Abstract
In this study, observations were carried out in the surface waters of Saraydüzü Dam Lake within Sinop provincial borders for 1 year to determine water quality. The basic 28 variables used to determine water quality were measured monthly at six stations. Taking into account the World Health Organization's drinking water standards, the water quality index (WQI) and Turkey’s Ministry of Forestry and Water Affairs Surface Water Quality Regulations (SWQR) were used in determining the water quality. In addition, irrigation water quality was examined. For this, sodium absorption rates (SAR), sodium percentage and residual sodium carbonate (RSC) values were calculated. WQI values in the lake were found to be between 17.62 and 29.88. Water quality parameters did not exceed the recommended limit values in all months and at all stations. According to these values, the Saraydüzü Dam Lake water belongs to the ‘very good’ class in terms of drinking water quality. The results obtained showed that there were no nitrogen or phosphate inputs that could harm the ecosystem in the lake and that there were no low/insufficient ambient oxygen conditions resulting from excessive oxygen consumption during the degradation process of organic matter. All water quality parametres are well below the permissible limits except some heavy metals according to SWQR. Cu, Zn and Fe were found to exceed the limit values. The water quality of irrigation water was found to be good in terms of SAR and sodium percentage, whereas RSC was observed to have varying qualities during the year and not be suitable for irrigation in some months. According to results of factor analysis (FA), pH, temperature, electrical conductivity, suspended solid matter (SSM), biological oxygen demand (BOD), total hardness (TH),total alkalinity (TA), calcium, nitrate, ammonium, mercury and dissolved oxygen are the main variables responsible for the processes in the ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, water pollution is a serious problem threatening both seas and inland waters. Pollutants discharged into the water disturb the balance of the ecosystem as well as leading to significant problems in terms of public health by impairing the quality of domestic and drinking water. Since rivers and lakes are the major water resources for domestic use and human consumption, accumulation of contaminants in these constitute a global health problem (Das Kangabam and Govindaraju 2017). Lakes especially are more susceptible to pollution than other water bodies because they are still waters and cannot clean themselves (Dalakoti et al. 2017).
Surface water quality in any region is under pressure from both natural processes (abrasion, rain, soil erosion, etc.) and anthropogenic inputs (agricultural, urban, industrial activities) (Wu et al. 2018; Zhao et al. 2012). Climate change also stimulates extreme cyanobacterial increases by affecting aquatic processes (Wu et al. 2015). Agricultural lands using fertilisers and pesticides, urban areas heavily applying herbicides and insecticides, industrial waste waters and eroded soils are typical nonpoint sources of nutrients, organic and inorganic (e.g. heavy metals) pollutants (Ali and Khairy 2016; He et al. 2012). In addition to the transportation of nitrogen and phosphorus from land to water, atmospheric deposition of these nutrients also contributes to the pollution of surface waters (Smith 2016).
Animals, vegetation and other organisms create an ecosystem in lakes and maintain the stability of the ecosystem (Tang et al. 2018). However, deterioration in water quality leads to the death of these organisms (Tang et al. 2018). Changes in the water level and increases in nutrients are important factors disturbing the ecosystem’s stability (Kong et al. 2017). For example, excessive nutrient increase stimulates algal proliferation, then organic matter production increases due to eutrophication, and the oxygen level decreases during decomposition of organic substances, thereby causing anoxic conditions to occur. This results in a reduction in the diversity and abundance of species in the environment. The bloom of toxic species in phytoplankton leads to poisoning and even deaths in the food chain.
Heavy metals are another pollutant group that degrades water quality and harms the ecosystem. It is known that anthropogenic sources accelerate heavy metal deposits in the water (Rupakheti et al. 2017). Heavy metals are toxic and permanent elements which can be hazardous when they reach humans through the food chain (Bing et al. 2013; Çevik et al. 2009; Kishe and Machiwa 2003; Luo et al. 2010). Some metals, such as Cu and Zn, are toxic in high doses, although they are ‘essential’ elements that are necessary for the life of organisms (Bai et al. 2011).
One of the most commonly used tools for assessing water quality is the water quality index (WQI). This method, first described by Horton (1965), is considered an indicator in water quality assessment (Das Kangabam and Govindaraju 2017). The comparison of measured variables in the water with acceptable values may lead to confusion, especially where multiple parameters need to be measured. WQI eliminates this problem by providing a single value after taking into account all the variables and is convenient for both scientists and authorities who make decisions concerning environmental policies.
Monitoring and control of the nutrients and heavy metals in water resources is a critical issue, both in terms of the ecosystem and public health. In this study, physicochemical water quality parameters including nutrient and heavy metal concentrations were investigated in the surface waters of the Saraydüzü Dam Lake, one of the largest dams in Turkey. The aim of this study was to determine the contamination level of the surface water in Saraydüzü Dam Lake using the WQI and Turkey’s Ministry of Forestry and Water Affairs Surface Water Quality Regulations (SWQR) to reveal the irrigation water quality and to determine the relationship among the variables and sources of the contaminants through multivariate statistical analyses.
Materials and methods
Study area
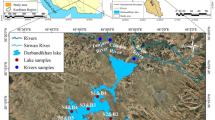
Saraydüzü Dam Lake is located in part of the Kızılırmak Basin in the province of Sinop. The dam was constructed for the purposes of energy and irrigation. The dam is 7 km from Saraydüzü village, 16 km from the town of Durağan, and 110 km from the city of Sinop. The capacity volume of the dam is 36.78 hm3 and the lake area is 217 km2. The deepest point of the dam lake is 69 m and the average depth is 12.3 m. The water source of the lake is Kızılırmak River. Sarayduzu Dam Lake is the fifth largest dam in Turkey. The dam is of great importance because it is used in the irrigation of agricultural lands around the Sarayüzü, Durağan and Boyabat townships. The dam lake is also located in a wildlife development area and meets the water needs of many wild animals. The dam is surrounded by steep slopes and the average elevation of the Saraydüzü Dam Lake is 305 m. The terrain is composed of relatively high mountain ranges with depressions where floods are common. The Kızılırmak River and Asarcik Stream form a valley. The high hills and mountains are partly wooded, partly covered in heather, and a large part is bare. A Black Sea-type climate is not dominant as Saraydüzü is located in the interior of the zone; therefore a Central Anatolian climate is characteristically seen. The hottest months of the region are July–August, and the coldest months are January and February. Eighty days of the year are rainy and there is 360–400 kg of rainfall per square meter (NADA 2013).
Sampling and analytical methods
From October 2016 to September 2017, surface water samplings were carried out monthly at six stations that were considered to represent the entire lake (Fig. 1). The water samples were taken with acid-cleaned 2.5-L sampling bottles from 15 to 20 cm below the water surface. Water samples to be used for heavy metal analysis were taken in polyethylene bottles previously washed with 50% HNO3 and deionised water, and acidified with 10 ml HNO3 per litre. The samples were transported to the laboratory in iceboxes and stored in the refrigerator at 4 °C until analysis.
Water temperature, dissolved oxygen (DO), pH, salinity and electrical conductivity (EC) values were measured in situ with a YSI 556 MPS. Chemical oxygen demand (COD), biological oxygen demand (BOD), total hardness (TH), nitrite nitrogen (NO22−-N), nitrate nitrogen (NO32−-N), ammonium nitrogen (NH4+-N), total alkalinity (TA), orthophosphate (PO43−-N), sulphite (SO32−), sulphate (SO42−), chloride (Cl−), calcium (Ca2+), magnesium (Mg2+), sodium (Na+) and potassium (K+) analyses were performed using standard methods (APHA 1998). Iron, lead, cadmium, zinc, nickel, copper and mercury analyses were performed using a PerkinElmer Optima 2000 DV ICP-OES.
WQI was calculated to assess water quality (Horton 1965; Kangabam et al. 2017; Şener et al. 2017). Thirteen parameters were selected from measured variables according to their importance for water quality. Cd, Cl, Cu, Pb, Hg, Ni, nitrate, nitrite, Na, TH, pH, sulphate and Zn were included in the index calculation. Each parameter was assigned a weight with 1 being the smallest weight and 5 being the largest weight (AW). In determining the weights, the importance of the relevant parameter was taken into consideration in terms of human health. Then, the relative weights (RW) were calculated by dividing the weight specified for each parameter by the sum of the weights for all parameters (Eq. 1).
The quality rating was then calculated by dividing the measured concentration (Ci) of each parameter by the limit values given in the World Health Organization (WHO) drinking water criteria (Si) (WHO 2011) (Eq. 2).
After the water quality sub-index was obtained by multiplying the quality grade by the relative weight of each variable (Eq. 3), the integrated WQI was obtained by totalling all the sub-indices (Eq. 4). This was evaluated according to WQI by (Ramakrishnaiah et al. 2009): WQI < 50 excellent, 50–100 good, 100–200 poor, 200–300 very poor and > 300 not suitable.
To determine irrigation water quality, the sodium absorption rate (SAR), sodium percentage (Na%) and residual sodium carbonate (RSC) parameters were calculated. SAR, Na% and RSC values were calculated according to Eqs. 5, 6 and 7, respectively (Ravikumar et al. 2013). The concentrations of the elements were calculated in mEq/L.
Factor analysis, correlation analysis and cluster analysis were performed on the data set to find the relations between variables and to determine their sources.
Results and discussion
Surface water properties
Descriptive statistics of the variables are given in Table 1. On the basis of the monthly averages of the measured variables, their changes during the year were examined (Fig. 2). According to these results, the dissolved oxygen values showed an increase in the period between October and April. In the December–June period, the values remained somewhat stable but then showed a decline from June to September. The amount of dissolved oxygen is influenced by many factors such as temperature, microbial population, biological processes, density of dissolved salts, pressure and sampling time (Tokatli 2013; Das Kangabam and Govindaraju 2017). DO increases in cold periods due to the fact that low temperatures increase oxygen solubility and living organisms reduce a large number of their activities that require oxygen consumption (Ali and Khairy 2016). Salinity, which showed a general declining trend in the October–March period, exhibited a rising trend in March and peaked with a value of 0.3667 psu in July. Subsequently, the values fell again. Increases in the spring–summer period may have been the result of evaporation.
The pH values showing a downward trend until February then entered a period of increase in March and continued to rise until the time of the last sampling. This may be related to the increased activity of photosynthetic organisms (Rostom et al. 2017). On the other hand, Zhang et al. (2016) associate high pH values with precipitation of Cd and other cations. Low pH values can pose a risk to the ecosystem because they expedite the uptake of toxic elements by aquatic biota (Faragallah et al. 2009). (Ustaoğlu and Tepe 2018) also represent that pH fluctuations in water may affect the toxicity of some compounds. pH values within safe limit (6.5–8.5) are considered safe for the skin, eyes, nose and ears (Srinivas Rao and Nageswara Rao 2010; Zhang et al. 2016) but there is no such risk in the case of Saraydüzü Dam Lake, since pH values are within the appropriate range according to WHO (2011) and SWQR (2016).
It was observed that surface water temperatures tended to follow a trend parallel to atmospheric temperatures, reaching a minimum value in February and maximum value in September.
EC was measured as 307.28 μS/s in October and reached its minimum value (152.81 μS/s) in March. The increase that started in March lasted until September. Although high EC values are associated with household waste, waste disposal, chemical sewage from agricultural fields and stock farming activities (Kangabam et al. 2017), low values indicate unharmed conditions (Sallam and Elsayed 2018). Considering that the values obtained in this study were low, the increases observed in the summer are thought to be the result of evaporation. Higher EC indicates higher salinity and higher salinity points out evaporation in summer (Jiang et al. 2015; Zhang et al. 2016). The similar relationship EC and temperature has been found by (Mutlu et al. 2016). The amount of suspended solids, which showed a sharp decline between October and January, remained at a more-or-less constant level until May, and then steeply increased between May and September. The increase in this period may represent growth in the phytoplankton community.
COD values, showing a decreasing tendency between October and February, started to rise in February and reached their maximum with a value of 3.06 mg/L in June. After a decrease in July, the values increased again. BOD exhibited a downward tendency between October and February and reached its minimum value in February (0.36 mg/L). After February, the values started to rise and reached their maximum value in September (1.54 mg/L). This increase in the summer months can be explained by increased primary production. COD is widely used to determine domestic, industrial and agricultural waste concentrations. Inorganic chemicals that consume oxygen during degradation cause increasing COD levels, whereas high BOD values are based on anthropogenic activities associated with fishing and domestic wastes (Sallam and Elsayed 2018; Zhao et al. 2012). DO concentration and the inverse correlation between BOD and DO (r = −0.74, p < 0.05) can be explained by the use of DO during oxidation of organic matter (Das Kangabam and Govindaraju 2017). The negative correlation between BOD and DO and positive correlation between BOD and COD (r = 0.68, p < 0.05) are reported to be an indication of anthropogenic effects (Dalakoti et al. 2017) but the low BOD and COD values and high DO value in this study demonstrate that the relations between these three variables are controlled by natural processes.
The chloride concentration reached its highest level (6.28 mg/L) in December, and then dropped to its minimum value (3.01 mg/L) in January. Following a small peak in February, the values receded in March and tended to increase until August. (Jindal et al. 2014) stressed that the annual average chlorine concentration of 6.88 mg/L they found in their study was an indication uncontaminated with any wastewater. The chloride concentration of Saraydüzü Dam Lake is consistent with this result.
The phosphate concentration, which reached its maximum value (0.51 mg/L) in November, showed a downward trend throughout the year except for a small peak in April and dropped to its minimum value (0.0014 mg/L) in July and August.
The sulphate concentration showed a downward trend until January, then entered an upward trend from January, except for March, and reached its maximum value (70.20 mg/L) in June. A downward trend was observed again until September.
The sulphide concentration had the same trend as sulphate, reaching its minimum value (0.33 mg/L) in January and its maximum value (2.63 mg/L) in June. Although the sodium concentration fluctuated a little from time to time, it was generally in an upward trend from the first sampling and reached its maximum value (75.74 mg/L) in June, then entered a downward trend until September.
Potassium concentration, which had two peaks in the October–June period, one in February (8.83 mg/L) and the other in June (16.48 mg/L), tended to decline from July to the end of the sampling period. Concentration of this ion generally is controlled by rock weathering (Li et al. 2017). TH showed a downward trend until December, but then went on to increase despite a small decline in March. The maximum value (232.87 mg/L) was reached in September. Dissolution of carbonates, evaporation and human activities may be responsible for increase in TH values (J. Wu et al. 2017). TA also exhibited the same trend as hardness. Magnesium concentration decreased between October and January, reaching its minimum value (15.49 mg/L) in January. The concentration increased until June and then decreased and remained constant until the end of the sampling period. Calcium showed similar trends to magnesium. The calcium concentration reached its minimum value (14.86 mg/L) in January and its maximum value (62.91 mg/L) in June. All organisms require magnesium and calcium as essential nutrient and these elements completely derived from rock weathering (Singh et al. 2016).
The nitrite concentration was at relatively low levels in the fall and winter months, and then increased in March and reached its maximum value (0.0042 mg/L) in June. Subsequently, it showed a decline until September. The nitrate concentration, showing a decline from October to February, was determined at the lowest level (BDL) in February. It entered an upward trend after February and reached its maximum level (3.62 mg/L) in September. Nitrite is an intermediate oxidation product between nitrate and ammonium (Belal et al. 2016) but nitrite is more toxic for animals and humans than nitrate. In addition, drinking water containing a high concentration of nitrate may cause methemoglobinemia, blue baby syndrome, gastric cancer, abnormal pains, central nervous system disorders and diabetes (Belal et al. 2016; Şener et al. 2017). However, in the case of the Saraydüzü Dam Lake, nitrite and nitrate concentrations were below the WHO (2011) standards and do not pose health risks.
Ammonium, which is an organic biodegradation product of nitrogen and can be used directly as food by plants (Belal et al. 2016), showed a decline between October and January and remained relatively stable until May. In May, the concentration began to increase and reached its maximum value (0.0016 mg/L) in September.
Rasoloariniaina 2017 reported that nitrogen accumulates as a result of evaporation. Increased nitrogen concentrations in the summer may be related to this situation. The iron concentration was determined at constant values throughout the year except for a peak in January. The lead concentration, which was at relatively low levels until February, increased in spring and reached its maximum value (1.92 μg/L) in July then decreased again. Copper concentration showed a fluctuating trend throughout the year. It peaked in November, February and June. Its maximum value (13 μg/L) was observed in June. Cadmium concentration increased in November, May, July and August. The minimum value (BDL) was determined in January, and the maximum value was determined as 0.3 μg/L in May, July and August. Mercury concentration, which had its maximum value in October, was at minimum levels between January and March. An upward trend was observed again in April. Nickel concentration showed an increase (3.33 μg/L) in November then decreased, reaching a minimum level between January and March. Then, the concentration reached a second peak in May and remained more or less constant until the end of the sampling period. For Zinc concentration, two important peaks were determined. The first of these was in November (13.0 μg/L) and the second was in June (12.17 μg/L). After June, zinc concentration showed a downward trend again until September.
WQI
The water quality of the Saraydüzü Dam Lake was evaluated using the WQI. For calculating the index, Cd, Cl, Cu, Pb, Hg, Ni, nitrate, nitrite, Na, hardness, pH, SO4 and Zn were taken into consideration. The limits of WHO (2011) were used in the calculation (Table 2). WQI values in the lake were found to be between 17.62 and 29.88. Water quality parameters did not exceed the recommended limit values in all months and at all stations. According to these values, the Saraydüzü Dam Lake water belongs to the ‘very good’ class in terms of drinking water quality. The smallest value was determined at St 6 in January, whereas the highest value was determined at St 3 in July. When the changes of WQI values within the year were examined, it was seen that the values were lower in the winter months and entered an upward trend in the summer (Fig. 3).
The findings obtained from the analyses were also evaluated according to Turkey’s Ministry of Forestry and Water Affairs SWQR. SWQR quality criteria are shown in Table 3. According to these criteria, the pH values are consistent with the given values of 6–9. EC values were between 151.92 and 307.7. In terms of these values, the water quality is very good.
The values for dissolved oxygen, chemical oxygen quantity (COD) and biological oxygen quantity (BOD) are consistent with the values in the ‘very good’ category. (Kangabam et al. 2017) indicated that high nitrate concentrations stem from agricultural lands where fertilisers are applied. However, the agricultural lands in the vicinity of Saraydüzü Dam Lake were not determined to have a negative effect on water quality in terms of nitrates. The maximum values obtained in the study for ammonium and nitrate nitrogen were 0.0019 mg NH4+-N/L and 3.68 mg NO3−-N/L, respectively, and these values indicate a quality classification of ‘very good’. The mean phosphate phosphorus concentration was determined as 0.09 mg o-PO4-P/L, the minimum phosphate concentration as 0.001 mg o-PO4-P/L, and the maximum phosphate value as 0.52 mg o-PO4-P/L. According to these values, the water quality is between ‘very good’ and ‘medium’ in terms of phosphate. According to the annual mean value, it is in the ‘good’ quality class. Fertilisers, domestic dishwashing water and detergents are the main sources of phosphorus. In addition to these, phosphate, which is accumulated in the sediment, can also be released into the water under suitable conditions (Ali and Khairy 2016; Horppila et al. 2017). In this context, periodic increases in phosphate levels may be due to the residential and agricultural areas around the lake. When assessed for trace metals, it was determined that the permissible annual mean values for Cu, Zn and Fe were exceeded, whereas critical values for Pb, Hg, Cd and Ni were not exceeded.
Evaluation of irrigation water quality
Saraydüzü Dam Lake waters are used for irrigation of the surrounding agricultural land. Therefore, the lake water was also evaluated in terms of irrigation water quality. For this purpose, the sodium adsorption ratio, the residual sodium carbonate amount and the sodium percentage were calculated using the elements found in the water. Sodium damages the structure of soil, decreases its permeability, and causes a hard crust on the soil surface. As a result of this, plant roots cannot breathe (Şener and Güneş 2015). The scale used in the evaluation of SAR values is given in Table 4 (Ravikumar et al. 2013). The SAR values of the Saraydüzü Dam Lake water were found to be between 0.98 and 2.08. Although the smallest value was observed during October, the highest value was observed in May. The mean SAR value was calculated as 1.56. According to these values, the lake water belongs to the ‘very good’ irrigation water class.
If the water contains high concentrations of bicarbonate ions, the risk of sodium damage increases. As calcium and magnesium are precipitated as carbonate, the relative sodium percentage increases (Ravikumar et al. 2013). To determine this risk, residual sodium carbonate value (RSC) was calculated. RSC values of < 1.25 indicate good, 1.25–2.50 indicate doubtful and > 2.50 indicate unsuitable conditions (Ravikumar et al. 2013). The smallest RSC value was found in October (1.12) and the highest value (4.94) was found in February. The annual mean RSC value is 2.72. According to the RSC values, the waters of Saraydüzü Dam Lake are safe for irrigation in October, borderline/doubtful in May–June–July–November and December, and unsuitable in other months. Water with a RSC value > 2.5 is not appropriate for use in irrigation because it will cause black alkaline soils (Uygan et al. 2006). To avoid the harmful effects of sodium on soil, it is desirable that the amount of sodium in irrigation water should not exceed 50–60% (Ravikumar et al. 2013). An increased amount of sodium is not advisable because it will cause base change with calcium and magnesium in the soil (Şener and Güneş 2015). The sodium percentage values obtained in the present study are in the range of 21.72–47.11 and in the ‘good-permissible’ range (Table 5).
Multivariate statistical analyses
Factor analysis was applied to the data set to express the relationship between the measured variables more clearly and to determine the processes that operate in the ecosystem. Four factors represented by eigenvalues > 1 were calculated. These four factors account for 86.86% of the total variance (Table 6). The first factor accounts for 46.99% of the total change and mainly consists of pH, temperature, EC, suspended solids, BOD, TH, TA, calcium, nitrate, ammonium and mercury as well as dissolved oxygen acting with them in the opposite direction. The second factor accounts for 23.88% of the total variance and mainly consists of salinity, sulphate, sulphite, sodium, potassium, nitrite, lead and copper. The third factor accounts for 10.97% of the total variance and consists of COD, chlorine, phosphate, magnesium, nickel and zinc. The final factor explains 5.03% of the variation and consists of iron and cadmium acting in opposite directions to each other.
In the first factor, the negative correlation of dissolved oxygen with temperature, pH, EC and BOD indicates natural processes. Temperature, pH and EC increase during the summer months, whereas oxygen concentration decreases. The presence of BOD in the first factor indicates that biological degradation is more effective than chemical oxidation. The presence of nitrate and ammonium, which are nitrogen forms used by plants, in the first factor indicates their primary importance for plant production. Although (Kıymaz and Karadavut 2014) expressed that the presence of TH, BOD, COD, DO, EC and ammonium in the same factor refers to contamination but WQI and SWQR results indicate very good quality in Saraydüzü Dam Lake. Uncumusaoğlu-Aydın (2018) suggested that a component including positively EC, BOD, temperature, SSM, ph, ammonium, TA, TH, Hg, nitrite and negatively DO can be pointed out organic pollution or seasonal effect of temperature. The presence of these parameters in the same factor can be explained by natural process in the present study. The natural source of these parameters may be surface runoff and soil leaching from the basin (Mutlu and Uncumusaoğlu-Aydın 2018). The second factor indicates a strong positive loading of major ions. This factor may represent agricultural applications, atmospheric deposition or basin geology and soil structure (Boyacioglu and Boyacioglu 2008; Kıymaz and Karadavut 2014; Mutlu and Uncumusaoğlu-Aydın 2018; Uncumusaoğlu-Aydın 2018; Zhang et al. 2016). Multivariate statistical analyses are widely used in determining sources of heavy metals and it is stated that metals having a positive component and/or high positive correlation share common sources and migration processes (Bai et al. 2011; Gao et al. 2013; Wang et al. 2012). Unlike the other heavy metals, it is interesting that mercury is contained within the first component and may point to a different source. Similarly, nickel, zinc and magnesium, and lead and copper appear to have common sources and processes. The presence of phosphate in third factor may demonstrate fertiliser or organic effluent contributions (Boyacioglu and Boyacioglu 2008; Iscen et al. 2008). The fact that iron and cadmium are in opposite directions in the last factor indicates that the cadmium source is not natural rocks. Cadmium may possibly be present in agricultural waters flowing into the lake.
According to the results of cluster analysis conducted by the nearest neighbour method, salinity, sulphate, sulphite, sodium, nitrite and potassium form a cluster. This cluster represents ions dissolved in the water. These ions may have common sources. pH, TE, BOD, nitrate and EC are other variables that appear to be interacting with each other. The parameters in this group are thought to represent natural processes of deterioration that vary depending on temperature and nitrate inputs. The similarity between SSD and ammonium may be for two reasons: comigration or phytoplankton increase induced by ammonia. The relationship between chlorine and magnesium also refers to common sources. The expected high similarity between TH and TA reflects the effect of carbonates on TA. Iron and DO appear to be distantly related to other parameters. The dendrogram of the cluster analysis is presented in Fig. 4.
In Pearson’s correlation analysis conducted to observe the relationships between variables, the test was performed after execution of LOG10 transformation to adjust the data to normal distribution. The data obtained from the correlation test are substantially in agreement with the data obtained from factor analysis and cluster analysis (Table 7). DO concentration is strongly and negatively correlated with pH, TE, EC, SSD, Hg and BOD. The pH, TE, EC, SSD and BOD show strong and positive correlations between themselves. Ustaoğlu and Tepe (2018) suggest that EC values depend on geological structure and precipitation amount. Relationships between EC, DO and TE indicate seasonal effects. The strong positive correlation of BOD and COD with temperature indicates that oxidation processes increase in the summer months. The positive correlation of pH with TE and SSD may be due to photosynthesis activity of the algae. The strong correlation between pH and TA points out ability of water to neutralise acids (Ustaoğlu and Tepe 2018). The result showing that phosphate is not related to nitrogenous nutrients suggests that they have different dynamics. Nitrite has a strong positive relationship with nitrate and nitrate with ammonium. There is a strong positive relationship between nitrite and sulphite–sulphate. The inverse relationship of Cu, Ni and Cd with Fe suggests that these metals may have a source other than lithogenic sources. The positive correlation of Cu, Ni and Cd among themselves may also reflect a common source. Zn and Ni also appear as elements sharing common processes. The positive correlation of Cu, Cd, Hg, Ni and Zn with nitrate may indicate a domestic or agricultural source. Strong correlation between calcium and TH indicates calcium contribution to hardness (Singh et al. 2016).
Conclusion
To determine the water quality of Saraydüzü Dam Lake, which is used to provide irrigation water to the surrounding area, samples were taken for 12 months from six stations representing the entire dam lake and water quality analyses were carried out. To determine water quality, WQI was calculated by taking into account WHO (2011) standards. WQI values in the lake were found to be between 17.62 and 29.88. According to these results, Saraydüzü Dam Lake surface water is in the ‘very good’ class. In the calculations made to determine irrigation water quality, the surface waters were found suitable in terms of SAR and sodium percentage content, whereas different properties were determined in terms of RSC throughout the year. According to SWQR, the water quality was found to be ‘very good’ in terms of DO, COD, BOD, nitrate and ammonium, but in terms of phosphate, it varied in range between the ‘very good’ and ‘medium’ classes. This suggests that there are no nutrient inputs from the surrounding domestic and agricultural areas that could damage the health of the ecosystem. The limit values for heavy metals Cu, Zn and Fe were exceeded. It would be beneficial to investigate the background values of the lake basin to clarify whether these high concentrations are due to its natural geological structure or anthropogenic factors. pH, temperature, EC, SSM, BOD, TH, TA, calcium, nitrate, ammonium, mercury and DO are the parameters that play important roles in variations in the ecosystem. Major ions are the second important group in the lake. Consequently, while surface water of the dam is suitable in terms of drinking water and environmental quality standards, it has some problems for irrigation use.
References
Ali, E. M., & Khairy, H. M. (2016). Environmental assessment of drainage water impacts on water quality and eutrophication level of Lake Idku, Egypt. Environmental Pollution, 216, 437–449. https://doi.org/10.1016/j.envpol.2016.05.064.
APHA. (1998). Standart methods for the ezamination of water and wastewater (20th ed.). Washington DC: American Public Health Association.
Bai, J., Cui, B., Chen, B., Zhang, K., Deng, W., Gao, H., & Xiao, R. (2011). Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecological Modelling, 222(2), 301–306. https://doi.org/10.1016/j.ecolmodel.2009.12.002.
Belal, A. A. M., El-Sawy, M. A., & Dar, M. A. (2016). The effect of water quality on the distribution of macro-benthic fauna in Western Lagoon and Timsah Lake, Egypt.I. The Egyptian Journal of Aquatic Research, 42(4), 437–448. https://doi.org/10.1016/j.ejar.2016.12.003.
Bing, H., Wu, Y., Nahm, W.-H., & Liu, E. (2013). Accumulation of heavy metals in the lacustrine sediment of Longgan Lake, middle reaches of Yangtze River, China. Environmental Earth Sciences, 69(8), 2679–2689. https://doi.org/10.1007/s12665-012-2090-4.
Boyacioglu, H., & Boyacioglu, H. (2008). Water pollution sources assessment by multivariate statistical methods in the Tahtali Basin, Turkey. Environmental Geology, 54(2), 275–282. https://doi.org/10.1007/s00254-007-0815-6.
Çevik, F., Göksu, M. Z. L., Derici, O. B., & Fındık, Ö. (2009). An assessment of metal pollution in surface sediments of Seyhan dam by using enrichment factor, geoaccumulation index and statistical analyses. Environmental Monitoring and Assessment, 152(1–4), 309–317. https://doi.org/10.1007/s10661-008-0317-3.
Dalakoti, H., Mishra, S., Chaudhary, M., & Singal, S. K. (2017). Appraisal of water quality in the lakes of Nainital District through numerical indices and multivariate statistics, India. International Journal of River Basin Management, 16, 1–11. https://doi.org/10.1080/15715124.2017.1394316.
Das Kangabam, R., & Govindaraju, M. (2017). Anthropogenic activity-induced water quality degradation in the Loktak lake, a Ramsar site in the Indo-Burma biodiversity hotspot. Environmental Technology, 1–10. https://doi.org/10.1080/09593330.2017.1378267.
Faragallah, H. M., Askar, A. I., Okbah, M. A., & Moustafa, H. M. (2009). Physico-chemical characteristics of the open Mediterranean sea water far about 60 km from Damietta harbor, Egypt. Journal of Ecology and The Natural Environment, 1(5), 106–119.
Gao, H., Bai, J., Xiao, R., Liu, P., Jiang, W., & Wang, J. (2013). Levels, sources and risk assessment of trace elements in wetland soils of a typical shallow freshwater lake, China. Stochastic Environmental Research and Risk Assessment, 27(1), 275–284. https://doi.org/10.1007/s00477-012-0587-8.
He, B., Oki, K., Wang, Y., Oki, T., Yamashiki, Y., Takara, K., Miura, S., Imai, A., Komatsu, K., & Kawasaki, N. (2012). Analysis of stream water quality and estimation of nutrient load with the aid of quick bird remote sensing imagery. Hydrological Sciences Journal, 57(5), 850–860. https://doi.org/10.1080/02626667.2012.683792.
Horppila, J., Holmroos, H., Niemistö, J., Massa, I., Nygrén, N., Schönach, P., Tapio, P., & Tammeorg, O. (2017). Variations of internal phosphorus loading and water quality in a hypertrophic lake during 40 years of different management efforts. Ecological Engineering, 103, 264–274. https://doi.org/10.1016/j.ecoleng.2017.04.018.
Horton, R. K. (1965). An index-number system for rating water quality. Journal of the Water Pollution Control Federation, 37(3), 300–306.
Iscen, C. F., Emiroglu, Ö., Ilhan, S., Arslan, N., Yilmaz, V., & Ahiska, S. (2008). Application of multivariate statistical techniques in the assessment of surface water quality in Uluabat Lake, Turkey. Environmental Monitoring and Assessment, 144(1), 269–276. https://doi.org/10.1007/s10661-007-9989-3.
Jiang, L., Yao, Z., Liu, Z., Wang, R., & Wu, S. (2015). Hydrochemistry and its controlling factors of rivers in the source region of the Yangtze River on the Tibetan Plateau. Journal of Geochemical Exploration, 155, 76–83. https://doi.org/10.1016/j.gexplo.2015.04.009.
Jindal, R., Thakur, R. K., Singh, U. B., & Ahluwalia, A. S. (2014). Phytoplankton dynamics and water quality of Prashar Lake, Himachal Pradesh, India. Sustainability Water Quality and Ecology, 3–4, 101–113. https://doi.org/10.1016/j.swaqe.2014.12.003.
Kangabam, R. D., Bhoominathan, S. D., Kanagaraj, S., & Govindaraju, M. (2017). Development of a water quality index (WQI) for the Loktak Lake in India. Applied Water Science, 7(6), 2907–2918. https://doi.org/10.1007/s13201-017-0579-4.
Kishe, M. A., & Machiwa, J. F. (2003). Distribution of heavy metals in sediments of Mwanza Gulf of Lake Victoria, Tanzania. Environment International, 28(7), 619–625. https://doi.org/10.1016/S0160-4120(02)00099-5.
Kıymaz, S., & Karadavut, U. (2014). Application of multivariate statistical analysis in the assessment of surface water quality in Seyfe Lake, Turkey. Journal of Agricultural Sciences (Turkey), 20, 152–163.
Kong, X., He, Q., Yang, B., He, W., Xu, F., Janssen, A. B. G., Kuiper, J. J., van Gerven, L. P. A., Qin, N., Jiang, Y., Liu, W., Yang, C., Bai, Z., Zhang, M., Kong, F., Janse, J. H., & Mooij, W. M. (2017). Hydrological regulation drives regime shifts: evidence from paleolimnology and ecosystem modeling of a large shallow Chinese lake. Global Change Biology, 23(2), 737–754. https://doi.org/10.1111/gcb.13416.
Li, P., Feng, W., Xue, C., Tian, R., & Wang, S. (2017). Spatiotemporal variability of contaminants in lake water and their risks to human health: a case study of the Shahu Lake Tourist Area, Northwest China. Exposure and Health, 9(3), 213–225. https://doi.org/10.1007/s12403-016-0237-3.
Luo, W., Lu, Y., Wang, T., Hu, W., Jiao, W., Naile, J. E., Khim, J. S., & Giesy, J. P. (2010). Ecological risk assessment of arsenic and metals in sediments of coastal areas of northern Bohai and Yellow Seas, China. AMBIO, 39(5–6), 367–375. https://doi.org/10.1007/s13280-010-0077-5.
Mutlu, E., & Uncumusaoğlu-Aydın, A. (2018). Analysis of spatial and temporal water pollution patterns in Terzi Pond (Kastamonu/Turkey) by using multivariate statistical methods. Fresenius Environmental Bulletin, 27(5), 2900–2912.
Mutlu, E., Demir, T., Yanik, T., & Sutan, N. A. (2016). Determınatıon of envıronmentally relevant water qualıty parameters ın Serefıye Dam-Turkey. Fresenius Environmental Bulletin, 25(12), 8.
NADA. (2013). North Anatolian Development Agency Saraydüzü District analysis report https://www.kuzka.gov.tr/Icerik/Dosya/www.kuzka.gov.tr_18_JT3S98BJ_sarayduzu_ilce_analizi.pdf. Accessed 21 March 2018.
Ramakrishnaiah, C. R., Sadashivaiah, C., & Ranganna, G. (2009). Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. Journal of Chemistry. Research article, 6, 523–530. https://doi.org/10.1155/2009/757424.
Rasoloariniaina, J. R. (2017). Physico-chemical water characteristics and aquatic macroinvertebrates of Lake Tsimanampesotse, south-western Madagascar. African Journal of Aquatic Science, 42(2), 191–199. https://doi.org/10.2989/16085914.2017.1357532.
Ravikumar, P., Mehmood, M. A., & Somashekar, R. K. (2013). Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Applied Water Science, 3(1), 247–261. https://doi.org/10.1007/s13201-013-0077-2.
Rostom, N. G., Shalaby, A. A., Issa, Y. M., & Afifi, A. A. (2017). Evaluation of Mariut Lake water quality using hyperspectral remote sensing and laboratory works. The Egyptian Journal of Remote Sensing and Space Science, 20, S39–S48. https://doi.org/10.1016/j.ejrs.2016.11.002.
Rupakheti, D., Tripathee, L., Kang, S., Sharma, C. M., Paudyal, R., & Sillanpää, M. (2017). Assessment of water quality and health risks for toxic trace elements in urban Phewa and remote Gosainkunda lakes, Nepal. Human and Ecological Risk Assessment: An International Journal, 23(5), 959–973. https://doi.org/10.1080/10807039.2017.1292117.
Sallam, G. A. H., & Elsayed, E. A. (2018). Estimating relations between temperature, relative humidity as independed variables and selected water quality parameters in Lake Manzala, Egypt. Ain Shams Engineering Journal, 9(1), 1–14. https://doi.org/10.1016/j.asej.2015.10.002.
Şener, Ş., & Güneş, D. (2015). Water quality and hydrogeochemical characterıstıcs of surface water and groundwaters in Aksu (Isparta) plain. Pamukkale University Journal of Engineering Sciences, 21(6), 260–269.
Şener, Ş., Şener, E., & Davraz, A. (2017). Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Science of the Total Environment, 584–585, 131–144. https://doi.org/10.1016/j.scitotenv.2017.01.102.
Singh, S. K., Singh, P., & Gautam, S. K. (2016). Appraisal of urban lake water quality through numerical index, multivariate statistics and earth observation data sets. International journal of Environmental Science and Technology, 13(2), 445–456. https://doi.org/10.1007/s13762-015-0850-x.
Smith, V. H. (2016). Effects of eutrophication on maximum algal biomass in lake and river ecosystems. Inland Waters, 6(2), 147–154. https://doi.org/10.5268/IW-6.2.937.
Srinivas Rao, G., & Nageswara Rao, G. (2010). Study of groundwater quality in Greater Visakhapatnam City, Andhra Pradesh (India). Journal of Environmental Science & Engineering, 52(2), 137–146.
SWQR. (2016). Turkey's Ministry of Forestry and Water Affairs Surface Water Quality Regulations. http://www.resmigazete.gov.tr/eskiler/2016/08/20160810-9.htm. Accessed 21 March 2018.
Tang, C., Yi, Y., Yang, Z., Zhang, S., & Liu, H. (2018). Effects of ecological flow release patterns on water quality and ecological restoration of a large shallow lake. Journal of Cleaner Production, 174, 577–590. https://doi.org/10.1016/j.jclepro.2017.10.338.
Tokatli, C. (2013). Use of statistical methods in water quality assessment: a case study of Balkan Arboretum Area in Trakya University (Edirne, Turkey). Journal of Applied Biological Sciences, 3, 79–83.
Uncumusaoğlu-Aydın, A. (2018). Statistical assessment of water quality parameters for pollution source identification in Bektaş Pond (Sinop, Turkey). Global NEST Journal, 20(1), 151–160.
Ustaoğlu, F., & Tepe, Y. (2018). Water quality and sediment contamination assessment of Pazarsuyu Stream, Turkey using multivariate statistical methods and pollution indicators. International Soil and Water Conservation Research. https://doi.org/10.1016/j.iswcr.2018.09.001.
Uygan, D., Hakgören, F., & Büyüktaş, D. (2006). Eskişehir Sulama Şebekesinde Drenaj Sularının Kirlenme Durumu ve Sulamada Kullanma Olanaklarının Belirlenmesi. Mediterranean Agricultural Sciences, 19(1), 47–58.
Wang, Y., Hu, J., Xiong, K., Huang, X., & Duan, S. (2012). Distribution of heavy metals in core sediments from Baihua Lake. Procedia Environmental Sciences, 16, 51–58. https://doi.org/10.1016/j.proenv.2012.10.008.
WHO. (2011). Guidelines for drinking-water quality, fourth edition. WHO. http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/. Accessed 1 March 2018.
Wu, T., Qin, B., Brookes, J. D., Shi, K., Zhu, G., Zhu, M., Yan, W., & Wang, Z. (2015). The influence of changes in wind patterns on the areal extension of surface cyanobacterial blooms in a large shallow lake in China. Science of the Total Environment, 518–519, 24–30. https://doi.org/10.1016/j.scitotenv.2015.02.090.
Wu, J., Xue, C., Tian, R., & Wang, S. (2017). Lake water quality assessment: a case study of Shahu Lake in the semiarid loess area of northwest China. Environmental Earth Sciences, 76(5), 232. https://doi.org/10.1007/s12665-017-6516-x.
Wu, Z., Wang, X., Chen, Y., Cai, Y., & Deng, J. (2018). Assessing river water quality using water quality index in Lake Taihu Basin, China. Science of the Total Environment, 612, 914–922. https://doi.org/10.1016/j.scitotenv.2017.08.293.
Zhang, Z., Wang, J. J., Ali, A., & DeLaune, R. D. (2016). Heavy metal distribution and water quality characterization of water bodies in Louisiana’s Lake Pontchartrain Basin, USA. Environmental Monitoring and Assessment, 188(11), 628. https://doi.org/10.1007/s10661-016-5639-y.
Zhao, Y., Xia, X. H., Yang, Z. F., & Wang, F. (2012). Assessment of water quality in Baiyangdian Lake using multivariate statistical techniques. Procedia Environmental Sciences, 13, 1213–1226. https://doi.org/10.1016/j.proenv.2012.01.115.
Acknowledgments
The authors would like to thank Şakir Fural for drafting the location map. We thank anonymous reviewers and Associate Editor Prof. Yu-Pin Lin for their helpful comments. Graham Lee is thanked for proof-reading the earlier version of the text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kükrer, S., Mutlu, E. Assessment of surface water quality using water quality index and multivariate statistical analyses in Saraydüzü Dam Lake, Turkey. Environ Monit Assess 191, 71 (2019). https://doi.org/10.1007/s10661-019-7197-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7197-6