Abstract
This study of Zerveli stream, Kastamonu, aims to present an assessment of its water quality and to determine the basic factors having a significant effect on the water to identify how these factors account for variations in water quality. Samples of surface water were obtained on a monthly basis between December 2016 and November 2017 at 11 stations along the watercourse. According to these samples, 28 different water quality parameters determining the water quality were evaluated. The results were assessed with respect to the environmental water quality and irrigation water quality. For this purpose, the water quality index (WQI), sodium absorption rate (SAR), sodium percentage (%Na), and residual sodium carbonate (RSC) parameters were calculated. WQI values within the year ranged between 17.26 (excellent) and 223.05 (very poor). Based on the monthly mean values, the water quality was found to be good in December, February, July, and August and poor in the remaining months. Water quality tended to deteriorate the greater the distance from the water source. According to factor analysis (FA), salinity, pH, temperature (T), electrical conductivity (EC), suspended solid matter (SSM), chemical oxygen demand (COD), biochemical oxygen demand (BOD), SO42−, SO32−, NO2−-N, NO−3-N, NH+4-N, and Cl are the main variables responsible for changes in the ecosystem. According to analysis of the irrigation water quality, the stream was found to be suitable for irrigation in terms of SAR (1.07–3.25) and %Na (37.58–61.89) but problematic in terms of RSC (3.80–5.58) values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing human population creates a greater need for water, and the gradual decrease in clean fresh water sources will become a serious problem in the future. A growing population increases the need for clean water resources, while at the same time it is one of the most important reasons for water pollution. For example, irrigation is a prerequisite for increasing, protecting, and diversifying agricultural production. However, it also has adverse effects on water quality because of salinity and agrochemicals polluting the running water sources (Isidoro and Aragüés 2007). Excessive fertilization of agricultural land causes nutrient enrichment and algal growth in the water sources, which may cause mortality in fish as well as oxygen depletion (Woli et al. 2008). In addition to the need for drinking water, sectors such as heavy industry, agriculture, aquaculture, livestock husbandry, forestry, hydropower production, transportation, and recreation also use water; therefore, water of a certain quality as well as healthy river ecosystems are necessary in order to meet their demand (Berndtsson and Bengtsson 2006; Effendi 2016).
Streams are dynamic systems that may undergo natural changes because of the increase in slope or the bedrock geology. They continuously carry dissolved and particulate matter, which is natural and anthropogenic in origin, and this continuously undergoes chemical and biological changes throughout its flow (Bakan et al. 2010). In this respect, water quality management plays an important role in controlling water pollution and planning the stream basin (Sarkar and Pandey 2015). As the importance of drinking water quality and raw water for aquatic life begins to be appreciated, the need for evaluating surface water quality gains more importance (Ouyang 2005). There are different methods used in analyzing water quality; the water quality index (WQI) is the most well-known and frequently used. Since its first use in 1965 by Horton (1965), WQI has remained an important indicator in water quality analyses (Das Kangabam and Govindaraju 2017). The advantage of this method is that, by using WQI, it is possible to decipher the complex evaluations caused by the comparison of calculated variables with quality standards one by one, as a single value.
Another method used in water quality assessment is multivariate statistical analysis. Principal components analysis (PCA) and cluster analysis are also useful tools used for pollution control and water resource management (Fan et al. 2010). PCA and factor analysis (FA) are multivariate statistical techniques used for identifying important constituents and factors that explain variations in the system (Ouyang 2005). Besides this, cluster analysis (CA) also defines natural clustering patterns by making use of similar samples (Azhar et al. 2015). All of these methods facilitate the identification of common sources, establish the processes and relations between variables used in assessing water quality, and reveal the basic variables playing an important role in water quality.
Zerveli stream is within the boundaries of the Küre Mountains National Park. In this respect, the monitoring of pollution and its ecological determination are important. A multilateral assessment of the water quality was carried out in order to determine which variables have the most significant effect on water quality.
Materials and methods
Study area
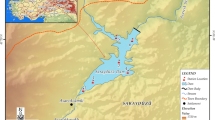
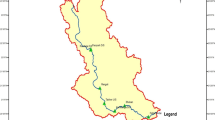
Zerveli stream (also known as Evrenye stream) is located in Kastamonu in the Black Sea Region of the Anatolian Peninsula, Turkey. Zerveli stream originates from the northern foothills of the Küre Mountains National Park extending parallel to the shore and then flows into the Black Sea (Fig. 1). The regime of the creek is irregular. Zerveli river reaches the sea by cutting through high hills. Küre Mountains National Park is one of the best examples of the Black Sea Moist Karst Forest ecosystems and is included in the 100 Forest Hotspots which need to be protected in Europe. The Küre Mountains National Park area forms part of the temperate zone forests of Northern Anatolia and the Caucasus, which host 157 endangered endemic plant species and 59 endangered plant taxa (Anonymous 2011).
Sampling and analytical methods
Surface water samples were collected between December 2016 and November 2017 from 11 stations representing the whole watercourse of the stream. Figure 1 is a map showing the locations of the sampling stations. Using 2.5-L sample bottles cleaned with 4% HCl, the water samples were taken from 15 to 20 cm below the water surface. Water samples to be used for the metal analysis were put in polyethylene bottles cleaned using 50% HNO3 and deionized water and then acidized with 10 mL HNO3/L. The samples were brought to the laboratory in a freezer and were kept refrigerated at 4 °C until analyses were carried out.
Water temperature (T), dissolved oxygen (DO), pH, chlorinity, and electrical conductivity (EC) were measured in situ by using a YSI556 MPS multimeter. Standard methods (APHA 1998) were used to analyze the COD, BOD, total hardness (TH), nitrite nitrogen (NO3−-N), ammonium nitrogen (NH4+-N), total alkalinity (TA), orthophosphate (PO43−-P), sulfite (SO32−), sulfate (SO42−), chloride (C1), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), and potassium (K+). The metal (Fe, Pb, Cd, Zn, Ni, Cu, and Hg) content of the water was measured using a Perkin Elmer Optima 2000 DV ICP-OES spectrometer. Cd, Cu, BOD, Pb, Hg, Ni, NO−3-N, NH+4-N, Zn, PO4, EC, and COD were included in the water quality index calculation. The weight of each parameter was assigned (minimum value 1, maximum value 5 [AW]). The significance of the respective parameters in terms of environmental health was taken into account in determining the weights (Horton 1965; Kangabam et al. 2017; Şener et al. 2017). Then, the total weight specified for the parameters was divided into the weights specified for each parameter in order to find the relative weight (RW) (Eq. 1).
Subsequently, the concentration (Ci) of each parameter was divided into the limit values (SWQR 2016) for the environmental quality standards (Si) designated by Turkey’s Ministry of Agriculture and Forestry to discover the quality rating (Qi) (Eq. 2).
The quality degree of each variable and RW were multiplied to obtain the water quality sub-index (SIi) (Eq. 3); then, all of the sub-indices were added to find the integrated WQI (Eq. 4). WQI was analyzed according to the criteria set by Ramakrishnaiah et al. (2009): WQI < 50, excellent; WQI = 50–100, good; WQI = 100–200, poor; WQI = 200–300, very poor; WQI > 300, not suitable.
In order to determine the irrigation water quality, the SAR, %Na, and RSC were calculated. SAR, %Na, and RSC values were calculated according to Eqs. 5, 6, and 7, respectively (Ravikumar et al. 2013). The concentrations of elements are expressed in meq/L.
To learn the relationships between the variables and to determine their sources, FA and CA were applied to the data set. The purpose of the factor analysis is to obtain a small number of factors which account for most of the variability in the 28 variables. The clusters are groups of variables having similar characteristics in CA.
The monthly changes in rainfall and air temperature were obtained from the General Directorate of Meteorology of Turkey.
Results and discussion
Temporal changes in water quality
Descriptive statistics of the variables are presented in Table 1. The temporal changes in monthly mean values are shown in Fig. 2. The annual fluctuations can be observed in several variables depending on season. The monthly changes in rainfall and air temperature are presented in Fig. 3. The DO concentration, which was higher in the winter and early spring, dropped in August–September together with the rising water temperatures. DO concentration level depends on various factors such as microbial activity, organic matter, pressure, temperature, sampling time, and flow minimization (Boskidis et al. 2010; Das Kangabam and Govindaraju 2017). The DO increase detected during periods of lower water temperature was caused by gas solubility, which increased during the lowest temperatures, and the decreased metabolic activity of organisms (Ali and Khairy 2016). The salinity values differed greatly between seasons.
The salinity, which was at low levels in December–March, rose along with the rising temperatures and reached a maximum level of 0.52 psu in August then started to fall. This rise and fall trend in salinity seems to be the effect of rain and evaporation. pH values were observed to be low during the winter and spring months. An increase that began in April reached its maximum value in October. The increase in pH was considered to be related to vitality activities, particularly the activity of photosynthetic organisms (Rostom et al. 2017; Singh et al. 2017). pH is an important variable that influences biotic composition of the system (Singh et al. 2017). Low pH causes an increase in toxic element uptake in aquatic organisms (Faragallah et al. 2009); however, the pH values of Zerveli stream were classified as excellent according to Turkey’s Agriculture and Forestry Surface Water Quality Regulations (SWQR).
The T values were compatible with general seasonal trends in atmospheric temperature; the lowest value was detected in February and the highest value in September. Although EC values somewhat increased during the summer, no significant fluctuations were detected throughout the year. High EC values are mainly related to domestic and agricultural waste, and low EC values indicate untainted environmental conditions (Kangabam et al. 2017; Sallam and Elsayed 2018). In this study, the values that increased in summer were considered to be related to increased temperatures, evaporation, and increased organic matter (Boskidis et al. 2010; Singh et al. 2017). The congruence between EC increase and BOD increase corroborates this hypothesis.
The SSM concentration, which was at low levels during the winter months, reached its minimum value in January. SSM, which began to increase consistently during the spring months, reached its maximum value in September. The SSM and rainfall data are not fully compatible. Increases in SSM concentration during months with low rainfall and high temperatures could be related to high phytoplankton levels in the SSM concentration. SSM can vary even in the same water course depending on rainfall, surface flows, and flow rate (Taşdemir and Göksu 2001). The increase in SSM concentration in the dry season may be related to evaporation and the lower water level. The spatial increase, which was analogous to the direction of flow, was the result of water erosion in the river bed.
COD values, while at very low levels in the winter season, increased in the spring months and reached their highest levels between August and October. The reasons for this were higher temperatures, biological activity, and decreased DO concentrations. The BOD values were similar to COD values. The chemicals consuming oxygen during disintegration, domestic waste, and fishery waste are responsible for the increase in BOD values (Sallam and Elsayed 2018; Zhao et al. 2012). Cl concentration, which was found to be rather regular and high between winter and the beginning of summer, was lower in the summer–autumn period. Phosphorus, which is an important element for all living beings, may cause excessive algae buildup that can harm several aquatic organisms (Bakan et al. 2010). In this regard, PO−4-P, of great importance to freshwater ecosystems, peaked three times during the year (July, October, and November). The PO−4-P concentration level was lower and more stable during the rest of the year.
SO42− and SO32− concentrations showed parallel proclivities throughout the year. Low values detected in the winter started to increase in the spring and they reached their maximum value in September. Na concentration, which began to increase in winter, reached its maximum level in June. Subsequently, it began to decrease in the following months to remain at similar levels until the end of the sampling period. K concentrations did not fluctuate significantly, except for a steep decrease in January. The increase of TH values ended in the April–June period, with a peak in June. Except for the aforementioned increase, they remained relatively stable throughout the year. TA values, which increased between April and June, fell back to their winter–spring values and, just as TH, had very low coefficient of variation values, which indicates that these variables were stable in distribution throughout the year. Mg and Ca values, which remained at low levels in winter, spring, and autumn, considerably increased in May and July.
The NO2−-N concentration, which was low in the winter season, reached its minimum value in January, then began to increase in March, reached its maximum value in July, and began to decrease again. The NO−3-N concentration, found to be at low levels in the December–April period, reached its lowest level in February. Then, NO−3-N concentration began to increase in the surface water after May and reached its maximum value in September. Following this peak, it then started to decrease. Except for small peaks in December and March, the NH+4-N concentration was found to be at low levels in the winter–spring period. With higher concentrations in May, NH+4-N reached its maximum level in July and then started to decrease in the following months. The nitrogen is thought to accumulate in water because of higher evaporation in the summer months (Rasoloariniaina 2017). Despite the partial oxidation status of NO2−-N, NH+4-N, and NO−3-N (Belal et al. 2016), it is more toxic than NO−3-N, and furthermore, high concentrations of NO−3-N cause various illnesses (Şener et al. 2017).
Primary nutrient sources in the river are agricultural-zootechnical activities and domestic and industrial discharge (Valeriani et al. 2015). Although there are settlements around the stream, NO2−-N and NO−3-N concentrations meet the water quality standards set by the World Health Organization. After falling to its minimum level in February, Fe concentration began to increase to its maximum level in May and then began to decrease and remained at stable levels until the end of the year. The concentration of Pb showed four significant peaks throughout the year and the first one was observed in January. This concentration then decreased and successively peaked again in April and May. Pb levels, which tended to decrease until September, reached their last significant peak in that month and then continued to decrease until the end of the sampling period.
Cu concentration, which was stable in the December–March period, reached its highest values in April and May. Following these rises, the Cu levels decreased gradually until October but then rose again in October and November. Except for their increase in September, October, and November, the concentrations of Cd remained stable throughout the year. Hg values, which were stable between December and February, started to rise in March and were calculated to be relatively higher in March through June. The concentration of Hg, which increased constantly until mid-autumn, reached its highest level in October. Ni concentration was at its highest level between March and June, and there were small fluctuations in the other months. Zn concentration, on the other hand, which showed a tendency to increase between winter and spring, reached its maximum level in May and then began to decrease at the beginning of summer.
Spatial changes in water quality
The spatial distribution patterns based on annual averages of variables are presented in Fig. 4. DO concentration was at a relatively low level in first station and maximum in second station. From this station to the last station (in a seaward direction), the DO values decreased. Higher oxygen levels are expected at locations closer to springs and lower DO levels away from rivers. The salinity values increased between the first station and the last one. This is because of the increase in salinity as the flowing water dissolves salt and sea water as it enters the river with the wind. pH concentration showed a trend similar to that of DO. pH concentration, which was relatively high in the first station, decreased in the second station. In the following stations, pH continuously increased until the point where the river meets the sea.
The mean T of the surface water of Zerveli stream did not show significant differences between stations but it remained at relatively lower levels in the first stations. Temperature variations in rivers are caused by fluctuations in water flow, and abiotic and biotic parameters. For example, the surface radiation and transmission from/to the air affects the substrate temperature in the area (Singh et al. 2017). The value of EC, which was at a relatively low level in the first station, did not show any significant change; however, the amount of dissolved matter increased, albeit to a small degree, at points closer to the sea. SSM concentration did not seem to change significantly between the first and last stations. The amount of SMM, which was at very low levels in the first station, started to increase starting from the second station and reached its maximum level in the 11th station, which is caused by matter being added to the stream as its waters run into the sea. COD and BOD values also increased starting from the spring and this trend is in harmony with the downtrend in DO. Cl, which reached its maximum concentration in the first station, was found to be at relatively low levels until the 7th station. However, it then increased towards the final station.
PO4-P concentration increased consistently between the first and final stations, which may indicate PO4-P input from the basin through which the waters run. SO42− and SO32− concentrations were close to each other in spatial distribution, as in temporal distribution. They increased starting from the first station right up to the last station. Na, K, TH, TA, and Mg values also increased closer to the end of the stream. Although TA did not show significant temporal differences, it exhibited significant spatial differences between stations. The TA value, which provides information about the natural salts in the steam water, also reflects the neutralizing capacity of the steam on acidic pollution increasing as a result of waste input and rain (Singh et al. 2017). The alkalinity value that increases at points closer to the sea is related to the effect of sea water. Ca values showed small differences between stations. NO−2-N, NO−3-N, and NH+4-N concentrations increased as the water flowed from its source towards points closer to the sea, which was likely caused by nitrogen input from anthropogenic sources. Of the metals, Fe, Pb, and Hg concentrations also increased continuously as the water flowed towards the sea. The regular increase in Cu, Cd, Ni, and Zn concentrations was interrupted by a small decrease in the 10th station.
Environmental assessment of water quality
In order to assess the water quality, WQI was calculated by using 12 different variables. The RW of parameters calculated for the variables used, and standard values obtained from the surface water environmental quality regulations of the Turkish Ministry of Agriculture and Forestry, are presented in Table 2.
The lowest WQI value (17.26) was found in February at the first station, whereas the highest WQI value (223.05) was observed in October at the 9th station. As can be seen, the WQI values ranged from excellent to very poor throughout the year. The monthly changes in WQI determined using monthly mean values are shown in Fig. 5.
According to this data, the water quality was found to be good in December, February, July, and August but poor in the other months. The input of matter from rains is considered to be the reason for the water quality decrease in months when the water quality was found to be poor. When comparing the stations in terms of water quality, the deterioration of quality was determined to increase after spring (Fig. 4). In the first station, a mean WQI value of 19.26 indicates excellent water quality. However, in the 11th station, the mean value of 176.62 indicates poor water quality. The water quality, which was good at the second, third, and fourth stations, fell into the poor category from the 5th station onwards.
According to standards set by the Ministry of Agriculture and Forestry, the environmental quality thresholds were exceeded only for Cu, Cd, Ni, and Zn (Table 2).
Assessing irrigation water quality
For multilateral evaluation of the Zerveli stream’s water quality, irrigation water quality parameters based on SAR, %Na, and RSC values were calculated. Na damages the structure of the soil and causes the soil surface to harden, which makes it harder for plant roots to take in air (Şener and Güneş 2015). SAR values were calculated to be 1.07–3.25. The scale used for evaluating SAR values is shown in Table 3 (Ravikumar et al. 2013).
The annual change in mean values showed that they never exceed 3.00 and values are of excellent quality, which means that the river is suitable for irrigation. When SAR value differences between stations are examined, a regular increase can be seen in the values from the first station to the last one. However, these values are still excellent in terms of irrigation water quality (Fig. 6). Na concentration in the ideal irrigation water must be lower than 50–60% (Ravikumar et al. 2013). An increase in Na concentration is desirable since it causes cation exchange in the calcium and magnesium in the soil (Şener and Güneş 2015). The Na values in Zerveli stream ranged between 37.58 and 61.89. Values used for assessing the %Na are shown in Table 4.
According to these values, it can be seen that the irrigation water quality is between good and doubtful. When yearly changes in mean values were examined, water quality was within acceptable limits. The mean %Na values of the stations were at good levels in the first station and within acceptable limits in the others (Fig. 5). If the water contains bicarbonate ions at high concentrations, then the damage risk arising from Na increases. The calcium and magnesium precipitate in the form of carbonate while the relative concentration of Na increases (Ravikumar et al. 2013). RSC values calculated in order to determine this risk varied between 3.80 and 5.58, which means that the water is not suitable for irrigation (Uygan et al. 2006). The waters with RSC > 2.5 were reported to cause black alkaline soil. When monthly mean values were examined, it was seen that the RSC values increased in the spring–summer months. When the stations were compared in terms of RSC levels, the lowest value (4.00) was found in the first station, and it increased as we move closer to the sea (Fig. 5). In the light of the aforementioned data, the stream water was found to be suitable for irrigation in terms of SAR and %Na but problematic in terms of RSC values.
Statistical observations
FA of the data was performed to determine the possible sources and transfer process of the variables. Four factors with eigenvalue > 1 were identified and these factors explained 87.22% of the total variance (Table 5). The first factor, consisting of salinity, pH, T, EC, SSM, COD, BOD, SO42−, SO32−, NO2−-N, NO−3-N, NH+4-N, and Cl (in a negative direction to the others), explained 54.52% of the total variance. BOD and COD are expressed positively while DO is expressed negatively, which suggests anthropogenic effects. However, since the water was found to be excellent according to environmental quality standards in terms of variables such as BOD, COD, and inorganic nitrogen salts, this factor was considered to represent natural degradation processes and other environmental factors that have an effect on them (Dalakoti et al. 2017). The second factor, consisting of Na, K, TH, TA, Mg, Ca, Fe, Pb, Cu, Hg, Ni, and Zn, explained 21.72% of the total variance. This factor also indicates possible common sources for these elements. The third factor explained 6.56% of the total variance and PO4-P was the prominent compound in this factor, which indicates a different source for orthophosphate than the other nutrients. The fourth factor explained 4.42% of the total variance and consists of negatively charged DO and Cd. Cd is included in this factor differently from the other metals and this indicates a different source for Cd.
In CA, the DO and Cl are positioned separately from the other variables. Similar to the results of FA, the salinity, EC, SSM SO32−, NO−3-N, NO2−-N, NH+4-N, BOD, SO42−, COD, and T form a cluster while Cd is distant from them. Another cluster consists of Na, K, TH, TA, Mg, Ca, Fe, Pb, Cu, Ni, and Zn. These variables also cluster among themselves; thus Na, TA, and TH form one group; K, Mg, and Ca form another group; and the metals (Fe, Cu, Pb, Zn) and Ni form a third group. PO4 is independent of all the other variables (Fig. 7).
Conclusion
According to the results of the monthly water quality analyses performed over 1 year in Zerveli stream, the WQI values varied between excellent and very poor. According to the monthly mean values, the water quality was good in December, February, July, and August but poor in the other months. The water quality was found to decrease as the distance from the spring increased. There are more settlements at points closer to the sea and therefore more anthropogenic input, which is thought to be the reason for this deterioration. The main reason for deterioration in water quality is increased Cd, Cu, and Zn concentrations. The stream water was found to be suitable for irrigation in terms of SAR and %Na; however, according to the RSC values, use of this water for irrigation purposes may have adverse effects on the soil.
References
Ali, E. M., & Khairy, H. M. (2016). Environmental assessment of drainage water impacts on water quality and eutrophication level of Lake Idku, Egypt. Environmental Pollution, 216, 437–449. https://doi.org/10.1016/j.envpol.2016.05.064.
Anonymous. (2011). Küre Mountains National Park Ecosystem-Based Functional Forest Management Plan Preparation Project 2011 - 2030. T.C. Ministry of Forestry and Water Affairs, General Directorate of Nature Conservation and National Park, 468 pages.
APHA. (1998). Standart methods for the ezamination of water and wastewater. 20th edition. Washington DC: American Public Health Association.
Azhar, S. C., Aris, A. Z., Yusoff, M. K., Ramli, M. F., & Juahir, H. (2015). Classification of river water quality using multivariate analysis. Procedia Environmental Sciences, 30, 79–84. https://doi.org/10.1016/j.proenv.2015.10.014.
Bakan, G., Özkoç, H. B., Tülek, S., & Cüce, H. (2010). Integrated environmental quality assessment of the Kızılırmak River and its coastal environment. Turkish Journal of Fisheries and Aquatic Sciences, 10(4), 453–462.
Belal, A. A. M., El-Sawy, M. A., & Dar, M. A. (2016). The effect of water quality on the distribution of macro-benthic fauna in Western Lagoon and Timsah Lake, Egypt. I. The Egyptian Journal of Aquatic Research, 42(4), 437–448. https://doi.org/10.1016/j.ejar.2016.12.003.
Berndtsson, J. C., & Bengtsson, L. (2006). Influence of different activities on water quality in a small basin. International Journal of River Basin Management, 4(4), 291–300. https://doi.org/10.1080/15715124.2006.9635298.
Boskidis, I., Gikas, G. D., Pisinaras, V., & Tsihrintzis, V. A. (2010). Spatial and temporal changes of water quality, and SWAT modeling of Vosvozis river basin, North Greece. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 45(11), 1421–1440. https://doi.org/10.1080/10934529.2010.500936.
Dalakoti, H., Mishra, S., Chaudhary, M., & Singal, S. K. (2017). Appraisal of water quality in the lakes of Nainital District through numerical indices and multivariate statistics, India. International Journal of River Basin Management, 16(2), 219–229. https://doi.org/10.1080/15715124.2017.1394316.
Das Kangabam, R., & Govindaraju, M. (2017). Anthropogenic activity-induced water quality degradation in the Loktak lake, a Ramsar site in the indo-Burma biodiversity hotspot. Environmental Technology, 1–10. https://doi.org/10.1080/09593330.2017.1378267.
Effendi, H. (2016). River water quality preliminary rapid assessment using pollution index. Procedia Environmental Sciences, 33, 562–567. https://doi.org/10.1016/j.proenv.2016.03.108.
Fan, X., Cui, B., Zhao, H., Zhang, Z., & Zhang, H. (2010). Assessment of river water quality in Pearl River Delta using multivariate statistical techniques. Procedia Environmental Sciences, 2, 1220–1234. https://doi.org/10.1016/j.proenv.2010.10.133.
Faragallah, H. M., Askar, A. I., Okbah, M. A., & Moustafa, H. M. (2009). Physico-chemical characteristics of the open Mediterranean Sea water far about 60 km from Damietta harbor, Egypt. Journal of Ecology and The Natural Environment, 1(5), 106–119.
Horton, R. K. (1965). An index-number system for rating water quality. Journal of the Water Pollution Control Federation, 37(3), 300–306.
Isidoro, D., & Aragüés, R. (2007). River water quality and irrigated agriculture in the Ebro Basin: an overview. International Journal of Water Resources Development, 23(1), 91–106. https://doi.org/10.1080/07900620601159743.
Kangabam, R. D., Bhoominathan, S. D., Kanagaraj, S., & Govindaraju, M. (2017). Development of a water quality index (WQI) for the Loktak Lake in India. Applied Water Science, 7(6), 2907–2918. https://doi.org/10.1007/s13201-017-0579-4.
Ouyang, Y. (2005). Evaluation of river water quality monitoring stations by principal component analysis. Water Research, 39(12), 2621–2635. https://doi.org/10.1016/j.watres.2005.04.024.
Ramakrishnaiah, C. R., Sadashivaiah, C., & Ranganna, G. (2009). Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. Journal of Chemistry. Research article., 6, 523–530. https://doi.org/10.1155/2009/757424.
Rasoloariniaina, J. R. (2017). Physico-chemical water characteristics and aquatic macroinvertebrates of Lake Tsimanampesotse, south-western Madagascar. African Journal of Aquatic Science, 42(2), 191–199. https://doi.org/10.2989/16085914.2017.1357532.
Ravikumar, P., Mehmood, M. A., & Somashekar, R. K. (2013). Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Applied Water Science, 3(1), 247–261. https://doi.org/10.1007/s13201-013-0077-2.
Rostom, N. G., Shalaby, A. A., Issa, Y. M., & Afifi, A. A. (2017). Evaluation of Mariut Lake water quality using hyperspectral remote sensing and laboratory works. The Egyptian Journal of Remote Sensing and Space Science, 20, S39–S48. https://doi.org/10.1016/j.ejrs.2016.11.002.
Sallam, G. A. H., & Elsayed, E. A. (2018). Estimating relations between temperature, relative humidity as independent variables and selected water quality parameters in Lake Manzala, Egypt. Ain Shams Engineering Journal, 9(1), 1–14. https://doi.org/10.1016/j.asej.2015.10.002.
Sarkar, A., & Pandey, P. (2015). River water quality modelling using artificial neural network technique. Aquatic Procedia, 4, 1070–1077. https://doi.org/10.1016/j.aqpro.2015.02.135.
Şener, Ş., & Güneş, D. (2015). Water quality and hydrogeochemical characteristics of surface water and groundwaters in Aksu (Isparta) plain. Pamukkale University Journal of Engineering Sciences, 21(6), 260–269.
Şener, Ş., Şener, E., & Davraz, A. (2017). Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Science of the Total Environment, 584–585, 131–144. https://doi.org/10.1016/j.scitotenv.2017.01.102.
Singh, H., Singh, D., Singh, S. K., & Shukla, D. N. (2017). Assessment of river water quality and ecological diversity through multivariate statistical techniques, and earth observation dataset of rivers Ghaghara and Gandak, India. International Journal of River Basin Management, 15(3), 347–360. https://doi.org/10.1080/15715124.2017.1300159.
SWQR (2016) Turkey’s Ministry of Agriculture and Forestry Surface Water Quality Regulations. http://www.resmigazete.gov.tr/eskiler/2016/08/20160810-9.htm
Taşdemir, M., & Göksu, Z. L. (2001). Some water quality criteria of Asi River (Hatay). Su Ürünleri Dergisi, 18(1) http://www.egejfas.org/egejfas/68354. Accessed 5 July 2018.
Uygan, D., Hakgören, F., & Büyüktaş, D. (2006). Eskişehir Sulama Şebekesinde Drenaj Sularının Kirlenme Durumu ve Sulamada Kullanma Olanaklarının Belirlenmesi. Mediterranean Agricultural Sciences, 19(1), 47–58.
Valeriani, F., Zinnà, L., Vitali, M., Spica, V. R., & Protano, C. (2015). River water quality assessment: comparison between old and new indices in a real scenario from Italy. International Journal of River Basin Management, 13(3), 325–331. https://doi.org/10.1080/15715124.2015.1012208.
Woli, K. P., Hayakawa, A., Nagumo, T., Imai, H., Ishiwata, T., & Hatano, R. (2008). Assessing the impact of phosphorus cycling on river water P concentration in Hokkaido. Soil Science and Plant Nutrition, 54(2), 310–317. https://doi.org/10.1111/j.1747-0765.2007.00243.x.
Zhao, Y., Xia, X. H., Yang, Z. F., & Wang, F. (2012). Assessment of water quality in Baiyangdian Lake using multivariate statistical techniques. Procedia Environmental Sciences, 13, 1213–1226. https://doi.org/10.1016/j.proenv.2012.01.115.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mutlu, E. Evaluation of spatio-temporal variations in water quality of Zerveli stream (northern Turkey) based on water quality index and multivariate statistical analyses. Environ Monit Assess 191, 335 (2019). https://doi.org/10.1007/s10661-019-7473-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7473-5