar, whereas sediments were passed through a sieve (2-mm diameter). Heavy metals were analyzed with the wet digestion method (Du Laing et al. 2003). Samples (0.5 g dry weight) were digested in 65% nitric acid in a microwave oven and finally filtered through 0.45 μm (Millipore). Heavy metals (Mn, Fe, Ni, Cu, Zn, Pb, and Cd) were measured using an atomic absorption spectrometer (Varian AA240FS).
Data analysis
The bioaccumulation factor (BAF) and translocation factor (TF) were calculated according to Bonanno (2013) as ratios: BAF = metal concentration in roots/metal concentration in sediment, and TF = metal concentration in a plant organ/metal concentration in roots. Pearson correlation was calculated between the plant organs, sediment, and water; r > 0.5 was considered to indicate a linear relation. One-way and two-way ANOVA were performed to study the difference between the organs and study sites. P < 0.05 was considered as significant. Necessary logarithmic transformations were done to fulfill the assumption of normality.
Results
Metal concentrations
The Mn sediment concentration varied between 691 and 265 μg/g−1 (Table 1). The respective values for Fe were 27,700–7900 μg/g−1, Ni 105–60 μg/g, Cu 130–12 μg/g−1, Zn 664–49 μg/g−1, Cd 3.5–1.2 μg/g−1, and Pb 101–25 μg/g−1. The difference among the wadis was significant for each metal.
The highest water Mn concentration was 0.29 μg/ml (Table 2). The highest concentration for Fe was 1.40 μg/ml, Ni 0.03 μg/ml, Cu 0.09 μg/ml, Zn 0.87 μg/ml, Cd 0.04 μg/ml, and Pb 0.44 μg/ml. The difference among the wadis was significant for Mn, Fe, Cu, Zn, and Pb whereas the difference was not significant for Ni and Cd.
Metal concentrations in the plants were highly variable (Table 3). In most cases, the concentrations in a plant decreased in the order of the following: roots > rhizome > leaves > stem. The single maximum concentration of Mn was 380 μg/g−1, for Fe 5700 μg/g−1, for Ni 63 μg/g−1, for Cu 140 μg/g−1, for Zn 560 μg/g−1, for Cd 4.6 μg/g−1, and for Pb 110 μg/g−1. The differences among the organs and among the study sites were mostly significant (Table 3).
Bioaccumulation, translocation, and correlations
Bioaccumulation factors indicated high variation among the metals in their accumulation into plant organs. In general, the highest bioaccumulation factors were observed for Cd. For Cd, the root to sediment relation was 1.2 indicating more Cd in the roots than in sediment dry matter (Fig. 2). Cd accumulated also in rhizome (0.9), stem (0.8), and leaves (0.7) the relations being near one. Relatively high roots to sediment relations were observed for Cu, Zn, and Pb, being 1, 0.9, and 1, respectively. The lowest organ to sediment relations was observed in general for Fe.
Translocation of the metals from roots to stems and leaves was highest for Cd (Fig. 3). The stem to roots relation was 0.7, and the leaves to roots relation was 0.6 for Cd. The relations were relatively high for Zn, being 0.7 and 0.6, respectively. The relations were the lowest for Fe (0.1).
Water metal concentrations did not correlate with the respective metal concentrations of stems, leaves, or rhizomes (r < 0.5). Water concentrations correlated only with root concentrations. Fe in roots and in water (r = 0.66) and Cu in roots and in water (r = 0.71) correlated positively (Table 4). Mn in roots and Mn water correlated negatively (r = − 0.61).
Sediment metal concentrations correlated with respective organ metal concentrations. Roots and sediment Cu correlated strongly positively (r = 0.82); Pb correlated slightly positively (r = 0.56) (Table 4). A strong positive correlation (r = 0.88) was observed for the Cu concentration between stem and sediment. A weaker correlation was observed between rhizome and sediment in the case of Cu (r = 0.59).
Metal concentration between the different organs correlated in many cases. The correlations were positive in all cases. The strongest correlations were observed between leaves and stem in the cases of Ni (r = 0.91), Zn (r = 0.73), and Cd (r = 0.68) (data not shown). Aboveground and belowground plant organs correlated variably depending on the metal. Especially, rhizome concentrations correlated with both stem and leaves concentrations. Rhizome and stem correlated in most cases, most strongly in the cases of Ni (r = 0.91) and Zn (r = 0.86). Positive correlations, although weaker, were observed for Mn, Cu, Cd, and Pb. The strongest correlations between rhizome and leaves were observed in the cases of Mn (r = 0.78), Ni (r = 0.93), and Zn (r = 0.75), and weaker correlations were observed for Cu, Cd, and Pb. Roots and stem correlated in the cases of Fe (r = 0.51) and Cu (r = 0.55). The highest correlations between roots and leaves were observed for Mn (r = 0.67) and Fe (r = 0.65) (Table 5).
Discussion
Contamination in the wadis
In general, the heavy metal concentrations in the Wadi Hanifah inside Riyadh and Aldawadmi cities were relatively high, and all sample sites can be considered more or less contaminated. Information about the contamination in the Arabian Peninsula is scarce. Now, we consider the Wadi Hanifah as Cd contaminated throughout. The Cd concentrations, which were mostly higher than 2 μg/g−1, are high compared with contaminated wetlands elsewhere (Deng et al. 2004; Obolewski et al. 2011; Scholes et al. 1999). Urban soils’ Cd contamination is mostly below 1 μg/g−1; in wastewater, the contamination may be up to 3 μg/g−1 (Khan et al. 2017). The concentrations were also higher than previously reported for Wadi Hanifah sediment (Abdel-Baki et al. 2011). The concentrations of P. australis tissues were among the highest compared with previously reported for P. australis growing in polluted wetlands, as reviewed by Vymazal and Březinová (2016). As a summary, the Wadis in Riyadh and Aldawadmi cities were contaminated with heavy metals and need remediation actions.
Accumulation
Bioaccumulation (BAF) and translocation factors (TF) have appeared to be useful in finding hyperaccumulator species, and in assessing their efficiency in phytoremediation (Ali et al. 2013; Klink et al. 2016). BAF indicate the metal transfer from soil or sediment to plant tissues when the factor is above one (Ali et al. 2013). We did not observe BAFs above one, except for Cd (roots to sediment relation). Moreover, based on the plant tissue concentrations that we observed, P. australis could not be considered as a hyperaccumulator of heavy metals in general. Only Fe and Zn concentrations exceeded the definition of a hyperaccumulator species according to Verbruggen et al. (2009).
Previous studies on P. australis have reported very high heavy metal bioaccumulation factors. A very high BAF (roots/sediment), namely eight was found for Zn and four for Ni (Klink et al. 2016). For Cd, however, the same study found BAF below one. Elsewhere, in experimental conditions, P. australis was assessed as a hyperaccumulator of Cu (Su et al. 2018). These results were different from our results; our BAFs for Zn, Ni, and Cu were below one. The results differed also in the case of Cd. We found the highest BAF for Cd, even over one. Our results are in accordance with two other studies. The accumulation of Cd into P. australis was observed in experimental conditions (Cicero-Fernández et al. (2017) and in a field (Bonanno, Vymazal, and Cirelli 2018). The contradictory results show the complexity of the issue and the need for field studies in different environmental conditions. Only few studies have even analyzed Cd, because of its low concentration, often under the detection limit.
As expected, the metal concentrations were generally highest in the roots. Previously, metal concentrations in the P. australis organs have been observed to decrease in many different orders, the root concentration being always the highest, however (G Bonanno 2011; Giuseppe Bonanno 2013; Klink 2017; Rzymski et al. 2014; Vymazal 2016; Vymazal and Březinová 2016). It also seems that the translocation depends on the metal (Giuseppe Bonanno et al. 2018). For instance, the majority of the heavy metals (Co, Cr, Cu, Fe, Mn, Ni, Zn) were largely retained in P. australis roots, while Cd and Pb were translocated to the leaves (Rzymski et al. 2014). All our TFs were below one, being highest for Zn and Cd (0.5–0.7). Our results indicate the highest translocation for Cd. The comparability of the translocation studies may be difficult and the differences might be explained by the growing phase of the plant, as suggested recently (Su et al. 2018).
The correlations between the metal concentrations in sediment, water, and P. australis organs have not been reported to any large extent previously. Bonanno (2013) and Bonanno et al. (2018) found that several metal concentrations of P. australis organs correlated positively with both sediment and water metal concentrations. We mostly found weak or moderate correlations, which were stronger between the organs and sediment than between the organs and water. The strongest correlations were observed for Cu. The stem Cu concentrations correlated also with both the roots and rhizome concentrations. On the other hand, the stem’s Cu BAF was relatively low, being only 0.2, thus, indicating an interpretation that is contradictory to that of the correlation result. Altogether, the positive correlations suggest that also Cu is transferred from sediment to aboveground plant organs. Moreover, most other metal concentrations correlated positively between rhizome and stem as well as between rhizome and leaves, although not between roots and stem. This indicates the translocation of Cu.
In comparison with our results, the values at the same, higher, or lower level have been reported (Giuseppe Bonanno 2013; Giuseppe Bonanno et al. 2018; Maddison et al. 2009; Teuchies et al. 2013). This shows that the accumulation may depend on the original sediment concentrations as well as on other complex environmental factors (Kabata-Pendias 2010; Mazumdar and Das 2015).
Implications to phytoremediation
We got indications that P. australis accumulates heavy metals to some extent, especially Cd, Zn, Pb, and Cu. Thus, we suggest that the species can be used in the phytoremediation of the heavy metal-polluted wadis in the Arabian Peninsula. Because it is highly recommended that native and endemic species instead of exotic species should be used in the phytoremediation (Leguizamo et al. 2017), we recommend that P. australis growing naturally in the wadis is used to plan practical research for the remediation actions.
All our results did not consistently prove for the accumulation of heavy metals to P. australis aboveground organs. However, as interpreted from a recent accumulation study by Vymazal (2016), the tissue concentrations or factors calculated for bioaccumulation and translocation alone do not tell the plant’s potential in phytoremediation. Our results altogether support previous views that P. australis could be used in phytoremediation, and especially in our wadis in the cases of Cd, Zn, and Pb and Cu. The utilization of P. australis is supported by recent findings that the species could be used together with bacteria to remediate areas with a mixture of pollutants (Fahid et al. 2020).
Although not a hyperaccumulator in our wadis, P. australis was able to grow with relatively high Cd concentration in its tissues. Cd is the most toxic and thus, the most hazardous contaminant. The concentrations above 5 μg g−1 are considered toxic to most plants (White and Brown 2010). Thus, the concentrations in our plants were not regarded as toxic. Taken a rough estimate about the dry biomass of P. australis leaves and stems (Vymazal 2016), it can be calculated that 1 to 12 mg of Cd per m2 could be removed using the phytoextraction by cutting and collecting the plants’ stems and leaves. For some reason, there is a high variation in the concentrations. However, the highest amounts that could be removed from the wadis can be considered remarkable.
Conclusions
Different environmental conditions probably explained largely why some of our results indicated higher, some lower, metal accumulation capacity of P. australis than previously reported. We support the previous view that P. australis is a good candidate to be utilized in the phytoremediation of wetlands. Our results indicated that the species would be efficient in phytoremediation especially in Cd-, Zn-, Cu-, and Pb-contaminated wadis in the Arabian Peninsula. Although P. australis appeared not to be a hyperaccumulator of heavy metals, it is a species growing naturally in the wadis in the Arabian Peninsula, and thus, fulfills the recommendation about the use of native over exotic species in phytoremediation (Leguizamo et al. 2017). Therefore, we recommend that practical experiments for the remediation actions should be started. We consider the wadis to be highly heavy metal contaminated. Because the big cities are lacking other recreational areas due to the extreme arid climate, the remediation actions are urgent.
References
Abdel-Baki, A. S., Dkhil, M. A., & Al-Quraishy, S. (2011). Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia. African Journal of Biotechnology, 10(13), 2541–2547.
Aldjain, I. M., Al-Whaibi, M. H., Al-Showiman, S. S., & Siddiqui, M. H. (2011). Determination of heavy metals in the fruit of date palm growing at different locations of Riyadh. Saudi Journal of Biological Sciences, 18(2), 175–180.
Al-Homaidan, A. A., Al-Ghanayem, A. A., & Alkhalifa, A. H. (2011). Green algae as bioindicators of heavy metal pollution in Wadi Hanifah Stream, Riyadh, Saudi Arabia. International Journal of Water Resources and Arid Environments, 1(1), 10–15.
Ali, H., Khan, E., & Sajad, M. A. (2013). Phytoremediation of heavy metals—concepts and applications. Chemosphere, 91(7), 869–881.
Bonanno, G. (2011). Trace element accumulation and distribution in the organs of Phragmites australis (common reed) and biomonitoring applications. Ecotoxicology and Environmental Safety, 74(4), 1057–1064.
Bonanno, G. (2013). Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicology and Environmental Safety, 97, 124–130.
Bonanno, G., & Cirelli, G. L. (2017). Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicology and Environmental Safety, 143, 92–101.
Bonanno, G., Vymazal, J., & Cirelli, G. L. (2018). Translocation, accumulation and bioindication of trace elements in wetland plants. Science of the Total Environment, 631, 252–261.
Bounessah, M., Al-Shayeb, S. M., Al-Ghefaili, K. M., & Abdulfatah, B. (2001). Assessment of lead levels in dust and date palm (Phoenix dactylifera L.) in 6-10 year-old school children environment in Riyadh City, Saudi-Arabia. Asian Journal of Chemistry, 13(4), 1435–1442.
Bragato, C., Brix, H., & Malagoli, M. (2006). Accumulation of nutrients and heavy metals in Phragmites australis (Cav.) Trin. ex Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environmental Pollution, 144(3), 967–975.
Cicero-Fernández, D., Peña-Fernández, M., Expósito-Camargo, J. A., & Antizar-Ladislao, B. (2017). Long-term (two annual cycles) phytoremediation of heavy metal-contaminated estuarine sediments by Phragmites australis. New Biotechnology, 38, 56–64.
Deng, H., Ye, Z. H., & Wong, M. H. (2004). Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environmental Pollution, 132(1), 29–40.
Du Laing, G., Tack, F. M. G., & Verloo, M. G. (2003). Performance of selected destruction methods for the determination of heavy metals in reed plants (Phragmites australis). Analytica Chimica Acta, 497(1–2), 191–198.
Fahid, M., Arslan, M., Shabir, G., Younus, S., Yasmeen, T., Rizwan, M., et al. (2020). Phragmites australis in combination with hydrocarbons degrading bacteria is a suitable option for remediation of diesel-contaminated water in floating wetlands. Chemosphere, 240, 124890.
Hanfi, M. Y., Mostafa, M. Y. A., & Zhukovsky, M. V. (2020). Heavy metal contamination in urban surface sediments: sources, distribution, contamination control, and remediation. Environmental Monitoring and Assessment, 192(1), 32.
Kabata-Pendias, A. (2010). Trace elements in soils and plants. CRC press.
Khan, M. A., Khan, S., Khan, A., & Alam, M. (2017). Soil contamination with cadmium, consequences and remediation using organic amendments. Science of the Total Environment, 601, 1591–1605.
Klink, A. (2017). A comparison of trace metal bioaccumulation and distribution in Typha latifolia and Phragmites australis: implication for phytoremediation. Environmental Science and Pollution Research, 24(4), 3843–3852.
Klink, A., Polechońska, L., Cegłowska, A., & Stankiewicz, A. (2016). Typha latifolia (broadleaf cattail) as bioindicator of different types of pollution in aquatic ecosystems—application of self-organizing feature map (neural network). Environmental Science and Pollution Research, 23(14), 14078–14086.
Lajayer, B. A., Moghadam, N. K., Maghsoodi, M. R., Ghorbanpour, M., & Kariman, K. (2019). Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environmental Science and Pollution Research, 26(9), 8468–8484.
Leguizamo, M. A. O., Gómez, W. D. F., & Sarmiento, M. C. G. (2017). Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—a review. Chemosphere, 168, 1230–1247.
Maddison, M., Soosaar, K., Mauring, T., & Mander, Ü. (2009). The biomass and nutrient and heavy metal content of cattails and reeds in wastewater treatment wetlands for the production of construction material in Estonia. Desalination, 246(1–3), 120–128.
Mazumdar, K., & Das, S. (2015). Phytoremediation of Pb, Zn, Fe, and Mg with 25 wetland plant species from a paper mill contaminated site in North East India. Environmental Science and Pollution Research, 22(1), 701–710.
Mbanga, O., Ncube, S., Tutu, H., Chimuka, L., & Cukrowska, E. (2019). Mercury accumulation and biotransportation in wetland biota affected by gold mining. Environmental Monitoring and Assessment, 191(3), 186.
Morari, F., Dal Ferro, N., & Cocco, E. (2015). Municipal wastewater treatment with Phragmites australis L. and Typha latifolia L. for irrigation reuse. Boron and heavy metals. Water, Air, & Soil Pollution, 226(3), 56.
Newete, S. W., & Byrne, M. J. (2016). The capacity of aquatic macrophytes for phytoremediation and their disposal with specific reference to water hyacinth. Environmental Science and Pollution Research, 23(11), 10630–10643.
Obolewski, K., SkorbiŁowicz, E., SkorbiŁowicz, M., Glińska-Lewczuk, K., Astel, A. M., & Strzelczak, A. (2011). The effect of metals accumulated in reed (Phragmites australis) on the structure of periphyton. Ecotoxicology and Environmental Safety, 74(4), 558–568.
Phillips, D. P., Human, L. R. D., & Adams, J. B. (2015). Wetland plants as indicators of heavy metal contamination. Marine Pollution Bulletin, 92(1–2), 227–232.
Rai, P. K. (2009). Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Critical Reviews in Environmental Science and Technology, 39(9), 697–753.
Rezania, S., Park, J., Rupani, P. F., Darajeh, N., Xu, X., & Shahrokhishahraki, R. (2019). Phytoremediation potential and control of Phragmites australis as a green phytomass: an overview. Environmental Science and Pollution Research, 26(8), 7428–7441.
Rzymski, P., Niedzielski, P., Klimaszyk, P., & Poniedziałek, B. (2014). Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environmental Monitoring and Assessment, 186(5), 3199–3212.
Salem, Z. B., Laffray, X., Ashoour, A., Ayadi, H., & Aleya, L. (2014). Metal accumulation and distribution in the organs of reeds and cattails in a constructed treatment wetland (Etueffont, France). Ecological Engineering, 64, 1–17.
Scholes, L. N. L., Shutes, R. B. E., Revitt, D. M., Purchase, D., & Forshaw, M. (1999). The removal of urban pollutants by constructed wetlands during wet weather. Water Science and Technology, 40(3), 333–340.
Su, F., Wang, T., Zhang, H., Song, Z., Feng, X., & Zhang, K. (2018). The distribution and enrichment characteristics of copper in soil and Phragmites australis of Liao River estuary wetland. Environmental Monitoring and Assessment, 190(6), 365.
Teuchies, J., Jacobs, S., Oosterlee, L., Bervoets, L., & Meire, P. (2013). Role of plants in metal cycling in a tidal wetland: implications for phytoremidiation. Science of the Total Environment, 445, 146–154.
Verbruggen, N., Hermans, C., & Schat, H. (2009). Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist, 181(4), 759–776.
Vymazal, J. (2016). Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Science of the Total Environment, 544, 495–498.
Vymazal, J., & Březinová, T. (2016). Accumulation of heavy metals in aboveground biomass of Phragmites australis in horizontal flow constructed wetlands for wastewater treatment: a review. Chemical Engineering Journal, 290, 232–242.
Vymazal, J., Švehla, J., Kröpfelová, L., & Chrastný, V. (2007). Trace metals in Phragmites australis and Phalaris arundinacea growing in constructed and natural wetlands. Science of the Total Environment, 380(1–3), 154–162.
Wang, J., & Chen, C. (2009). Biosorbents for heavy metals removal and their future. Biotechnology Advances, 27(2), 195–226.
White, P. J., & Brown, P. H. (2010). Plant nutrition for sustainable development and global health. Annals of Botany, 105(7), 1073–1080.
Funding
This work was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Homaidan, A.A., Al-Otaibi, T., El-Sheikh, M.A. et al. Accumulation of heavy metals in a macrophyte Phragmites australis: implications to phytoremediation in the Arabian Peninsula wadis. Environ Monit Assess 192, 202 (2020). https://doi.org/10.1007/s10661-020-8177-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-8177-6