Abstract
The taxonomy-based response pattern of macroinvertebrates to pollution gradient is well established, with tolerant taxa increasing in impacted conditions, while sensitive taxa increase with decreasing deterioration, typical of rural pollution. This study identified rural indicator and sensitive traits of Ephemeroptera, Plecoptera and Trichoptera (EPT) taxa by examining their trait distribution pattern in relation to rural pollution. Physicochemical parameters and EPT were sampled seasonally from August 2016 to April 2017. Eight sites were selected and categorised into three site groups. Site group 1 served as the least impacted site group and Site group 2 as the moderately influenced, whereas Site group 3 was the most impacted. Seven traits were selected and categorised into 27 trait modalities. The response of EPT traits to physicochemical parameters was analysed using the simultaneous analysis of the information contained in three tables: R (environmental characteristics of samples), L (taxa distribution across samples) and Q (species traits) (RLQ) and confirmed with fourth-corner analysis. Three trait attributes, large (10–20 mm), swimming, shredding, streamlined body shape and large body size (≥ 10–20), were considered tolerant signature traits of semi-urban pollution. These trait attributes were associated with the influenced Site group 3 and indicated a significant positive affinity with at least one physicochemical indicator of increasing semi-urban pollution (NH4-N, NO3-N, NO2-N, PO4-P, EC, turbidity, temperature and pH). Conversely, small body size (< 10 mm), operculate gills, spherical body shape and a preference for sediments were correlated with the least influenced Site group 1 and were considered sensitive traits of semi-urban disturbance. Overall, this study provided critical insights into EPT responses to disturbance, revealing that semi-urban activities influenced EPT traits differently in the Tsitsa River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic disturbances in rivers and streams draining rural and urban catchments constitute a significant threat to freshwater ecosystem integrity (Genito et al., 2002; Tiecher et al., 2017). Semi-urban landscapes are dynamic and under fast transformation from rural into urban land, and rivers draining such landscapes are often characterised by pollution from poorly managed agricultural activities (e.g. cattle grazing and subsistence crop production), informal settlements, industrial advancement and a fast-growing human population (Siegloch et al., 2017). These activities deliver excessive sediments, nutrients and toxicants from fertilisers, animal manure and pesticide applications (Rubach et al., 2012), leading to deterioration in water physicochemistry and ecological integrity of freshwater systems (Cornejo et al., 2020; Gerth et al., 2017). These effects may sometimes be minimal in semi-urban catchments compared to urban rivers, and prolonged pollution from increasing activities in rivers within and around urban catchments can result in severe changes in both biological communities and ecosystem functioning of freshwater ecosystems (Díaz et al., 2008). However, little information exists regarding the effects of anthropogenic pollution on the community structure of aquatic biota in a semi-urban catchment, particularly in Africa, as most studies focus more on urban pollution (Stepenuck et al., 2002; Edegbene et al., 2020a; Odume, 2020).
Minimal anthropogenic influence and natural variability of freshwater ecosystems often co-occur in near-natural to semi-urban river systems (Gong et al., 2020; Rosenberg & Resh, 1993; Yang et al., 2020). As such, a critical challenge facing resource managers is the detection of minimal changes in water quality deterioration and unentangling the differences between anthropogenic pollution and natural variability in riverine systems, thus, hampering the abilities of most biomonitoring approaches to detect when anthropogenic pollution start to impair ecosystem structure and function of semi-urban catchments (Gieswein et al., 2019). So, it is critical to investigate the response patterns of aquatic communities to anthropogenic activities in semi-urban catchments, particularly in South Africa, where population growth and rural–semi-urban–urban migration is growing at an alarming rate and expected to reach 80% of the population by 2050 (Mlambo, 2018). Therefore, a study of this kind using a trait-based approach will stimulate the development of an effective biomonitoring strategy for semi-urban catchments and help resource managers to indicate early deterioration, which can inform appropriate mitigation strategies.
Trait-based studies based on macroinvertebrates are among the most explored aquatic biota for biomonitoring rivers (Yadamsuren et al., 2020). Anthropogenic pollution has been reported to negatively affect the distribution patterns of macroinvertebrate traits in riverine systems, with traits responding differential along stress gradients (Desrosiers et al., 2019; Ding et al., 2017; Edegbene et al., 2020a, 2021; Ntloko et al., 2021). However, trait-based studies using the whole macroinvertebrate dataset may be challenging due to the complexity of the dataset and a dearth of autecological information for most macroinvertebrate taxa, particularly Afrotropical species. For example, information on life-history traits of the majority of Afrotropical macroinvertebrates remains sparse (Akamagwuna, 2018; Edegbene et al., 2020b). Further, biomonitoring studies in Africa are confronted with resource scarcity and taxonomic expertise, limiting the majority of the trait-based studies in the region to family-level analysis (Edegbene et al., 2020a, b; Odume, 2020). These challenges result in the loss of critical ecological information, biased results and interpretation and limit the utility and realisation of the full potentials of the trait-based studies in Africa.
The Ephemeroptera, Plecoptera and Trichoptera (EPT) are the most diverse and abundant orders of macroinvertebrates in Africa and have received considerable fundamental research, both taxonomy-wise, life-history and behaviour in the continent (Akamagwuna, 2018; Barber-James & Gattolliat, 2012; Barber-James et al., 2008; Palmer, 1991; Palmer & Keeffe, 1992; Palmer et al., 1993a, b). Thus, more taxonomic and functional information is available for these taxa compared to other macroinvertebrates, particularly in South Africa (Masese & Raburu, 2017). Further, the EPTs represent the most sensitive orders of macroinvertebrates, and they can reflect minimal and severe changes in water quality deterioration, making them suitable to assess pollution impacts in a semi-urban catchment. Thus, given that traits mediate organisms’ responses to environmental condition and the EPTs are well established in Africa, we asked whether the EPTs are reliable indicators of semi-urban pollution in the Tsitsa River. We addressed this question by exploring the distribution patterns of traits in the studied riverine systems. We expected that, like taxonomy-based indicators that are well established, tolerant and sensitive signature traits of the EPTs would be identified for long-term monitoring of river and streams draining semi-urban catchments.
The Tsitsa River catchment in the Eastern Cape of South Africa is located in a semi-urban catchment and is among the few rivers in the country in their near-natural condition (Le Roux & Sumner, 2013). However, increasing pollution from nearby rural communities, informal settlements, domestic (e.g. fishing and ritual practices), subsistence and commercial agricultural activities and urban advancement continue to affect the river system (Gwapedza et al., 2020; Parwada & Tol, 2017; Waal et al., 2017). For example, the proposed multi-purpose dam and a hydropower station in the Tsitsa River by the South African Department of Environment, Forestry and Fisheries (DEFF) intended to improve the livelihood of the nearby communities are likely to stimulate rural–semi-urban migration, increasing the population and, hence, anthropogenic impacts on water quality of the river systems (Parwada & Tol, 2017). Previous studies (e.g. Akamagwuna, 2018; Van Tol et al., 2018) have observed water quality deterioration in the catchment, resulting from agricultural activities, except few physicochemistry in a relatively good ecological condition. As such, the increasing water quality deterioration can have a significant adverse effect on aquatic communities in the Tsitsa River systems as well as impacts on human society by increasing the costs of water treatment (Gwapedza et al., 2020; Van Tol et al., 2018). However, only a few studies have investigated the effect of pollution on biological communities and the ecological functioning of the river system. Therefore, ecological monitoring of the river system can help resource managers to form appropriate management strategy, bringing us closer to achieving the United Nations Sustainable Development Goals (SDGs) on the provision of sustainable clean water and livelihood (https://sdgs.un.org/goals). The objectives of this study were to (i) explore the distribution patterns of the EPT traits along a semi-urban pollution gradient in Tsitsa River and (ii) identify signature traits of EPT taxa that would serve as reliable indicators for long-term monitoring of riverine systems draining semi-urban catchments.
Materials and methods
Study area
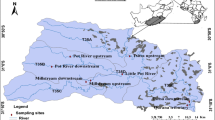
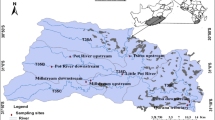
This study was conducted between upstream (S 30° 56′ 51.5″, E 28° 27′ 16.2″) and downstream (S 31° 8′ 34.69″, E 28° 40′ 26.29″) of the Tsitsa River Catchment. Eight sites in five streams, Tsitsa, Qurana tributary, Pot River, Little Pot River, and Millstream, were selected for this study (Fig. 1). The Tsitsa River is situated in the Eastern Cape Province of South Africa, with a catchment area of 4924 km2. The river flows into the river at Port St. Johns after joining the Mzimvubu River. The soil in the catchment is characterised by the highly erodible Beaufort sandstone that is made of basalt material and alluvial deposits (Akamagwuna et al., 2019). The annual rainfall ranges from 700 to 1000 mm, with peak rainfall occurring during the summer months of November to February (Akamagwuna et al., 2019). The river is an invaluable ecological asset to the communities that live around it; ecosystem services like water and aquatic resources are provided by the river system. The river serves as the most important source of water supply for subsistence agricultural activities, including livestock farming and rural settlements that depend on it for drinking and recreational activities (Van Tol et al., 2018). Vegetation in the Tsitsa River includes sub escarpment grassland and savanna bioregions, dominated by moist grasslands and Acacia sp. (Libala et al., 2020).
Land-use delineation and classification of sites
Land-use cover (%) for each site and catchment was evaluated by screening a digital land-use data map of South Africa. The South Africa land cover map is based on a simple classification system that consists of 35 land-cover classes. Land-use types were determined using ArcGis 10.2 ( Environment Systems Research Institute (ESRI), 2013). We obtained the sub-catchment area above each site, and the land-cover areas (%) were obtained for the forest, pasture, agriculture and settlements that were obtained and used to classify the sites into three site groups (Fig. 1). Rural settlements, agricultural and forest lands were the main land-use activities in the catchment. The percentage of rural and agricultural land-cover areas that drain each site was used to categorise the sites into three land-cover categories. The three categories represent a gradient of water quality and riparian deterioration conditions, which include Site group 1 (Upper and Lower pot River, and Little pot River), Site group 2 (Millstream up and down), and Site group 3 (Qurana tributary, and Tsitsa River up and down). Site groups 3 and 1 were drained by > 70% human settlements and forest lands, respectively, whereas a combination of human settlement and indigenous forests drained Site group 2 (Fig. 1). A similar method was employed by Edegbene et al. (2020a), who used selected environmental factors to classify river sites into three impact categories, namely, least impacted sites (LIS), moderately impacted sites (MIS) and heavily impacted sites (HIS). In the present study. Site group 1 was considered as the least impacted site group. The three sites within this group were mainly located in a game forest reserve and a few sparsely distributed pasture land for cattle grazing (Fig. 1). They were also situated in privately owned farmland and thus were subjected to minimal pollution from the surrounding catchment area (Fig. 1). Site group 2 was considered as moderately impacted; the sites within this group were located in an area with a high proportion of agricultural cultivated land and rural settlements particularly Millstream downstream site that is about 5 m from the biggest town (Maclear) in the catchment (Fig. 1). This may constitute the main cause of pollution in this site group. Site group 3 was considered as a highly impacted site group. This is due to the high proportion of rural settlements, agricultural and grazing land in watersheds of sites (Tsitsa up and down, and Qurana tributary) in this group that may serve as the main sources of diffuse pollution, especially nutrients and sediments (Fig. 1). All eight selected sampling sites had macroinvertebrate sampling habitats fully represented.
Physicochemical parameters
Physicochemical parameters were measured seasonally from August 2016 to April 2017, completing one hydrological season. Parameters measured on-site per site and season were dissolved oxygen (DO) (mg/l), pH, electrical conductivity (EC, mS/m), temperature (°C) and turbidity (NTU). The Hanna meter probe (model H198) was used to measure DO, EC and temperature. The portable turbidity meter (model 966) was used to measure turbidity. Water samples were collected to measure parameters, including nitrate-nitrogen (NO3-N), nitrite-nitrogen (NO2-N), orthophosphate-phosphorus (PO4-P), ammonium-nitrogen (NH4-N) and total inorganic nitrogen (TIN) in the laboratory. Water samples were analysed within 24 h of collection in the laboratory. NO3-N and NO2-N were analysed, according to the American Public Health Association (1998), using a Biotek microplate reader at 540 nm. PO4-P and NH4-N were analysed using the Merck spectroquant® phosphate and ammonium test kits, respectively. The protocol used by Odume and Mgaba (2016) was used to calculate total inorganic nitrogen (TIN) by adding the nitrogen concentrations of NO3-N, NO2-N and NH4-N.
EPT taxa
A 1000 µm handle long mesh-net was used to sample macroinvertebrates according to the South African Scoring System version 5 (SASS 5) protocol (Dickens & Graham, 2002). Kick samples of macroinvertebrates were quantitatively collected from each site from three distinct biotopes: stones, sediments, and vegetation. The stone biotope comprises stone in and out of the current, while sediments comprise gravel, sand and mud. Vegetation consists of aquatic and marginal vegetation. On each sampling occasion, macroinvertebrate samples were collected for 3 min from stones, 1 min from sediments and 2 min from vegetation biotopes. At every sampling season, three replicate samples were collected from different reaches for each biotope and preserved in 75% ethanol with a 500 ml polyethene bottle. Samples were transported to the laboratory for sorting, enumeration and identification of EPT specimens into genus or species level under dissecting microscope (X10 and X40) using keys described by De Moor et al. (2003a, b).
EPT traits
Seven traits, categorised into 27 modalities, were selected (Table 1). The seven traits are those related to body structure and physiology, such as body form, body size and gill type, behaviour (locomotion and feeding habit) and ecology and trophic features (biotope preferences and food preferences; Table 1). These traits have demonstrated responses to anthropogenic disturbances or natural environmental gradient (Kuzmanovic et al., 2017; Pease et al., 2015), and thus, they were deemed suitable for this study. Trait information was obtained primarily from available trait literature in South Africa (Day & De Moor, 2002a, b; De Moor et al., 2003b; Odume et al., 2018; Palmer et al., 1993a, 1996) and supplemented by literature information on traits from elsewhere (Merritt et al., 2008; Poff et al., 2006). Trait information was retrieved at the genus or species level. The fuzzy coding approach was used to represent the link between trait attributes and each taxon (Chevenet et al., 1994; Table S1, Supplementary Information). The method uses affinity scores that account for the possible functional differences between species within a family and developmental stages of individuals (Chevenet et al., 1994). Additionally, the fuzzy coding method solves the problem from the direct allocation of a taxon to a single trait attribute (Mondy & Usseglio-Polatera, 2014). Affinity scores of each taxa ranging from scores of 0 to 5 (i.e. 0, 1, 3 and 5) were used to describe organisms’ affinity to trait attributes. Zero affinity of a given taxon to a particular trait attribute was given a score of 0, while a score of 5 indicates the high affinity of a taxon to a given trait attribute. Low affinity was indicated by 1, and 3 indicates moderate affinity of a taxon to a given trait attribute (Chevenet et al., 1994). Each trait score was multiplied by the relative abundance of EPT taxa as earlier used by Odume (2020) and Edegbene et al. (2021).
Data analysis
Physicochemical parameters
Canonical analysis of principal coordinates (CAP; Anderson et al., 2008), based on Euclidean distance, examined the influence of physicochemical variables in structuring sites and confirmed the site classification. CAP is a variant of principal coordinates analysis (PCOA), which can find axes through the multivariate cloud of points that best discriminate among groups. Two-way multivariate analysis of variance (PERMANOVA; Anderson et al., 2008) tested the significant influence of land-cover categories and seasons on physicochemical parameters. Multiple post hoc comparison tests identified significantly different LUC and season pairs. Before PERMANOVA, Shapiro–Wilk’s and Levene’s tests in R (R Core Team, 2020) were used to test the normality and homogeneity of variance of the physicochemical dataset. CAP and PERMANOVA were conducted in Primer v6.
Exploring the spatio-temporal response patterns of EPT taxa and traits along an agricultural pollution gradient
The RLQ analysis (R, environmental characteristics of samples; L, taxa abundances across samples; Q, taxa traits) was used to examine the response patterns of EPT traits among the three site categories and seasons. The RLQ is a step-wise multivariate technique routinely conducted on three separate ordination analyses, each on three data matrices (Dolédec et al., 1996). The first ordination (correspondence analysis, COA) is conducted on the taxa abundance (L-table), the second ordination (principal component analysis, PCA) on the environmental data (R-table), and the third ordination (Hill-Smith analysis, H–S) on the trait data. The second ordination links the taxa data set to the environmental variable data using the sample score results of the CA as row weights, whereas the third ordination provides a link between the taxa and trait data using the taxon scores from the CA as row weights. Finally, RLQ analysis simultaneously conducts ordinations on the three separate analyses (CA, PCA and H–S). Monte-Carlo 999 permutations tested the statistical significance of the RLQ model. Data used for RLQ included the combined EPT taxa and environmental variable at each site, leading to taxon and variable site information for every season and fuzzy coded trait data.
Identifying potential indicator EPT traits along Semi-urban pollution gradient in the Tsitsa River
Two criteria were used to identify potential indicator semi-urban pollution traits in the Tsitsa River. First, from the RLQ ordination results, EPT traits that exhibited strong positive associations with the highly disturbed sites (Site group 3) were deemed potential tolerant traits of semi-urban pollution, and traits that strongly associated with the least disturbed sites (Site group 1) were considered sensitive traits of semi-urban pollution. Second, the fourth-corner analysis was used to confirm the potential indicator traits of semi-urban pollution traits. The fourth-corner test is a multivariate analysis that tests the relationships between multiple traits and physicochemical parameters by revealing trait attributes that either positively or negatively correlates significantly with a given physicochemical parameter. Thus, traits that were positively associated with Site group 3 on the RLQ plot and also either significantly negatively correlated with DO or positively correlated with any of nutrients (PO4-P, NH4-N, NO3-N and NO2-N), EC, turbidity, temperature and pH were confirmed as tolerant traits of semi-urban pollution. While, traits that were strongly associated with Site group 1 in the RLQ plot and also either exhibited significant positive correlations DO or negatively correlated with any of increasing nutrients (PO4-P, NH4-N, NO3-N and NO2-N), EC, turbidity, temperature and pH on the fourth-corner test were considered semi-urban pollution sensitive traits. The RLQ, fourth-corner and associated analyses (COA, PCA and Hill-Smith) were conducted using the ade4 package in R v4 (Dray & Dufour, 2007; R Core Team, 2020). The approach adopted in the present study followed similar reasoning in recent studies to identify signature traits of urban pollution (Edegbene et al., 2020a; Odume, 2020) and part of a larger project to assess water deterioration and sediment stress on the Tsitsa River (see Akamagwuna et al., 2019).
Results
EPT trait distribution patterns
The CAP analysis indicated that the physicochemical parameters differed distinctly among the three groups (Fig. 2), and the percentage classification of sites to their parent group was 50% (Tables 1 and 2). The highly impacted Site groups 3 were clearly separated from other site groups, confirming the classification of sites based on the land-use cover. Two-way PERMANOVA revealed a significant difference in physicochemical variables among the three site categories (F = 6.39, p = 0.016) but did not differ significantly among seasons (p > 0.05; Table 3).
A total of 24 taxa (genus and species) were identified and assigned to each trait attributes across three site groups and four seasons (see Supplementary information, Table S1). The RLQ analysis result revealed that axis 1 with eigenvalue of 1.6 accounted for 70.5% of the total variance in the RLQ model, indicating that axis 1 accounted for most of the variability in the analysis. The second axis with eigenvalue of 0.5 explained 20.9% variance; hence, the first two axes accounted for a cumulative variance of 91.4%, which indicates a good ordination (Table 3). Axis 1 enabled the separation of influenced Site group 3 from the moderately influenced and least impacted site group (Site group 1; Fig. 3). Traits such as burrowing, operculate gills, filter-feeding, filamentous gills, cylindrical body shape, abdominal gills, very small body size (< 5 mm) and a preference for vegetation were positively associated with Site group 1. These traits were mainly represented by the occurrence of taxa belonging to the order Trichoptera (e.g. Hydropsyche spp., Cheumatopscyhe spp., Cheumatopsyche afra and Cheumatopsyche thomasetti; Fig. 3). Conversely, traits like swimmers, streamlined body shape, grazers, clingers, shredders, detritus, a preference for stone habitat and large body size (≥ 10–20) size were positively associated with Site groups 3 and were represented by the majority of the taxa observed in the Tsitsa River, especially Baetidae family (Fig. 3).
RLQ analysis showing the distribution pattern of EPT in relation to site groups (a), traits (b), and taxa (c) based on the analysed physicochemical parameters. Abbreviations: SG1_W (site group 1, winter), SG1_SP (site group 1, spring), SG1_SU (site group 1, autumn), SG2_W (site group 2, winter), SG2_SP (site group 2, spring), SG2_SU (site group 2, summer), SG2_A (site group 2, autumn), SG3_W (site group 3, winter), SG3_SP (site group 3, spring), SG3_SU (site group 3, summer) and SG3_A (site group 3, autumn). Traits: filamentous gill (FIL_G), plate-like gill (PL_G), operculate gill (OP_G), abdominal gill (ABD_G), burrowers (BUR), crawlers (CRA), sprawlers (SPR), clingers/climbers (CLI), swimmers (SWI), detritus (FPOM, DET1), detritus (CPOM, DET2), macrophytes/algae (MAC), animal materials (AN_M), filter feeders (FI_F), grazers/scrapers (GRA), shredders (SHR), predators (PRE), small (< 5) (SIZE1), medium (> 5–10) (SIZE2), large (> 10–20) (SIZE3), stone (STO), sediment (GSM), vegetation (VEG), flattened (FLA), spherical (SPH), streamlined (STR) and cylindrical (CYL). Taxa: Acanth_sp = Acanthiops sp., Acanth_tsi = Acanthiops tsitsa, Adeno_sp = Adenophlebia sp., Adeno_aur = Adenophlebia auriculata, Afro_sp = Afronurus sp., Afro_ber = Afronurus bernardi, Baet_sp = Baetis sp., Caen_sp = Caenis sp., Pseudo_pisc = Pseudocloeon piscis, Pseudo_glau = Pseudocloeon glaucum, Pseudo_sp = Pseudocloeon sp., Pseudo_vin = Pseudocloeon vinosum, Apheni_sp = Aphanicera sp., Cheum_sp = Cheumatopsyche sp., Proso_amamz = Prosopistoma amamzamanya and Hydrop_sp = Hydropsyche sp
Of the 27 trait attributes evaluated, the fourth-corner result showed that three trait attributes, large body size (10–20 mm), streamlined body and a preference for stone biotope, indicated a significant association with at least one physicochemical parameters indicating semi-urban pollution (Fig. 4). Large body size (10–20 mm) showed a positive association with EC, and organisms with streamlined body shape were positively associated with PO4-P and TIN, and organisms having a preference for stone were positively associated with pH (p < 0.05; Fig. 4).
Results of the combined fourth-corner analysis illustrating associations between EPT trait attributes and the measured physicochemical parameters in the Tsitsa River, during the study period (August 2016–April 2017). Abbreviations: filamentous gill (FIL_G), plate-like gill (PL_G), operculate gill (OP_G), abdominal gill (ABD_G), burrowers (BUR), crawlers (CRA), sprawlers (SPR), clingers/climbers (CLI), swimmers (SWI), detritus (FPOM, DET1), detritus (CPOM, DET2), macrophytes/algae (MAC), animal materials (AN_M), filter feeders (FI_F), grazers/scrapers (GRA), shredders (SHR), predators (PRE), small (< 5), (SIZE1), medium (> 5–10) (SIZE2), large (> 10–20) (SIZE3), stone (STO), sediment (GSM), vegetation (VEG), flattened (FLA), spherical (SPH), streamlined (STR) and cylindrical (CYL)
Identifying and classifying indicator traits of semi-urban pollution
From the RLQ analysis and further correlations of traits with physicochemical variables using fourth-corner analysis showed that swimming, shredding, streamlined body shape and large body size (≥ 10–20), which were positively associated with Site group 3 on the RLQ plot, exhibited significant positive correlations with nutrients (PO4-P, NH4-N, NO3-N and NO2-N), EC, turbidity, temperature and pH (Fig. 4). Shredders significantly correlated with PO4-P and TIN, large body size (≥ 10–20) correlated with EC, whereas streamlined and stone showed significant correlations with PO4-P and pH, respectively. Thus, these traits were considered tolerant of semi-urban pollution in Tsitsa River systems. Small body size (< 10 mm), operculate gills, spherical body shape and a preference for sediments were deemed sensitive to semi-urban pollution. These traits were positively associated with Site group 1 in the RLQ plot and were significantly negatively correlated to environmental indicators of semi-urban pollution (i.e. PO4-P, NH4-N, NO3-N and NO2-N), EC, turbidity, temperature and pH; Fig. 4). Small body size (< 10 mm) indicated a positive association with EC, whereas spherical body shape and sediments were positively associated with pH.
Discussion
The identification of trait-based indicators of pollution is an invaluable step towards the development of a trait-based tool for biomonitoring of rivers draining semi-urban catchments, particularly in a region where trait-based studies are underexplored. In Africa, most rivers drain semi-urban landscape, and the majority of the existing biomonitoring studies have focused mainly on family-level macroinvertebrate taxonomic metrics (Dalu et al., 2017; Petersen et al., 2017; Agboola et al., 2019). Traits, unlike taxonomy, are less constrained by geographical and spatial differences and, thus, can be applied across wide spatial and temporal scales (Verberk et al., 2013; Voß & Schäfer, 2017). Further, trait-based indicators can provide insights into mechanisms structuring functional characteristics of aquatic biota. In the present study, we examined how semi-urban pollution affects the distribution of EPT traits, intending to identify potential indicator traits for biomonitoring of rivers draining semi-urban catchments. Traits such as swimming, streamlined body shape, large body size (≥ 10–20) and shredding were identified as potentially semi-urban pollution signature traits. These traits were more associated with the impacted Site group 3 and exhibited a significant positive correlation with at least one of the physicochemical indicators of semi-urban pollution (i.e. PO4-P, TIN, NH4-N, NO3-N and NO2-N, EC, turbidity, temperature and pH).
The association of actively swimming and streamlined body shape taxa with evidence of increasing pollution in this study is consistent with other studies (e.g. Castro et al., 2018; Shieh et al., 2012) that have found macroinvertebrates that can swim and possess streamlined body shape to increase significantly in heavily polluted sites. Contrarily, Edegbene et al. (2021) recently reported swimmers to be sensitive to urban pollution in their study conducted in selected rivers in the Niger Delta region of Nigeria and attributed the sensitivity of active swimmers to their ability to escape from impeding pollution and seek refuge. Mobility and habitat-related traits are associated with dispersal capacity, habitat utilisation and competition (Brederveld et al., 2011). For example, most active swimmers can move away from areas of increased perturbation to seek refuge and escape from predators (Buendia et al., 2013). The majority of the swimming and streamlined body shaped animals possess high body flexibility, enabling efficient habitat utilisation (Shieh et al., 2012). Further, high flexibility provides advantages to taxa in sites with a high percentage of fine sediments, enabling the use of small interstices within the bed sediments (Descloux et al., 2014; Gayraud & Philippe, 2001; Lamouroux et al., 2004). Thus, the association of swimmers and streamlined body shape with semi-urban pollution indicates that active mobility and flexible body shape can offer species resilience (Shieh et al., 2012). These explanations suggest why the majority of the streamlined and active swimming EPT taxa such as Oligoneuriopsis spp., Oligoneuriopsis lawrencei, Aphanicerca spp., Pseudocloeon glaucum and Acanthiops tsitsa were also distributed Site group 3 in the present study.
Functional feeding groups are commonly reported to exhibit high responses to anthropogenic pollution. For example, macroinvertebrate shredders are strongly affected by the nutritional quality of leaf litter as food, particularly during high sediments deposition and destruction of riparian vegetation (Ferreira et al., 2014; Masese et al., 2014; Tomanova et al., 2006). However, EPT shredders proved resilient to semi-urban pollution in the Tsitsa River, consistent with other studies that have found macroinvertebrate shredders to increasing in disturbed sites. The majority of EPT shredders belonging to Hydpropchychidae and Baetidae observed in this study can use various specialised gills and tegument for intake of oxygen, which can confer resilience on them under depleting DO. Further, most EPT shredders are generalist feeders, which can increase diversities of feeding mechanisms, hence favouring high competition for food in polluted sites. This explanation suggests the significant association of shredding with nutrient variables (PO4-P and TIN) and the strong positive association of the majority of shredding EPT taxa such as Euthrauus spp. and Aphanicerca spp. with Site group 3.
Large body size (≥ 10–20 mm) was strongly associated with Site group 3 and EC, indicating that the animals with the large body may offer species resilience to dissolved solids from semi-urban pollution. Thus, a large body was identified as a tolerant indicator trait of semi-urban pollution in the present study. Nevertheless, it has been well established that organisms with small body sizes are commonly found in environments subject to high levels of anthropogenic disturbance, while large-bodied animals are less associated with pollution (Feio & Dolédec, 2012; Kuzmanovic et al., 2017). Large body size animals usually have reduced surface-area-to-volume ratio, which can decrease contact for the uptake of dissolved salts and other toxicants such as metals (Larsen & Ormerod, 2010; Feio et al., 2015). The reduced surface-area-to-volume ratio of large body-sized animals may suggest the increased occurrence of the large body (> 40 mm) in Site group 3, and small body size (< 10 mm) being identified as a potential indicator of semi-urban pollution. The associations of large body size (≥ 10–20 mm) of EPT in the Tsitsa River are consistent with a recent study by Odume (2020), who observed macroinvertebrates with large body size (≥ 10–20) to significantly increase in heavily polluted urban sites in the Swartkops River, Eastern Cape Province of South Africa.
In addition to small body size (< 10 mm), traits such as operculate gills, spherical body shape and a preference for sediments were deemed sensitive to semi-urban pollution. These traits were considered rural sensitive traits because they indicated a strong significant negative association with at least one of the physicochemical indicators of semi-urban pollution. Morphological and respiratory traits such as spherical body shape and gills are reported to be sensitive to unstable microhabitats (Ding et al., 2017; Doretto et al., 2018; Magbanua et al., 2010). Likely, elevated sediments and acidity from fertilisers and pesticides use affected organisms that need large and stable interstitial spaces and high oxygen availability (Descloux et al., 2014; Zhang et al., 2018). Thus, potentially resulting in the filtering of specific un-adapted traits such as operculate gills, spherical body shape and preferring sediments. Gayraud and Philippe (2001) showed that the proportions of spherical organisms were negatively correlated to interstitial space availability. Further, it has been observed that at extreme acidic condition, precipitated metals may coat the stream substrate and cause unstable habitat for macroinvertebrates (MacCausland & McTammany, 2007).
Conclusion
The results of this study showed that EPT traits were differentially affected by semi-urban pollution. The EPT taxa are commonly reported to be sensitive to environmental disturbances, but recent studies have shown that certain species of the EPT can be tolerant of disturbance. The present study provided a valuable contribution to trait-based biomonitoring by providing significant insights into the responses of the EPT traits to semi-urban pollution. Traits such as swimming, streamlined body shape, large body size (≥ 10–20) and shredding were strongly associated with Site group 3 and environmental indicators (PO4-P, TIN, NH4-N, NO3-N and NO2-N, EC, turbidity, temperature and pH) of semi-urban pollution. Operculate gills, spherical body shape and a preference for sediments were associated with the least impacted sites and were sensitive to semi-urban pollution in the present study. Overall, by identifying species-level traits-based indicators, this study provided an important step towards the development of a generally applicable macroinvertebrate trait-based tool for biomonitoring of river systems draining semi-urban catchments in Africa, where such tools remain scarce.
Data availability
Data is contained within the article or Supplementary Material.
References
Agboola, O. A., Downs, C. T., & O’Brien, G. (2019). Macroinvertebrates as indicators of ecological conditions in the rivers of KwaZulu-Natal. South Africa. Ecological Indicators, 106, 105465. https://doi.org/10.1016/j.ecolind.2019.105465
Akamagwuna, F. C. (2018). Taxonomic and trait-based responses of the orders ephemeroptera, plecoptera, odonata, and trichoptera (EPOT) to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. Rhodes University, M.S.c thesis.
Akamagwuna, F. C., Mensah, P. K., Nnadozie, C. F., & Odume, O. N. (2019). Evaluating the responses of taxa in the orders Ephemeroptera, Plecoptera and Trichoptera (EPT) to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. Environmental Monitoring and Assessment, 191(11). https://doi.org/10.1007/s10661-019-7846-9
American Public Health Association. (1998). Standard methods for the examination of water and wastewater (20th edn). American Public Health Association, 1.
Anderson, M., Gorley, R. N., & Clarke, K. R. (2008). PERMANOVA + for PRIMER user manual. PRIMER-E Ltd1, 1, 1:218.
Barber-James, H. M., & Gattolliat, J. L. (2012). How well are Afrotropical mayflies known? Status of current knowledge, practical applications, and future directions. Inland Waters, 2(1), 1–9. https://doi.org/10.5268/IW-2.1.447
Barber-James, H. M., Gattolliat, J. L., Sartori, M., & Hubbard, M. D. (2008). Global diversity of mayflies (Ephemeroptera, Insecta) in freshwater. Hydrobiologia, 595(1), 339–350. https://doi.org/10.1007/s10750-007-9028-y
Brederveld, R. J., Jähnig, S. C., Lorenz, A. W., Brunzel, S., & Soons, M. B. (2011). Dispersal as a limiting factor in the colonization of restored mountain streams by plants and macroinvertebrates. Journal of Applied Ecology, 48(5), 1241–1250. https://doi.org/10.1111/j.1365-2664.2011.02026.x
Buendia, C., Gibbins, C. N., Vericat, D., Batalla, R. J., & Douglas, A. (2013). Detecting the structural and functional impacts of fine sediment on stream invertebrates. Ecological Indicators, 25, 184–196. https://doi.org/10.1016/j.ecolind.2012.09.027
de Castro, D. M. P., Dolédec, S., & Callisto, M. (2018). Land cover disturbance homogenizes aquatic insect functional structure in neotropical savanna streams. Ecological Indicators, 84, 573–582. https://doi.org/10.1016/j.ecolind.2017.09.030
Chevenet, F., Doledec, S., & Chessel, D. (1994). A fuzzy coding approach for the analysis of long-term ecological data. Freshwater Biology, 31(3), 295–309. https://doi.org/10.1111/j.1365-2427.1994.tb01742.x
Cornejo, A., Pérez, J., López-Rojo, N., Tonin, A. M., Rovira, D., Checa, B., Jaramillo, N., Correa, K., Villarreal, A., Villarreal, V., García, G., Pérez, E., Ríos González, T. A., Aguirre, Y., Correa-Araneda, F., & Boyero, L. (2020). Agriculture impairs stream ecosystem functioning in a tropical catchment. Science of the Total Environment, 745, 140950. https://doi.org/10.1016/j.scitotenv.2020.140950
Dalu, T., Wasserman, R. J., Tonkin, J. D., Alexander, M. E., Dalu, M. T. B., Motitsoe, S. N., Manungo, K. I., Bepe, O., & Dube, T. (2017). Assessing drivers of benthic macroinvertebrate community structure in African highland streams: An exploration using multivariate analysis. In Science of the Total Environment (Vols. 601–602, pp. 1340–1348). https://doi.org/10.1016/j.scitotenv.2017.06.023
Day, J. A., & de Moor, I. J. (2002a). Guides to the freshwater invertebrates of southern Africa. Volume 5: Non-arthropods (the protozoans, Porifera, Cnidaria, Platyhelminthes, Nemertea, Rotifera, Nematoda, Nematomorpha, Gastrotrichia, Bryozoa, Tardigrada, Polychaeta, Oligochaeta and Hirudinea. Water Research Commision, 5. https://doi.org/WRCReport No.TT167/02
Day, J. A., & de Moor, I. J. (2002b). Guides to the freshwater invertebrates of southern Africa. Volume 6: Arachnida and Mollusca (Araneae, Water Mites and Mollusca). Water Research Commision, 6. https://doi.org/.WRCReport No.TT182/02
De Moor, I. J., Day, J. A., & De Moor, F. C. (2003a). Guides to the freshwater invertebrates of southern Africa: Insecta: Ephemeroptera, Odonata and Plecoptera. Water Research Commision, 7. https://doi.org/(report no.TT207/03.)
De Moor, I. J., Day, J. A., & De Moor, F. C. (2003b). Guides to the freshwater invertebrates of southern Africa. Volume 8: In- secta II: Hemiptera, Megaloptera, Neuroptera, Trichoptera and Lepidoptera. Water Research Commision, 8. https://doi.org/WRCReportNo.TT214/03
Descloux, S., Datry, T., & Usseglio-Polatera, P. (2014). Trait-based structure of invertebrates along a gradient of sediment colmation: Benthos versus hyporheos responses. Science of the Total Environment, The, 466–467, 265–276. https://doi.org/10.1016/j.scitotenv.2013.06.082
Desrosiers, M., Usseglio-Polatera, P., Archaimbault, V., Larras, F., Méthot, G., & Pinel-Alloul, B. (2019). Assessing anthropogenic pressure in the St. Lawrence River using traits of benthic macroinvertebrates. Science of the Total Environment, 649, 233–246. https://doi.org/10.1016/j.scitotenv.2018.08.267
Díaz, A. M., Alonso, M. L. S., & Gutiérrez, M. R. V. A. (2008). Biological traits of stream macroinvertebrates from a semi-arid catchment: Patterns along complex environmental gradients. Freshwater Biology, 53(1), 1–21. https://doi.org/10.1111/j.1365-2427.2007.01854.x
Dickens, C. W., & Graham, P. M. (2002). The South African Scoring System (SASS) Version 5 rapid bioassessment method for Rivers. African Journal of Aquatic Science, 27(1), 1–10. https://doi.org/10.2989/16085914.2002.9626569
Ding, N., Yang, W., Zhou, Y., González-Bergonzoni, I., Zhang, J., Chen, K., Vidal, N., Jeppesen, E., Liu, Z., & Wang, B. (2017). Different responses of functional traits and diversity of stream macroinvertebrates to environmental and spatial factors in the Xishuangbanna watershed of the upper Mekong River Basin, China. Science of the Total Environment, 574, 288–299. https://doi.org/10.1016/j.scitotenv.2016.09.053
Dolédec, S., Chessel, D., ter Braak, C. J. F., & Champely, S. (1996). Matching species traits to environmental variables: A new three-table ordination method. Environmental and Ecological Statistics, 3(2), 143–166. https://doi.org/10.1007/BF02427859
Doretto, A., Piano, E., Bona, F., & Fenoglio, S. (2018). How to assess the impact of fine sediments on the macroinvertebrate communities of alpine streams? A selection of the best metrics. Ecological Indicators, 84, 60–69. https://doi.org/10.1016/j.ecolind.2017.08.041
Dray, S., & Dufour, A. B. (2007). The ade4 package: Implementing the duality diagram for ecologists. Journal of Statistical Software, 22(4), 1–20. https://doi.org/10.18637/jss.v022.i04
Edegbene, A., Arimoro, F. O., & Odume, O. N. (2020a). How does urban pollution influence macroinvertebrate traits in forested riverine systems? Water, 1, 2–17. https://doi.org/10.3390/w12113111
Edegbene, A. O., Arimoro, F. O., Odume, O. N., Ogidiaka, E., & Keke, U. N. (2021). Can macroinvertebrate traits be explored and applied in biomonitoring riverine systems draining forested catchments? Frontiers in Water, 3(607556). https://doi.org/10.3389/frwa.2021.607556
Edegbene, O., Arimoro, O., & Odume, O. (2020b). Exploring the distribution patterns of macroinvertebrate signature traits and ecological preferences and their responses to urban and agricultural pollution in selected rivers in the Niger Delta ecoregion, Nigeria. Aquatic Ecology, 9, 1–18. https://doi.org/10.1007/s10452-020-09759-9
Environment Systems Research Institute. (2013). ArcGIS Version 10. Environment Systems Research Institute Inc.
Feio, M. J., Dol Edec, S., & Graça, M. A. S. (2015). Human disturbance affects the long-term spatial synchrony of freshwater invertebrate communities. Environmental Pollution, 196, 300–308. https://doi.org/10.1016/j.envpol.2014.09.026
Feio, M. J., & Dolédec, S. (2012). Integration of invertebrate traits into predictive models for indirect assessment of stream functional integrity: A case study in Portugal. Ecological Indicators, 15(1), 236–247. https://doi.org/10.1016/j.ecolind.2011.09.039
Ferreira, W. R., Ligeiro, R., Macedo, D. R., Hughes, R. M., Kaufmann, P. R., Oliveira, L. G., & Callisto, M. (2014). Importance of environmental factors for the richness and distribution of benthic macroinvertebrates in tropical headwater streams. Freshwater Science, 33(3), 860–871. https://doi.org/10.1086/676951
Gayraud, S., & Philippe, M. (2001). Does subsurface interstitial space influence general features and morphological traits of the benthic macroinvertebrate community in streams? Archiv Fur Hydrobiologie, 151(4), 667–686. https://doi.org/10.1127/archiv-hydrobiol/151/2001/667
Genito, D., Gburek, W. J., & Sharpley, A. N. (2002). Response of stream macroinvertebrates to agricultural land cover in a small watershed. Journal of Freshwater Ecology, 17(1), 109–119. https://doi.org/10.1080/02705060.2002.9663874
Gerth, W. J., Li, J., & Giannico, G. R. (2017). Agricultural land use and macroinvertebrate assemblages in lowland temporary streams of the Willamette Valley, Oregon, USA. Agriculture, Ecosystems & Environment, 236, 154–165. https://doi.org/10.1016/j.agee.2016.11.010
Gieswein, A., Hering, D., & Lorenz, A. W. (2019). Development and validation of a macroinvertebrate-based biomonitoring tool to assess fine sediment impact in small mountain streams. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2018.10.180
Gong, P., Liu, H., Cai, C., Wang, G., Xin, Y., & Dai, X. (2020). Alkaline-thermally treated penicillin V mycelial residue amendment improved the soil properties without triggering antibiotic resistance. In Waste Management (Vol. 105, pp. 248–255). https://doi.org/10.1016/j.wasman.2020.02.008
Gwapedza, D., Nyamela, N., Hughes, D. A., Slaughter, A. R., Mantel, S. K., & van der Waal, B. (2020). Prediction of sediment yield of the Inxu River catchment (South Africa) using the MUSLE. International Soil and Water Conservation Research, . https://doi.org/10.1016/j.iswcr.2020.10.003
Kuzmanovic, M., Dolédec, S., de Castro-Catala, N., Ginebreda, A., Sabater, S., Muñoz, I., & Barceló, D. (2017). Environmental stressors as a driver of the trait composition of benthic macroinvertebrate assemblages in polluted Iberian rivers. Environmental Research, 156, 485–493. https://doi.org/10.1016/j.envres.2017.03.054
Lamouroux, N., Dolédec, S., & Gayraud, S. (2004). Biological traits of stream macroinvertebrate communities: effects of microhabitat, reach, and basin filters. Journal of the North American Benthological Society, 23(3), 449–466. https://doi.org/10.1899/0887-3593(2004)023%3c0449:btosmc%3e2.0.co;2
Larsen, S., & Ormerod, S. J. (2010). Low-level effects of inert sediments on temperate stream invertebrates. Freshwater Biology, 55(2), 476–486. https://doi.org/10.1111/j.1365-2427.2009.02282.x
Le Roux, J. J., & Sumner, P. D. (2013). Water erosion risk assessment in South Africa: A proposed methodological framework. Swedish Society for Anthropology and Geography, 95(4), 323–336. https://doi.org/10.1111/geoa.12018
Libala, N., Palmer, C. G., & Odume, O. N. (2020). Using a trait-based approach for assessing the vulnerability and resilience of hillslope seep wetland vegetation cover to disturbances in the Tsitsa River catchment, Eastern Cape. South Africa. Ecology and Evolution, 10(1), 277–291. https://doi.org/10.1002/ece3.5893
MacCausland, A., & McTammany, M. E. (2007). The impact of episodic coal mine drainage pollution on benthic macroinvertebrates in streams in the Anthracite region of Pennsylvania. Environmental Pollution, 149(2), 216–226. https://doi.org/10.1016/j.envpol.2006.12.030
Magbanua, F. S., Townsend, C. R., Blackwell, G. L., Phillips, N., & Matthaei, C. D. (2010). Responses of stream macroinvertebrates and ecosystem function to conventional, integrated and organic farming. Journal of Applied Ecology, 47(5), 1014–1025. https://doi.org/10.1111/j.1365-2664.2010.01859.x
Masese, F. O., & Raburu, P. O. (2017). Improving the performance of the EPT Index to accommodate multiple stressors in Afrotropical streams. African Journal of Aquatic Science, 42(3), 219–233. https://doi.org/10.2989/16085914.2017.1392282
Masese, F. O., Kitaka, N., Kipkemboi, J., Gettel, G. M., Irvine, K., & McClain, M. E. (2014). Litter processing and shredder distribution as indicators of riparian and catchment influences on ecological health of tropical streams. Ecological Indicators, 46, 23–37. https://doi.org/10.1016/j.ecolind.2014.05.032
Merritt, R. W., Cummins, K. W., & Berg, M. B. (2008). An introduction to the aquatic insects of North America. (4th ed.). Kendall/Hunt Publishing Company.
Mlambo, V. (2018). An overview of rural-urban migration in South Africa: Its causes and implications. Archives of Business Research, 6(4), 63–70. https://doi.org/10.14738/abr.64.4407
Mondy, C. P., & Usseglio-Polatera, P. (2014). Using fuzzy-coded traits to elucidate the non-random role of anthropogenic stress in the functional homogenisation of invertebrate assemblages. Freshwater Biology, 59(3), 584–600. https://doi.org/10.1111/fwb.12289
Ntloko, P., Palmer, C. G., Akamagwuna, F. C., & Odume, O. N. (2021). Exploring macroinvertebrates ecological preferences and trait-based indicators of suspended fine sediment effects in the Tsitsa River and its tributaries, Eastern Cape. South Africa. Water, 13(6), 798. https://doi.org/10.3390/w13060798
Odume, O., & Mgaba, N. (2016). statistical analysis of macroinvertebrate community assemblage structure in relation to river health assessment of an urban river, Eastern Cape, South Africa. Aquatic Ecosystem Health and Management, 4988(May 2017). https://doi.org/10.1080/14634988.2016.1255098
Odume, O. N. (2020). Searching for urban pollution signature and sensitive macroinvertebrate traits and ecological preferences in a river in the Eastern Cape of South Africa. Ecological Indicators, 108, 105759. https://doi.org/10.1016/j.ecolind.2019.105759
Odume, O., Ntloko, P., Akamagwuna, F., Dallas, H., & Barber-James, H. (2018). A trait database for South African macroinvertebrates. Water Research Commission, K1/7157.
Palmer, C. G. (1991). Benthic assemblage structure, and the feeding biology of sixteen macro invertebrate taxa from the Buffalo River, Eastern cape, South Africa. Rhodes University, Ph.D. thesis.
Palmer, C. G., Maart, B., Palmer, A. R., & O’keeffe, J. H. (1996). An assessment of macroinvertebrate functional feeding groups as water quality indicators in the Buffalo River, eastern Cape Province, South Africa. Hydrobiologia, 318, 153–164. https://springerlink.bibliotecabuap.elogim.com/content/pdf/10.1007%2Fbf00016677.pdf
Palmer, Carolyn G, & Keeffe, J. H. O. (1992). Feeding patterns of four macroinvertebrate taxa in the headwaters of the Buffalo River , eastern Cape. Hydrobiologia, 157–173.
Palmer, C. G., O’Keeffe, J., & Palmer, A. (1993). Macroinvertebrate functional feeding groups in the middle and lower reaches of the Buffalo River Eastern Cape, South Africa. II. Functional morphology and behaviour. Freshwater Biology, 29, 455–462.
Palmer, C. G., O’Keeffe, J., Palmer, A., Dunne, T., & Radloff, S. (1993). Macroinvertebrate functional feeding groups in the middle and lower reaches of the Buffalo River, Eastern Cape, South Africa. I. Dietary variability. Freshwater Biology, 29(3), 441–453. https://doi.org/10.1111/j.1365-2427.1993.tb00778.x
Parwada, C., & Tol, J. Van. (2017). Soil properties influencing erodibility of soils in the Ntabelanga area, Eastern Cape Province, South Africa. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science, 67(1), 67–76. https://doi.org/10.1080/09064710.2016.1220614
Pease, A. A., Taylor, J. M., Winemiller, K. O., King, R. S., Pease, A. A., Winemiller, Á. K. O., Taylor, J. M., & King, Á. R. S. (2015). Ecoregional, catchment, and reach-scale environmental factors shape functional-trait structure of stream fish assemblages. Hydrobiologia, 753, 265–283. https://doi.org/10.1007/s10750-015-2235-z
Petersen, C. R., Jovanovic, N. Z., Le Maitre, D. C., & Grenfell, M. C. (2017). Effects of land use change on streamflow and stream water quality of a coastal catchment. Online) = Water SA, 43(1). https://doi.org/10.4314/wsa.v43i1.16
Poff, N. L., Olden, J. D., Vieira, N. K. M., Finn, D. S., Simmons, M. P., & Kondratieff, B. C. (2006). Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. Journal of the North American Benthological Society, 25(4), 730–755. https://sites.biology.colostate.edu/poff/Public/poffpubs/Poffetal_2006_FunctionalTraitNiche_JNABS.pdf
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/R
Rosenberg, D. M., & Resh, V. H. (1993). Freshwater biomonitoring and benthic macroinvertebrates. Chapman and Hall.
Rubach, M. N., Baird, D. J., Boerwinkel, M.-C., Maund, S. J., Ivo, @bullet, @bullet, R., & Van Den Brink, P. J. (2012). Species traits as predictors for intrinsic sensitivity of aquatic invertebrates to the insecticide chlorpyrifos. https://springerlink.bibliotecabuap.elogim.com/content/pdf/10.1007/s10646-012-0962-8.pdf
Shieh, S. H., Wang, L.-K., & Hsiao, W.-F. (2012). Shifts in functional traits of aquatic insects along a subtropical stream in Taiwan. Zoological Studies. http://zoolstud.sinica.edu.tw/Journals/51.7/1051.pdf
Siegloch, A. E., Schmitt, R., Spies, M., Petrucio, M., & Hernandez, M. I. M. (2017). Effects of small changes in riparian forest complexity on aquatic insect bioindicators in Brazilian subtropical streams. Marine and Freshwater Research, 68(3), 519–527. https://doi.org/10.1071/MF15162
Stepenuck, K. F., Crunkilton, R. L., & Wang, L. (2002). Impacts of urban landuse on macroinvertebrate communities in southeastern Wisconsin streams. Journal of the American Water Resources Association, 38(4), 1041–1051. https://doi.org/10.1111/j.1752-1688.2002.tb05544.x
Tiecher, T., Minella, J. P. G., Caner, L., Evrard, O., Zafar, M., Capoane, V., Le Gall, M., & dos Santos, D. R. (2017). Quantifying land use contributions to suspended sediment in a large cultivated catchment of Southern Brazil (Guaporé River, Rio Grande do Sul). Agriculture, Ecosystems and Environment, 237, 95–108. https://doi.org/10.1016/j.agee.2016.12.004
Tomanova, S., Goitia, E., & Helesˇic, J. H. (2006). Trophic levels and functional feeding groups of macroinvertebrates in neotropical streams. Hydrobiologia, 556(3), 251–264. https://doi.org/10.1007/s10750-005-1255-5
van der Waal, B., Rowntree, K., Roux, J., Buckle, J., Biggs, H., Braack, M., Kawa, M., Wolff, M., Palmer, T., Sisitka, L., Powell, M., Clark, R., Ntshudu, M., Mtati, N., Tol, J. Van, & Zijl, G. Van. (2017). THE TSITSA PROJECT Restoration and sustainable land management plan infrastructure for improved livelihoods and futures. Department of Environmental Affairs, 5.
Van Tol, J. J., Akpan, W., Maroyi, A., Mutengwende, N., Huchermeyer, N., Ngesi, S., Nqandeka, H. M., Mamera, M., Bradley, G., & Rowntree, K. M. (2018). The Mzimvubu Water Project: Baseline indicators for long-term impact monitoring. In WRC project No. K5/2433 (Issue 2433).
Verberk, W. C. E. P., van Noordwijk, C. G. E., & Hildrew, A. G. (2013). Delivering on a promise: Integrating species traits to transform descriptive community ecology into a predictive science. Freshwater Science, 32(2), 531–547. https://doi.org/10.1899/12-092.1
Voß, K., & Schäfer, R. B. (2017). Taxonomic and functional diversity of stream invertebrates along an environmental stress gradient. Ecological Indicators, 81, 235–242. https://doi.org/10.1016/j.ecolind.2017.05.072
Yadamsuren, O., Morse, J. C., Hayford, B., Gelhaus, J. K., & Adler, P. H. (2020). Macroinvertebrate community responses to land use: A trait-based approach for freshwater biomonitoring in Mongolia. Hydrobiologia, 847(8), 1887–1902. https://doi.org/10.1007/s10750-020-04220-2
Yang, Y., Yi, Y., Zhou, Y., Wang, X., Zhang, S., & Yang, Z. (2020). Spatio-temporal variations of benthic macroinvertebrates and the driving environmental variables in a shallow lake. Ecological Indicators, 110(November 2019), 105948. https://doi.org/10.1016/j.ecolind.2019.105948
Zhang, Y., Cheng, L., Tolonen, K. E., Yin, H., Gao, J., Zhang, Z., Li, K., & Cai, Y. (2018). Substrate degradation and nutrient enrichment structuring macroinvertebrate assemblages in agriculturally dominated Lake Chaohu Basins, China. Science of the Total Environment, 627, 57–66. https://doi.org/10.1016/j.scitotenv.2018.01.232
Acknowledgements
We acknowledge the Water Research Commission of South Africa for funding this project under project no. K1/7157. The Rhodes University Council is also acknowledged for partial funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akamagwuna, F.C., Ntloko, P., Edegbene, A.O. et al. Are Ephemeroptera, Plecoptera and Trichoptera traits reliable indicators of semi-urban pollution in the Tsitsa River, Eastern Cape Province of South Africa?. Environ Monit Assess 193, 309 (2021). https://doi.org/10.1007/s10661-021-09093-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09093-z