Abstract
Excessive delivery of fine sediment has been implicated as the main water quality stressor in the Tsitsa River catchment. This study evaluates the responses of the taxa Ephemeroptera, Plecoptera and Trichoptera (EPT) to suspended sediment and grain size distribution in eight selected study sites in the Tsitsa River catchment. The sampling of macroinvertebrates took place seasonally from August 2016 to April 2017 and EPT identified to either genus or species level. To avoid site redundancy, the sites were clustered into four groups, with groups 1 and 2 being more impacted when compared to groups 3 and 4. The results of the sediment grain size analysis revealed that sediment grain size distribution ranged from 0.121 to 5.61 μm; percent clay and percent silt were the most dominant sediment fractions across all groups. Among the EPT metrics examined, Shannon index, Simpson’s index, evenness, Ephemeroptera abundance, EPT abundance and Trichoptera abundance were sensitive, differentiating between sediment groups 3 and 4 (less impacted groups) from 1 and 2 (highly impacted groups). Site groups 3 and 4 supported more EPT species, in terms of the number of individuals and diversity. Species such as Caenis sp., Pseudocloeon glaucum, Oligoneuropsis lawrencei and Baetis sp. were considered sediment-tolerant, displaying strong positive association with influenced groups. Taxa such as Hydropsyche sp., Pseudocloeon sp., Cheumatopsyche sp. and Afronurus sp. were considered sediment-sensitive. Overall, the present study revealed that genera/species within the order EPT were differentially sensitive to fine sediment stress and grain sizes, proving to be useful bioindicators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sedimentation of freshwater ecosystems remains a major water quality stressor of concern globally (Wilkes et al. 2017). Excessive delivery of fine sediments (grain size l < 2000 μm in diameter) into freshwater ecosystems may negatively influence ecosystem structure and function through various biophysical processes such as clogging of biological organs, filling of interstitial spaces, smothering and deterioration of the quality of food and habitats (Von Bertrab et al. 2013). The severity of fine sediment effects on aquatic organisms can be influenced by a range of factors including geochemical composition, the distribution of grain sizes, the sensitivity of the receiving environment, exposure duration, intensity and rate of deposition (Bilotta and Brazier 2008; Jones et al. 2012). For example, smaller grain size fractions (< 63 μm) are known to be more detrimental to macroinvertebrates than larger fractions (> 63 μm) because of their capacity to accumulate high concentrations of contaminants and clog fine biological structures (Bilotta and Brazier 2008; Leitner et al. 2015).
Over 70% of the land surface area in South Africa has been reported to be affected by various degrees of soil erosion (Le Roux et al. 2007; Le Roux et al. 2008; Collins et al. 2016), making most riverine systems draining such catchments prone to sedimentation. Stream sedimentation in South Africa has been aggravated by various interacting factors such as soil erosivity, steepness of slopes, flow and rainfall variabilities (Grenfell and Ellery 2009; Basson et al. 2010). The influence of elevated sediment delivery in the riverine ecosystem is more pronounced in the northern part of the Eastern Cape, where the Tsitsa River and its tributaries are situated. The river systems are composed mainly of dispersive soils that can be eroded easily during high flow and exacerbated by inappropriately managed communal livestock grazing practices, making sedimentation an important water quality stressor in the catchment (Gordon et al. 2013; Odume et al. 2018). However, little is known about how macroinvertebrates are affected by excessive sedimentation of the Tsitsa River systems.
Macroinvertebrates of the orders Ephemeroptera, Plecoptera and Trichoptera (EPT) are commonly used in biomonitoring studies because they are perceived to be sensitive to a range of aquatic stressors, and their taxonomic and functional diversities (Akamagwuna et al. 2019; Siegloch et al. 2017). Taxa within the EPT taxa have been used specifically in previous studies to assess fine sediment stress on aquatic biota, but field-based responses have been inconsistent in terms of sediment effects on taxa richness, abundance, diversities and responses of specific species (Pollard and Yuan 2010; Sutherland et al. 2012; Descloux et al. 2014). For example, taxa such as Baetis sp. have been reported in previous studies to display both negative and positive correlations to fine sediment load (Conroy et al. 2016). The inconsistent responses reported in the literature may be due to the co-occurrence of sediment stress with other aquatic stressors, thus requiring field-based studies where sedimentation is the primary stressor of water quality. In the Tsitsa River catchment, despite the fact that elevated fine sediment delivery is the primary water quality stressor and the EPT are good biological indicators, no study has characterised the responses of the EPT taxa to sediment stress, with particular emphasis on grain size distribution. The objectives of this study, therefore, are to (i) assess the responses of the EPT taxa to sediment stress and grain size distribution and (ii) identify sensitive EPT metrics to sediment stress, useful for routine biomonitoring.
Materials and methods
Study area
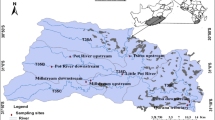
The study was carried out in five streams in the Tsitsa River catchment in the Eastern Cape Province, South Africa (Fig. 1). The five streams include the Tsitsa, millstream, Qurana, Pot and Little Pot Rivers. The Tsitsa River catchment covers an area of about 4924 km2. Average annual precipitation in the area ranges from 700 to 1000 mm, with the peak in precipitation occurring during the summer months of November to February (Midgeley et al. 1994). The landscape of the area is highly erodible, consisting of erodible Beaufort sandstones. The erodible nature of the area is exacerbated by poor land management and intensive agriculture and livestock farming. The largely rural communities that live in the catchment depend on the river for ecosystem service such as water for domestic use and for ritual purposes.
Study sites
A total of eight sites in five river systems were selected for sampling (Fig. 1). Site selection was done to reflect a gradient of sediment stress on the bases of initial site visual inspection and turbidity measurement. Sites in the Tsitsa River were sites 1 and 2, and they were considered to be highly impacted sites. They receive sediments from the surrounding area due to livestock grazing and gully erosion that were evident within the river channels and surrounding area. Site 3 was selected from the Qurana River. This site was also degraded due to gullies and cattle grazing and hence was selected as one of the highly influenced sites.
Sites selected in the Pot and Little Pot rivers were sites 4, 5 and 6. The three sites flow through a privately owned land that is protected, limiting access for livestock grazing, making them not as degraded as sites 1, 2 and 3. The three sites were considered to be less influenced; however, site 5 was considered more influenced than site 4 because it is situated downstream of the Pot River and receives sediment delivery from the upstream of the river. Sites 7 and 8 were situated in the up- and downstream of Millstream; both sites were selected as moderately influenced sites, due to less evidence of gully erosion, with main sources of sediment coming from livestock grazing and forestry. Macroinvertebrate sampling habitats were adequately represented in all sampling sites. Cluster analysis was performed on the eight study sites based on fine sediment grain sizes (Akamagwuna et al. 2019). The analysis returned four groups; the four groups formed a gradient of sediment impact from group 1 comprising sites 1 and 3 > group 2 (sites 2 and 7) > group 3 (sites 4, 5 and 8) and > group 4 (site 6). All other analyses in this paper were based on the four site groups from cluster analysis.
Environmental variables
The physical and chemical variables measured on-site at each of the sampling sites were conductivity in millisiemens/metre, dissolved oxygen (DO) in milligrams/litre, temperature (°C), turbidity in nephelometric turbidity unit (NTU) and pH. Conductivity, temperature, DO and pH were measured using the Hanna multiparameter probe, model H198. Turbidity was measured in nephelometric turbidity unit (NTU) using the portable turbidity meter, model 966. All variables were measured once per season from winter, August 2016, to autumn, April 2017.
The disturbance method was used to sample suspended sediments from the sampling spot as invertebrate according to Jones et al. (2015). Samples were collected from riffles and pools with an open-ended cylindrical polyethylene container (75 × 48.5 cm dimension). The open-ended container was gently inserted into an undisturbed patch to a depth of 10 cm. A wooden pole of 15 cm in length was used to vigorously agitate the water column for about 1 min after which fine sediment suspension was collected and filtered into a 250-ml bottle using a mesh net of 2 mm in size. Collected samples were preserved in a cooler box containing ice and taken to the laboratory for grain size characterisation. The samples were analysed for grain size characteristics using the Mastersizer 3000, model 1040, in the water quality laboratory of the Geography Department, Rhodes University (see Akamagwuna et al. 2019 for details of the grain size analysis). All samples were analysed within 48 h after each season sampling. The GRADISTAT version 8 (Blott and Pye 2012) was used to classify the output result from the Mastersizer 3000 into different grain size proportions, by applying the Folk and Ward (1957) statistics (Table 1). Grain size statistics including mean, mode, range and standard deviation were computed in micrometres (μm).
Macroinvertebrate sampling and EPT identification
Benthic macroinvertebrate samples were sampled at each study site in accordance with the South African Scoring System version 5 protocol (SASS5) (Dickens and Graham 2002). Macroinvertebrates were sampled using a 30 × 30-cm, 1000-μm net from three different habitats: stones, comprising stones in and out of current; vegetation, comprising aquatic and marginal vegetation; and sediments, comprising gravel, sand and mud. At every sampling season, samples were collected in three replicates from each of the three habitats per site. Samples were preserved in 70% alcohol and then taken to laboratory. The samples were sorted into different families, and then EPT specimens were separated. EPT specimens were further identified to their genus and, where possible, species levels using regional identification guides (De Moor et al. 2003a, 2003b). Representative specimens of the identified EPT genus/species were taken to the Albany Museum in Grahamstown for specialist confirmation.
Statistical analysis
Difference between site groups based on sediment grain size distribution
The differences between the four groups of sites in terms of water quality variables were evaluated using one-way analysis of variance (ANOVA). When statistically significant difference was confirmed by ANOVA, Tukey’s test was used to check the groups that were different. The differences in sediment grain size across the groups and seasons were assessed with two-way permutational multivariate analysis of variance (PERMANOVA) using the groups and seasons as statistical factors. Before ANOVA and PERMANOVA, Shapiro-Wilk’s and Levene’s tests were used to test the normality and homogeneity of variance in the dataset. Data were transformed logarithmically or normalised when assumptions were violated. Groups and seasons that were different when a significant difference was revealed by PERMANOVA and ANOVA were identified using the Bonferroni comparison test.
Identifying sensitive EPT metrics to sediment stress and grain size distribution
Twelve metrics in three classes: abundance (absolute number of EPT individuals), richness (number of EPT taxa observed) and composition and diversity (Table 2) were screened in order to identify metrics that are sensitive to sediment stress. Screening of metric sensitivity follows a single criterion: the metric’s potential to discriminate between groups differentially impacted by sediment stress and grain size distribution. The level of overlap of medians and inter-quartile ranges (IQRs) between the groups, retrieved through cluster analysis, was used as an indicator of metric sensitivity (Jun et al. 2012; Wang et al. 2015). Metrics whose IQRs do not overlap between the less impacted groups 3 and 4 and the highly impacted groups 1 and 2 were considered sensitive. In addition, metrics whose IQRs overlap, but whose medians were outside the IQRs, were also considered sensitive (Odume et al. 2012).
Relating EPT species and genera to grain size distribution
The canonical correspondence analysis (CCA) was used to assess the associations between sediment grain sizes and EPT taxa. The detrended correspondence analysis (DCA) was carried out to verify the gradient length of the EPT dataset before conducting CCA. Determination of the data gradient length was necessary to assess whether the dataset met the unimodal distribution requirement of a CCA. The DCA returned a gradient length > 4 standard deviations (SD) indicating that CCA was suitable. Multi-colinearity of the predictor variables was assessed using variance inflation factors (VIF). None of the VIFs tested were above 10, indicating the regression coefficients may have provided reliable estimation of the effects of all the explanatory variable in the dataset. The statistical significance of the CCA axes and sediment grain sizes was investigated using the Monte Carlo test, 1000 permutations at alpha = 0.05. CCA, DCA and VIF were undertaken using the vegan package version 2.4.3 in R software version 3.4.1 (Oksanen 2017; R Development Core team 2019).
Results
Environmental variables
The mean, standard deviation and range values of physico-chemical variables that were recorded for this study over the four seasons (winter, spring, summer and autumn) were generally low and are presented in Table 3. Conductivity was the only variable that showed a significant difference between the groups and seasons. The sediment grain size distribution recorded in this study ranged from ~ 0.121 to 5.375 μm. Grain size statistics computed, including grain size mean and range, standard deviation and proportions of each grain size within the groups, are presented in Table 4. Other grain size statistics such as standard deviation and percent fractions of each sediment class are presented in Table 4. Among the percentage fractions recorded, sand fractions were the lowest sizes recorded, with most of the site groups having less than 0.006% of this fraction. Clay was the highest grain size fraction recorded in this study, consistently high at all site groups (Table 4). The sediment grain sizes and turbidity were significantly different between the site groups and seasons (P < 0.05) (Table 4). The dry and wet seasons were significantly different (P < 0.05). Post hoc test showed that group 1 was significantly different from groups 2, 3 and 4 (P < 0.05). The dry (winter and spring) seasons differed from the wet (summer and autumn) seasons (P < 0.05) (Table 5).
EPT assemblage structure
Twenty (genera and species) of EPT were recorded during the study period (Table 6). Ephemeroptera taxa were the most abundant across all site groups, followed by Trichoptera taxa, and the least abundant taxa recorded was Plecoptera taxa. Of the 20 species and genera recorded in this study, 18 belong to the order Ephemeroptera, one genus in Plecoptera and two genera belong to the order Trichoptera. Euthraulus sp. was the most abundant genus recorded at all site groups, followed by Caenis sp. Site group 3 supported the highest number of taxa, with a total number of 30 genera/species, which were mostly observed during the dry season, whereas the least taxa richness observed was at site group 2 with a total number of 22 species and genera.
EPT taxa individuals varied temporally and spatially. A total number of 2001 taxa individuals were observed at all site groups during the study period. Of this density, site group 3 had the highest number of individuals, 697, which were mostly recorded during the wet season (443). The least number of individuals was recorded at site 2 with a total of 309 individuals. Overall, site groups 3 and 4 supported more taxa and number of richness of individuals compared to site groups 1 and 2. During the study period, the wet season seems to have supported more EPT abundance and richness species and genera than the dry season (Table 6).
EPT metrics
Of the 12 metrics examined, 6 were considered to have a satisfactory discriminatory ability, differentiating between groups 3 and 4 (less sediment-impacted site groups) and groups 1 and 2 (Fig. 2). The 6 metrics include Shannon’s and Simpson’s indexes, Ephemeroptera abundance, EPT abundance, evenness and Trichoptera abundance. Conversely, Margalef’s index, EPT richness, Ephemeroptera richness, the relative abundance of Trichoptera, Trichoptera richness and relative abundance of Ephemeroptera were considered not sensitive to fine sediment grain sizes (Fig. 3). Except for the evenness index and Ephemeroptera richness, EPT metrics that discriminated between site group 4 and the other site groups showed significant differences between the groups. Bonferroni’s post hoc tests revealed that site group 4 was different from site group 2 in terms of Shannon and Simpson’s indexes, EPT abundance and Ephemeroptera abundance and further differed from site group 3 in terms of Ephemeroptera abundance.
Relating EPT genera/species to sediment grain size distribution and turbidity
The CCA ordination results of EPT assemblage composition and the percent grain size fractions of suspended sediments and turbidity showed that the first three axes of the CCA model explained 49.69% variation in the EPT grain size relationship. Axes 1 and 2 with eigenvalues 0.23 and 0.12 explained 26.57% and 12.38% variations, respectively, and the third axis with an eigenvalue of 0.09 explained 10.73% (Table 7). The Monte Carlo unrestricted test revealed that the CCA model was statistically significant (P < 0.05) and the first CCA axis was statistically significant (F = 1.6145; P < 0.05).
The CCA result revealed that the proportions of very fine sand (F = 2.79; P < 0.05) and fine silt (F = 2.49; P < 0.05) were the statistically significant grain sizes explaining the responses of EPT taxa to suspended sediment in the CCA model. The CCA ordination plot showed that except for the proportion of very fine silt and clay, all suspended sediment grain size fractions and turbidity were negatively associated with axis 1 (Fig. 4). This axis enabled the clear separation of the dry seasons from the wet seasons, with wet seasons (summer and autumn) displaying negative associations with axis 1, with taxa such as Baetis sp., Prosopistoma amamzamanya, Pseudocloeon glaucum, Pseudocloeon vinosum, Caenis sp. and Adenophlebia auriculata indicating a strong negative association.
CCA triplot showing the correlation between suspended sediment grain size fractions including turbidity and EPT taxa during the study period (August 2016–April 2017) across site groups. Abbreviations: Acanth_sp (Acanthiops sp.), Acanth_tsi (Acanthiops tsitsa), Adeno_sp (Adenophlebia sp.), Adeno_aur (Adenophlebia auriculata), Afro_sp (Afronurus sp.), Afro_ber (Afronurus bernardi), Baet_sp (Baetis sp.), Caen_sp (Caenis sp.), Pseudo_pisc (Pseudocloeon piscis), Pseudo_glau (Pseudocloeon glaucum), Pseudo_sp (Pseudocloeon sp.), Pseudo_vin (Pseudocloeon vinosum), Apheni_sp (Aphenicera sp.), Cheum_sp (Cheumatopsyche sp.), Proso_amamz (Prosopistoma amamzamanya) and Hydrop_sp (Hydropsyche sp.). Site groups: SG1 (site group 1), SG2 (site group 2), SG3 (site group 3) and SG4 (site group 4). Seasons: wi (winter), sp (spring), su (summer) and au (autumn). Suspended sediment grain size fractions: V_fine sand (very fine sand), V_ coarse silt (very coarse silt), C_coarse silt (coarse silt), M_silt (medium silt) and F_silt (fine silt)
Hydropsyche sp., Cheumatopsyche sp., Pseudocloeon sp., Acanthiops sp. and Adenophlebia sp. were related with groups 3 and 4, especially during the dry season (winter and spring), indicating a positive association with axis 1. Grain sizes that favoured the assemblage distribution of these taxa towards the positive side of axis 1 were very fine silt and clay. Group 1 was mostly negatively associated with axis 2 and was negatively associated with all fractions of suspended sediment grain sizes except clay and turbidity. Taxa that indicated a negative association with axis 2 include Acanthiops sp., Adenophlebia auriculata and Afronurus bernardi.
Discussion
Sediment grain size characteristics
Sediment particle sizes are a critical aspect of stream substrates that can influence the assemblage distribution of macroinvertebrates (Wood and Armitage 1997; Bilotta and Brazier 2008). The results of the suspended sediment grain size distribution obtained in this study indicated that the predominant grain sizes in the Tsitsa River catchment were clay and silt grain size fractions, consistently higher than other grain sizes across the four study sites and seasons. However, percent clay and silt fractions were predominantly higher at site groups 2 and 4 than at site groups 1 and 3. The soil in the Tsitsa River erodes easily in the presence of running water, especially in the upper catchment, which may represent a significant cause of sediment input into these river systems (Pretorius and Le Roux 2016). Previous studies in the catchment have implicated suspended sediment as the main water quality stressor affecting the Tsitsa River and its tributaries (Gordon et al. 2013). For example, Madikizela and Dye (2003) who investigated the assemblage structure and distribution of macroinvertebrates in the Umzimvubu River, which the Tstsa River and its tributaries form the major sub-catchment, showed variable and high concentrations of suspended sediments in the sites investigated. The study further found that water quality variable values were largely low except for suspended solids, which are similar to the low values of water physico-chemical variables observed in this study. The low concentrations of water physico-chemical variables and varying distribution of suspended fine sediments provided a basis for assessing differences in assemblage distribution of EPT in relation to fine sediment. The high proportions of clay and silt fractions recorded in this study may have ecological implication for the distribution of EPT species as smaller grain sizes can easily penetrate and clog sensitive biological structures related to gills and filter-feeding nets (Jones et al. 2012).
Turbidity was highest at site groups 1 and 2, which may have led to the low abundance and species richness of EPT at these two site groups. Turbidity is an important water quality variable that affects all macroinvertebrate communities. Elevated turbidity levels recorded at site groups 1 and 2 could be detrimental to taxa feeding on periphyton and macrophytes, and visual impairment through elevated turbidity (Waters 1995). The results are consistent with those of a previous study by Gordon et al. (2013) who investigated the effects of sediment as a physical water quality stressor on macroinvertebrates in the Tsitsa River. The study showed that sites with high turbidity had low diversity of macroinvertebrates.
EPT assemblage structure
The result of this study showed that EPT communities were largely affected by differences in the intensity of sediment load and distribution of grain sizes in the studied river systems. Groups classified to be highly impacted by suspended sediment stress were seen to have a lower taxonomic richness and diversity. The findings of the present study are in accordance with those of previous studies by Murphy et al. (2017) and Mathers et al. (2017) who have also documented changes in the taxonomic composition of macroinvertebrate communities between sites with different intensities of sediment stress.
Spatial and temporal changes associated with hydrological regimes form a key factor in structuring the assemblages of biological communities in streams and river ecosystems (Larsen et al. 2009; Mathers et al. 2017). The results of the present study provided evidence to support the temporal assemblage variability of macroinvertebrate communities in response to fine sediments. The findings of this study revealed that fine sediment and turbidity stress had severe negative effects on the taxonomic richness and diversity of EPT taxa in the studied river systems across the study period. Kosnicki and Sites (2011) also reported temporal variability in the assemblages of macroinvertebrate communities due to fine sediment stress. Temporal responses of EPT to suspended sediments may be attributed to the seasonal changes in hydrological and rainfall regimes, with wet summer and autumn seasons having high sediment input from erosion. The Tsitsa River catchment has its peak rainfall during the summer months, which may have implications on the amount of sediments delivered into the river channel due to erosion and the consequent effect on biological communities.
Sensitivities of EPT metrics to fine sediments
The majority of EPT metrics calculated for the Tsitsa River and its tributaries differed in their sensitivity to suspended fine sediment stress. All EPT metrics in the abundance category and diversity measures including Shannon index, Simpson’s index and evenness were affected by increasing fine sediment grain size distribution, distinguishing between less sediment-impacted site groups 3 and 4 and other site groups. The majority of metrics in the composition (i.e. relative abundance) and richness were not sensitive to suspended fine sediment stress and grain size distribution. Previous studies have also reported similar results, with a significant decline in the density of certain macroinvertebrate taxa due to elevated delivery of sediment, especially those of the EPT taxa (Angradi 1999; Larsen et al. 2011). Pollard and Yuan (2010) have noted that taxa of EPT are expected to decrease in response to increased fine sediments and reduced substrate stability. In this study, EPT abundance was useful in discriminating between the site groups.
Macroinvertebrate metrics that use measures of diversity or relative composition of taxa have previously been used to discriminate between affected and unaffected sites (Buendia et al. 2013; Odume et al. 2012). In this study, the Shannon diversity index and Simpson’s index significantly decreased with an increase in fine sediment load and grain sizes. The results of the present study are in congruence with Buendia et al. (2013), who also found a significant decline of Shannon index as fine sediment increased in the Isabena streams, Central Pyrenees, UK. The low values for Shannon index and Simpson index in the impacted site groups may be an indication that fine sediment can have severe effects on EPT, especially those taxa such as Hydropsyche sp., Cheumatopsyche sp. and Adenophlebia sp. that are well known to be affected by fine sediment particles through clogging of feeding nets and gills. Overall, some of the EPT metrics proved useful in discriminating between site groups impacted, providing more evidence for their inclusion in developing a sediment-specific biomonitoring index.
Responses of EPT taxa to suspended sediment stress
A number of previous studies have found deleterious effects of sediment on macroinvertebrates, with several taxa showing species-specific responses across different sediment grain size classes (Jones et al. 2012; Wagenhoff et al. 2012). The CCA ordination showed that EPT taxa displayed taxon-specific responses to fine sediment stress, with taxa within the same order or genus showing varying responses to sediment disturbances. For example, Baetis sp., Caenis sp. and Pseudocloeon glaucum belonging to the order Ephemeroptera were positively related to the highly influenced groups 1 and 2. These taxa possess characteristics such as operculate gill and burrowing abilities that may allow them to survive in areas with increased sediment entrainment (Akamagwuna et al. 2019). In contrast, less disturbed site groups 3 and 4 were dominated with the presence of Acanthiops sp., Adenophlebia sp. and Pseudocloeon piscis including Cheumatopsyche sp. The present study suggests that EPT species/genera are differentially sensitive to sediment stress and grain size distribution, and could, therefore, be useful biological indicators.
Conclusion
In the studied river systems, elevated sediments appeared to be the primary water quality stressor. The EPT species/genera responded differentially to sediment stress and grain size distribution, and these responses were seasonally mediated. Species such as Caenis sp., Pseudocloeon glaucum, Oligoneuropsis lawrencei and Baetis sp. were considered sediment-tolerant, displaying strong positive association with sediment-influenced site groups, especially during the wet season, where sediment delivery into the rivers was relatively high, whereas EPT such as Hydropsyche sp, Pseudocloeon sp., Cheumatopsyche sp. and Afronurus sp. showed strong positive relationships with less influenced groups during the dry season. Overall, the findings of this study provided evidence to support the growing body of knowledge indicating sediment is a key water quality stressor and the differential effects of grain size on biological assemblages, particularly the EPT.
References
Akamagwuna, F. C., Mensah, P. K., Nnadozie, C. F., & Odume, O. N. (2019). Trait-based responses of Ephemeroptera, Plecoptera, and Trichoptera to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. River Research and Applications, 35(7), 777–1072. 1–14. https://doi.org/10.1002/rra.3458.
Angradi, T. R. (1999). Fine sediment and macroinvertebrate assemblages in Appalachian streams: A field experiment with biomonitoring applications. Journal of the North American Benthological Society, 18(1):49–66.
Basson GR, Rooseboom A, Msadala V, Gibson L, & Roux JLE (2010). Sediment yield prediction for South Africa: 2010 edition.
Bilotta, G. S., & Brazier, R. E. (2008). Understanding the influence of suspended solids on water quality and aquatic biota. Water Research, 42(12), 2849–2861. https://doi.org/10.1016/j.watres.2008.03.018.
Blott, S. J., & Pye, K. (2012). Particle size scales and classification of sediment types based on particle size distributions: review and recommended procedures. Sedimentology, 59(7), 2071–2096. https://doi.org/10.1111/j.1365-3091.2012.01335.x.
Buendia, C., Gibbins, C. N., Vericat, D., Batalla, R. J., & Douglas, A. (2013). Detecting the structural and functional impacts of fine sediment on stream invertebrates. Ecological Indicators, 25, 184–196. https://doi.org/10.1016/j.ecolind.2012.09.027.
Cho, W. S., Park, Y. S., Park, H. K., Kong, H. Y., & Chon, T. S. (2011). Ecological informatics approach to screening of integrity metrics based on benthic macroinvertebrates in streams. Annales de Limnologie International Journal of Limnology, 47, S51–S62.
Collins, A. L., Pulley, S., Foster, I. D. L., Gellis, A., Porto, P., & Horowitz, A. J. (2016). Sediment source fingerprinting as an aid to catchment management: a review of the current state of knowledge and a methodological decision-tree for end-users. Journal of Environmental Management, 194, 86–108. https://doi.org/10.1016/j.jenvman.2016.09.075.
Conroy, E., Turner, J. N., Rymszewicz, A., Bruen, M., Sullivan, J. J. O., & Kelly-quinn, M. (2016). An evaluation of visual and measurement-based methods for estimating deposited fine sediment. International Journal of Sediment Research, 31(4), 368–375. https://doi.org/10.1016/j.ijsrc.2016.04.002.
De Moor, I. J., Day, J. A., & de Moor, F. C. (2003a). Guides to the freshwater invertebrates of Southern Africa. Water Research Commission, 7
De Moor, I. J., Day, J. A., & de Moor, F. (2003b). Guides to the freshwater invertebrates of Southern Africa: Insecta: Ephemeroptera, Odonata and Plecoptera. Water Research Commission, 7:(Report No.TT 207/03.).
Descloux, S., Datry, T., & Usseglio-Polatera, P. (2014). Trait-based structure of invertebrates along a gradient of sediment colmation: benthos versus hyporheos responses. Science of the Total Environment, 466–467, 265–276. https://doi.org/10.1016/j.scitotenv.2013.06.082.
Dickens, C. W., & Graham, P. M. (2002). The South African Scoring System (SASS) Version 5 rapid bioassessment method for rivers. African Journal of Aquatic Science, 27(1), 1–10. https://doi.org/10.2989/16085914.2002.9626569.
Folk, R. L., & Ward, W. X. C. (1957). Brazos River Bar: a study in the significance of grain size parameters (vol. 27). Retrieved from https://pubs.geoscienceworld.org/sepm/jsedres/article-pdf/27/1/3/2802405/3.pdf
Gordon, A. K., Niedballa, J., & Palmer, G. C. (2013). Sediment as a physical water quality stressor on macro-invertebrates : a contribution to the development of a water quality guideline for suspended solids. WRC Report No. 2040/1/13.
Grenfell, S., & Ellery, W. (2009). Hydrology, sediment transport dynamics and geomorphology of a variable flow river: the Mfolozi River. South Africa, 35(3) Retrieved from http://www.wrc.org.za.
Jones, J. I., Murphy, J. F., Collins, A. L., Sear, D. A., Naden, P. S., & Armitage, P. D. (2012). The impact of fine sediment on macro-invertebrates. River Research and Applications, 28(8), 1055–1071. https://doi.org/10.1002/rra.1516.
Jones, I., Growns, I., Arnold, A., McCall, S., & Bowes, M. (2015). The effects of increased flow and fine sediment on hyporheic invertebrates and nutrients in stream mesocosms. Freshwater Biology, 60(4), 813–826. https://doi.org/10.1111/fwb.12536.
Jun, Y. C., Won, D. H., Lee, S. H., Kong, D. S., & Hwang, S. J. (2012). A multimetric benthic macroinvertebrate index for the assessment of stream biotic integrity in Korea. International Journal of Environmental Research and Public Health, 9(10), 3599–3628. https://doi.org/10.3390/ijerph9103599.
Kaller, M. D. & Hartman K. J. (2004). Evidence of a threshold level of fine sediment accumulation for altering benthic macroinvertebrate communities. Hydrobiologia, 518, 95–104.
Kosnicki, E., & Sites, R. (2011). Seasonal predictability of benthic macroinvertebrate metrics and community structure with maturity-weighed abundances in Missouri Streams, USA. Ecological Indicators, 11(2), 704–714.
Larsen, S., Vaughan, I. P., & Ormerod, S. J. (2009). Scale-dependent effects of fine sediments on temperate headwater invertebrates. Freshwater Biology, 54(1), 203–219. https://doi.org/10.1111/j.1365-2427.2008.02093.x.
Larsen, S., G. Pace, & S. J. Ormerod (2011). Experimental effects of sediment deposition on the structure and function of macroinvertebrate assemblages in temperate streams. River Research and Applications, 27, 257–267.
Le Roux, J. J., Newby, T. S., Sumner, P. D., Le Roux, J. J., Newby, T. S., & Sumner, P. D. (2007). Monitoring soil erosion in South Africa at a regional scale: review and recommendations. South African Journal of Science, 103(7–8), 329–335.
Le Roux, J., Morgenthal, T., & Malherbe, J. (2008). Water erosion prediction at a national scale for South Africa. Water SA, 34(3), 305–314 Retrieved from http://www.wrc.org.za/Knowledge Hub Documents/Water SA Journals/Manuscripts/2008/03/WaterSA_2008_03_2243.pdf.
Leitner, P., Hauer, C., Ofenböck, T., Pletterbauer, F., Schmidt-Kloiber, A., & Graf, W. (2015). Fine sediment deposition affects biodiversity and density of benthic macroinvertebrates: a case study in the freshwater pearl mussel river Waldaist (Upper Austria). Limnologica - Ecology and Management of Inland Waters, 50, 54–57. https://doi.org/10.1016/j.limno.2014.12.003.
Madikizela, B. R., & Dye, A. H. (2003). Community composition and distribution of macroinvertebrates in the Umzimvubu River, South Africa: a pre-impoundment study. BR Madikizela & AH Dye Community composition and distribution of macroinvertebrates in the Umzimvubu River. South Africa: a pre-impoundment study. African Journal of Aquatic Science, 28(2), 137–149. https://doi.org/10.2989/16085910309503778org/10.2989/16085910309503778.
Mathers, K. L., Rice, S. P., & Wood, P. J. (2017). Temporal effects of enhanced fine sediment loading on macroinvertebrate community structure and functional traits. Science of the Total Environment, 599–600, 513–522. https://doi.org/10.1016/j.scitotenv.2017.04.096.
Midgeley, D. C., Pitman, W. V. William V., Middleton, B. J., South Africa. Water Research Commission., & Water Resources 1990 Joint Venture (South Africa). (1994). Surface water resources of South Africa, 1990 (1st ed. 1994.). [Pretoria]: Water Research Commission. Retrieved from http://rhodes.worldcat.org/title/surface-water-resources-of-south-africa-1990/oclc/85953108&referer = brief_results
Murphy, J. F., Jones, J. I., Arnold, A., Duerdoth, C. P., Pretty, J. L., Naden, P. S., et al. (2017). Can macroinvertebrate biological traits indicate fine-grained sediment conditions in streams? River Research and Applications, 33(10), 1606–1617. https://doi.org/10.1002/rra.3194.
Odume, O. N., Muller, W. J., Arimoro, F. O., & Palmer, C. G. (2012). The impact of water quality deterioration on macroinvertebrate communities in the Swartkops River, South Africa: a multimetric approach. African Journal of Aquatic Science, 37(2), 191–200. https://doi.org/10.2989/16085914.2012.670613.
Odume, O., Ntloko, P., Akamagwuna, F., Dallas, H., & Barber-James, H. (2018). A trait database for South African macroinvertebrates. Water Research Commission, (K1/7157).
Oksanen, J.. (2017). Vegan: an introduction to ordination. Retrieved from https://cran.r-project.org/web/packages/vegan/vignettes/intro-vegan.pdf
Pollard, A. I., & Yuan, L. L. (2010). Assessing the consistency of response metrics of the invertebrate benthos: a comparison of trait- and identity-based measures. Freshwater Biology, 55(7), 1420–1429. https://doi.org/10.1111/j.1365-2427.2009.02235.x.
Pretorius, S. N., & Le Roux, J. J. (2016). Sediment yield modelling in the upper Tsitsa Catchment, Eastern Cape, South Africa. Retrieved from https://repository.up.ac.za/bitstream/handle/2263/60852/Pretorius_Sediment_2017.pdf?sequence = 1&isAllowed = y
R Development Core team (2019). R statistical computing environment. Vienna: R Foundation for Statistical Computing. Retrieved from http://www.r-project.org/R.
Siegloch, A. E., Schmitt, R., Spies, M., Petrucio, M., & Hernández, M. I. M. (2017). Effects of small changes in riparian forest complexity on aquatic insect bioindicators in Brazilian subtropical streams. Marine and Freshwater Research, 68(3), 519–527. https://doi.org/10.1071/MF15162.
Sutherland, A. B., Culp, J. M., & Benoy, G. A. (2012). Evaluation of deposited sediment and macroinvertebrate metrics used to quantify biological response to excessive sedimentation in agricultural streams, 50–63. https://doi.org/10.1007/s00267-012-9854-1, Evaluation of Deposited Sediment and Macroinvertebrate Metrics Used to Quantify Biological Response to Excessive Sedimentation in Agricultural Streams
Turley, M. D., Bilotta, G. S., Extence, C. A., & Brazier, R. E. (2014) Evaluation of a fine sediment biomonitoring tool across a wide range of temperate rivers and streams. Freshwater Biology, 59(11), 2268–2277
Von Bertrab, M. G., Krein, A., Stendera, S., Thielen, F., & Hering, D. (2013). Is fine sediment deposition a main driver for the composition of benthic macroinvertebrate assemblages? Ecological Indicators, 24, 589–598. https://doi.org/10.1016/j.ecolind.2012.08.001.
Wagenhoff, A., Townsend, C. R., & Matthaei, C. D. (2012). Macroinvertebrate responses along broad stressor gradients of deposited fine sediment and dissolved nutrients: a stream mesocosm experiment. Journal of Applied Ecology, 49(4), 892–902. https://doi.org/10.1111/j.1365-2664.2012.02162.x.
Wang, X., Zheng, B., Liu, L., & Wang, L. (2015). Development and evaluation of the lake multi-biotic integrity index for Dongting Lake, China. Journal of Limnology, 74(3), 1–5. https://doi.org/10.4081/jlimnol.2015.1186.
Waters, T. F. (1995). Sediment in streams. American Fisheries Society.
Wilkes, M. A., Mckenzie, M., Murphy, J. F., & Chadd, R. P. (2017). Assessing the mechanistic basis for fine sediment biomonitoring: inconsistencies among the literature, traits and indices. River Research and Applications. https://doi.org/10.1002/rra.3139.
Wood, P. J., & Armitage, P. D. (1997). Biological effects of fine sediment in the lotic environment. Environmental Management, 21(2), 203–217. https://doi.org/10.1007/s002679900019.
Acknowledgements
We thank Dr. Helen James of the Albany Museum, Grahamstown, for help with taxonomic identification.
Funding
This study was funded by the Water Research Commission (WRC) (project no.: K1/7157), the Unilever Center for Environmental Water Quality (UCEWQ), Rhodes University Research Grant and the AfriAlliance Group on IWRM and Ethics through funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 689162.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akamagwuna, F.C., Mensah, P.K., Nnadozie, C.F. et al. Evaluating the responses of taxa in the orders Ephemeroptera, Plecoptera and Trichoptera (EPT) to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. Environ Monit Assess 191, 664 (2019). https://doi.org/10.1007/s10661-019-7846-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7846-9