Abstract
Content of potentially toxic elements was examined in soils from Srem (Vojvodina), to evaluate industrial facilities as pollution sources. Based on the distribution of the elements, the results of sequential extraction, enrichment factor (EF), ecological risk factor (Er), ecological risk index (RI), and statistical analysis, the current ecological status of the soils was determined. Elements in soils around the industrial facilities can be grouped into the five significant components derived by the principal component analysis (PCA), which explains 78.435% of the total variance. Al, Fe and Mg, and K and Mn are associated with two lithogenic components, respectively. Anthropogenic origin is identified for Hg and Cd. Mixed sources, geogenic and anthropogenic, are identified within two PCA components; one wich includes As, Pb, B, Zn, and the other: K and Cr, Ni and Cu. Cluster analysis (CA) corroborated the results obtained by PCA. The preliminary results revealed that the soils studied in a vicinity of industrial facilities in Srem have been exposed to different degrees of pollution. Among the characterized studied elements, Pb, Cd, Hg, Cu, Ni, and Cr are the main contaminants. Based on calculated EF, the studied soils show minor to severe enrichment with heavy metals. Ecological risk assessment results indicate that Cd and Hg carry the highest ecological risk level, and Zn and Cr the lowest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils are a medium in which heavy metals and other contaminants accumulate from various sources, especially in the surface layer. The accumulation of heavy metals in such environment presents a potential risk to humans by the transfer of these elements into the aquatic environment, or uptake by plants entering the food chain. Element content in soils and sediments depends both on the nature of the parent material, due to processes such as erosion, transformation or transport, and on various anthropogenic activities. Activities in urban areas, including emissions from transport (exhaust gases, tyre wear, particles formed by road erosion), industrial waste (from power plants, fuel combustion, metallurgy, automobile repair plants, chemical industry, etc.), household waste, and erosion of buildings or sidewalks, etc., can be a source of soil pollution (Su et al. 2014). Industrial pollution usually contaminates surface layers 0–40 cm, and in the other hand, elevated values that originate from the natural sources, i.e., geological matrix, are accommodated at all depths (Krishna and Govil 2007). Thus, soil and atmosphere around urban and rural areas that could contain the metals originating from industrial sources are as follows: Hg, Cr, Cu, Pb, Zn, Ni, Cd, and As (Lee et al. 2005; Yongming et al. 2006; Baez et al. 2007; Lu et al. 2007; Slezakova et al. 2007; Ghariani et al. 2009; Maura de Miranda et al. 2012; Nagajyoti et al. 2010; Wuanna and Okiemen 2011; Li and Feng 2012; Su et al. 2014).
In agroecosystems, which are observed in this paper as well, there are two main input pathways for microelements as potential pollutants: through air (aerosol, suspended particles, resuspended particles, and newly formed particles in the atmosphere, etc.) and through soil (agrochemistry use, solid waste, etc.), while their output is due to crop removal, plant use in food and textile industries, leaching and erosion of soils.
The actual concentration of the elements in soils is affected by anthropogenic element fluxes, while the background (BG) concentration of microelements in the soil does not change much because of short-term agricultural activities (Bradford et al. 1996). The main sources of heavy metals in agricultural soils, in addition to the aforementioned impacts from cities, are exhaust gases from transport, landfill, sewage sludge, fertilizers, and pesticides (Montagne et al. 2007), as well as irrigation by impure water. Once agrochemicals, fertilizers, especially phosphates, and pesticides are applied, the soil may become contaminated with Cd, As, Pb, Hg, Cr, Zn, and Cu. Zn and Cu appear in manure due to supplements added to animal nutrition (Mico et al. 2016; Huang and Jin 2008; Cai et al. 2012; Pinto et al. 2015; Yong et al. 2015). Besides that, impure water irrigation may enrich the soil with Fe, Mn, Zn, Pb, Cu, and Cr (Shomar et al. 2005; Khan et al. 2010).
As the samples used for this study were collected mostly from a set of agricultural soil surfaces surrounding the industrial facilities, potential sources of pollution could be agrichemicals, including irrigation, apart from industrial activities. Additionaly, possible pollution sources include traffic activities within the nearby urban areas and contamination from the combustion of fossil fuels, both within the industries as well as in private households, including city stoves during the autumn and winter periods. In many studies, it has been established that increase in Cr, Ni, Co, and Cu contents originates from natural sources—parent rocks (Bradford et al. 1996; Rodrigues Martin et al. 2006; Cai et al. 2012; Li and Feng 2012; Naveedullah et al. 2013; Giuseppe et al. 2014; Panagopoulos et al. 2015; Pinto et al. 2015).
Furthermore, within the framework of GEMAS project, many samples (above 2000) of agricultural soils were collected across almost the entire European continent, resulting in a publishing of a set of the elements. Albanese et al. (2015) presented Co, Cr, Cu, and Ni distribution in agricultural and grazing land soil of Europe. The association of these elements suggests the presence of mafic and ultramafic parent rocks (rocks which can be associated with ancient oceanic crust), and their influence on concentration is a well-known fact for soils in Serbia, northern Greece, and Italy. Here, the studied area of Vojvodina has a different geological matrix (sedimentary rocks) relative to other parts of Serbia. Consequently, we assumed that there are some differences in contents of the elements, in particular of Co, Ni, Cu, and Cr. Previous studies of soils explained a presence of Cr and Ni in the territory of Vojvodina as a consequence of the geochemical characteristics of the lithological matrix (bedrock) (Ubavić et al. 1993; Sekulić et al. 2005; Sakan et al. 2007, 2010, 2011; Štrbac 2014; Nešić et al. 2008; Ninkov et al. 2010; Maksimović et al. 2012) where in the content level of Cu determined in the sediments and soils is in good correlation with Ni (Relić et al. 2005). Higher Ni content, particularly in the alluvial-diluvial soils of Srem and the Sava river coastline, Dozet et al. (2011) explained by alluvial processes which may have transported nickel from ultramafic rocks (as a bedrock) exposed at Fruška Gora Mountain towards a hypsometrically lower relief. Uniform Co content in soil samples (agricultural or non-agricultural) (Brankov et al. 2006) indicates an insignificant anthropogenic effect. By this paper, we improve the previous explanation of the possible natural sources of these trace elements.
On the other hand, some researchers (Jakšić et al. 2012; Zeremski-Škorić et al. 2015) observed higher values of Ni and Cr content attributed as a consequence of anthropogenic impact (especially in surface layers) generated by the sewage sludge, composts, and fertilizers. Elevated concentrations of copper seem to appear due to the application of copper compound fungicides (Ninkov et al. 2010, 2012). In the aforementioned reports, the mean content of the toxic elements Pb, As, Cd, and Zn is below the maximum allowed content (MAC) according to the domestic standards; however, the main reasons for increased values in some localities are consequence of the application of agrochemistry (Ninkov et al. 2010) including traffic, in the case of Pb (Popović et al. 2008). Even though aforementioned researcher made some progress, there are the leak of the data that can be used in geochemical mapping and determination of status of nutrition value and the presence of unhealthy elements in soils of Serbia and Vojvodina. In order to characterize the concentration level of the trace elements, and influence to quality and safety of food and food crops, than hazardous natural and anthropogenic sources that have existed, more information are required.
The initial guideline of this paper is a potentially higher impact of the industrial facilities to the level of toxic trace elements. Here, we included boron, an element that has been recognized as an essential element for plants much earlier. It is one of the seven essential micronutrients and is, therefore, extremely important in the production of commercial crop plants. Although many studies of boron content in the soils have existed, the method of sequential extraction as described in our work has never been applied to this element. We have opinion that it is important to characterize the form of this element in soils and that the information in this paper might be useful in other studies in the future.
The presented article provides an approach to evaluate the origin, pollution status, and environmental risk of the toxic elements in soils surrounding industrial facilities in Srem (Vojvodina), using the following: enrichment factor (EF), ecological risk factor (Er), ecological risk index (RI), statistical methods (principal component analysis (PCA) and cluster analysis (CA)) and risk element speciation by applying sequential extraction. In addition, geochemical background content (BG) for the elements is determined in the investigated area. These BG values should be a representative of the geological settings, bedrock, and chemical compositions of studied region and they must be determined locally. Currently, published studies that characterized concentrations and patterns of spatial distribution of heavy metals, as well as their background values in soils located at Srem are extremely limited. Moreover, in this research, the EF values are calculated by using Co as element for normalization. Such approach in assessment of anthropogenic impact in soils is relatively new, since it has not been applied often in researches.
Definition of the environmental status of Srem is important because the entire Vojvodina, as well as Srem, is a typical agricultural region with a dominant percentage of agricultural land. We expect that the results of this study would provide answers to the questions related to the origin of various toxic elements. Furthermore, the results of this research should contribute to the creation of an environmental database of the soils of the Vojvodina region, Serbia, and Europe.
Material and methods
Study area
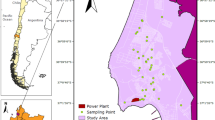
The Srem administrative area is situated in the north-western part of Serbia, in southwest Vojvodina. The northern boundary is presented by the Danube River, and the western boundary is the state line with Croatia, while the rivers Sava and Danube (further downstream) form the eastern edge.
The Srem area is part of the Pannonian basin, where tectonic activity, i.e., faulting and subsidence of the ancient ‘Pannonian land’, was not part of the processes that can be associated with the Fruška Gora domain. As a consequence of tectonic activity during Neogene, a set of sedimentary successions associated with shallow lakes were accommodated. Neogeneis followed by localization of Quaternary deposits: initiation of the Danube River bed (upper Pleistocene) formation by termination of lacustrine sedimentation and dry-out. These processes are followed by accumulation of a eolian material which is further deposited by the rapid climatic changes in the glacial and inter-glacial periods, further resulting in the rhythmical alteration of glacial and fluvial erosion. These processes shaped the present-day geomorphological units: (1) the mountain chain of Fruška Gora and (2) ‘Ravni Srem’ area, composed of (a) loess deposits of Fruška Gora and the Zemun Plateau, (b) loess terraces of the southern realm of the Srem loess, and (c) alluvial planes of the rivers Sava and Danube (Nikić et al. 2010). Among the other geological formations, surface Quaternary deposits, late Pleistocene deposits, and Holocene deposits have the most prominent role. The surface geology of Fruška Gora Mt. is rather different to the surrounding geomorphological units. Fruška Gora Mountain is composed of metamorphic and partially magmatic rocks, which differentiates the research point in a vicinity of Beočin from other sampling locations. The northern slope, where Beočin (point 8, Fig. 1) is situated, consists of marly tuffites and tuffaceous marl (marine facies) with a high calcium carbonate content (Arsikin and Čongradac 1979).
Other sampling localities, designated 1–7 (Fig. 1), are positioned within the sediments of the Ravni Srem. Holocene deposits are presented by alluvial terraces, terraces of alluvial flood plains, alluvium and diluvium. A large part of the deposits across the investigated area can be associated with the Pleistocene: shallow lacustrine systems, including swamp deposits, proluvial-diluvial deposits, and loess-swamp deposits accommodated in lower terrace flat planes. In the Fruška Gora domain, proluvial deposits are in the form of conus, and accumulations of intermittent water flow (between Čerević and Beočin), while diluvial deposits are associated with the mild hill-slopes. Diluvial deposition and accumulation processes were very intensive in the vicinity of Ruma and other settlements on the northern slopes of Fruška Gora (Cvetković 2010). Processes associated with proluvial-diluvial deposition could have some influence on the present-day soils associated with the majority of the sampling points; thereby, the soil samples can be related to the latest Quaternary sedimentary successions.
This study observes mostly agricultural soils surrounding the industrial facilities in eight localities (Fig. 1): (1) Šimanovci town, samples S1–S5 (S1: plant for manufacturing cauldrons and cutting metal, S2–S3: printing house, S4–S5: plant for pesticides and facade material); (2) Pećinci, samples S6–S8 (sugar refinery); (3) StaraPazova, samples S9–S14 (S9: salt plant, S10: printing house, S11: solid metal waste, S12–S14: metal processing factory); (4) Inđija, S15–S20 (S15–S16: tobacco factory, S17–S18: paint and lacquer factory, S19–S20: animal feed manufacturing, battery plants, tin sheet manufacturing); (5) Ruma, S21–S26 (S21–S22: leather industry, S23–S26: tyre industry); (6) SremskaMitrovica, S27–S32 (S27–S28: plant for wood processing, cellulose and paper, S29: urban area, S30–S32: sugar refinery); (7) Šid, S33–S41 (S33–35: paint and lacquer factory, S36–S37: insulation material factory, S38 and S41: oil refinery, S39–S40: animal food factory and printing house); (8) Beočin, S42–S45 (cement industry).
Sampling and analytical method
Forty-five soil samples (S1–S45) were collected in October 2010 from agricultural and non-agricultural land of 15 industrial zones of Srem. Sampling was performed using plastic shovels, at various depths depending on whether samples were of agricultural or non-agricultural soils (10–30 or 30–50 cm, respectively), according to the GEMAS (Geological Mapping of Agricultural and Grazing Soils) sampling methodology (EuroGeoSurveys Geochemistry Working Group 2008). Composite samples of 1 kg were divided into 200-g portions. The representative samples were divided for granulometric, mineralogical, and chemical analysis.
The other set of samples was obtained by digging seven boreholes at different depths. For the purpose and aim of the study, seven soil samples from shallow layers (0.2 or 0.3 m), as well as six samples from the deepest layers from the same boreholes (6.8, 6.0, 6.0, 7.4, 7.9, and 5.0 m) were considered. After sampling, all samples were frozen at − 20 °C until analysis. Chemical specification was conducted by modification of the Tessier’s sequential extraction (Tessier et al. 1979) which included the five phases: F1—“ion-exchangeable”-adsorbed and water soluble metal forms, and much less metal bound to carbonate (1MCH3COO(NH4)); F2—metal bound to carbonate and easily reducible species (0.01 M HCl and 0.1 M NH2OH·HCl); F3—metal bound to moderately reducible phases or the Fe-oxide fraction (0.2 M H2C2O4 and 0.2 M (NH4)2C2O4); F4—organic matter and sulfides (30% H2O2 adjusted to pH 2 with HNO3, followed by extraction with 3.2 M CH3COO(NH4)); F5—“residual” fraction (6 M HCl) (Sakan et al. 2011; Relić et al. 2005).
The concentrations of trace elements Cu, Cd, Pb, Cr, Cu, Co, Ni, Pb, Fe and Mn, B, As, and major elements Al, Ca, Mg, and K from the five extracts were determined by ICP/AES (inductively coupled plasma atomic emission spectrometry; iCAP-6500 Duo, Thermo Scientific, UK), and the concentration of Hg was determined using a mercury hydride generation system (Perkin Elmer 6500, MHS-15). Light metal contents represent macro components in the soil matrix; it was used to understand the interactions of light metals with the elements studied, using statistical analysis and correlations. The organic matter content (% of humus) was determined by the Kotzman method.
Quality control, accuracy, and precision of the measurement and concentration values were performed using a certified reference material, Loamy Soil 3(CRM034-50G;Fluka Analytical), after aqua regia digestion (USЕРА 3050 b). The recoveries (%) of the elements were as follows: Cd 94.2, Cr 104.4, Cu 101.5, Ni 100.5, Pb 97.3, Zn 101.6, Co 99.0, Hg 76.7, Fe 103.3, Mn 99.9, B 101.8, As 102.8, Al 109.8, Ca 100.5, Mg 96.4, and K 104.2.
Granulometry and mineralogical analysis
Grain sizes were detected by sieving and by the pipette method. Sieving was performed using 4, 2, 1, 0.5, 0.25, 0.125, and 0.063 mm sieves; for the pipette method, a fraction finer than 0.063 mm size (at a temperature of 20 °C) was taken. For analysis of samples, defined pipetting time intervals were used to divide particles of 0.02, 0.01, 0.005, and 0.002 mm size; samples were classified according to Konta (1973).
The fraction of soils coarser than 0.063 mm was examined in immersion fluid under a binocular loupe or polarization microscope in emitted light (OrtolixII Pol-Bk and LeitzWeltzlar, Germany), in order to observe the main coarser components of the geological matrix and the presence of rock fragments (especially igneous rocks).
Statistical analysis
Descriptive statistics, including mean, standard deviation, maximum, minimum, and median values, were used to present results. Correlation coefficients (Pearson’s coefficient) can be used to understand relations between elements and their similarity or dissimilarity with regard to origin, geochemical affinity, and ways of transformation. Multivariate statistical analysis, PCA, was applied to check for some logical patterns in the data that might be explained. CA is usually used in combination with PCA; its primary purpose is to classify the objects of a system into categories—clusters—based on their similarities (Suresh et al. 2012). The objective is to find an optimal grouping of parameters within each cluster as similarities and differentiation between distinct clusters as dissimilarities. Descriptive statistics, CA, and PCA were carried out using SPSS 21 for Windows.
Assessment of soil pollution status
Determination of the extent or degree of pollution by a given heavy metal requires comparison of the pollutant metal concentration with that of reference material—unpolluted or pristine substances comparable with the samples studied (Abrahim and Parker 2008). Two major methods for determining BG content are common: direct (geochemical) and indirect (statistical) (Pinto et al. 2015). The geochemical method refers to the investigation of samples unaffected by industrial activities—at a certain distance from anthropogenic pollution sources, or deep core samples (Dung et al. 2013)—and uses simple descriptive statistics, such as median or mean, to estimate BG content (Pinto et al. 2015). The research in this paper is based on the geochemical method. The area distant from sources of anthropogenic pollution chosen to determine BG values is the area of southwestern Srem covered with oak wood in which seven boreholes were drilled (point BG, Fig. 1). The reference BG values were taken as the mean of the concentration of the element from the samples of boreholes at a depth of 0.2 or 0.3 m (BG1) and at the deepest point (6.8, 6.0, 6.0, 7.4, 7.9, and 5.0 m) of these boreholes (BG2). The value taken for final comparison of the results was the average (BGavr), the arithmetic mean of BG1 and BG2 values (Table 1).
Also, according to the organic matter content, the samples examined were from chernozems; for comparison, the mean value and the range of values (Table 1) of the concentration in the chernozems are given as well (Kabata Pendias 2011).
As it is known that heavy metals of anthropogenic origin are mostly concentrated in mobile phases and have affinity for easily soluble geochemical fractions (Lu et al. 2007; Sakan et al. 2010), the distribution between phases after sequential extraction can be an indicator of anthropogenic impact in some areas.
Another criteria to quantify anthropogenic influence and risk level can be EF. This factor represents the actual contamination level of recent soil compared to older soil (e.g., at deeper depths). An EF of approximately 1 is considered an indicator of lithological origin, while values above 10 indicate anthropogenic origin (Nolting et al. 1999). According to some authors, an EF < 5 (Li and Feng 2012) or < 2 (Wang et al. 2006) is not taken as significant, because increasing values can reflect the difference between the reference sample and the composition of the local material. EF is calculated using the formula:
where M is the concentration of a target element and Y is the concentration of the elements chosen for normalization. EF values can be interpreted as follows: EF < 1 no enrichment, i.e., absence of anthropogenic effects, EF 1–3 minor enrichment, EF 3–5 moderate enrichment, EF 5–10 moderately severe enrichment, EF 10–25 severe enrichment, EF 25–50 very severe enrichment, and EF > 50 extremely severe enrichment (Acevedo-Figueroa et al. 2006). In geochemical studies, different elements are used to correct differences in the particle size (grain size) and mineralogy. The most common is Al (Rubio et al. 2000; Sakan et al. 2009; Ra et al. 2014); others such as Fe (Acevedo-Figueroa et al. 2006), Ca (Loska et al. 2004), or Sc (Šparica 2012) are also used. In this paper, Co was used as the element for normalization, since it is recommended in other studies (Matthai and Birch 2001; Sakan et al. 2015; Grygar and Popelka 2016).
Er is the potential ecological risk of a single metal, calculated using the following:
where Тir is the biological toxicity factor of different metals as a constant: Тir(Hg) = 40, Тir(Cd) = 30, Тir(As) = 10, Тir(Co) = Тir(Cu) = Тir(Ni) = Тir(Pb) = 5, Тir(Zn) = 1, and Тir(Cr) = 2, and Cir is the polluted coefficient of many metals (given as the ratio of concentration measured in the sample and in the reference sample). Eir values can be interpreted as follows: Er < 40 low ecological risk, 40 ≤ Er < 80 moderate ecological risk, 80 ≤ Er < 160 considerable ecological risk, 160 ≤ Er < 320 high ecological risk, and Er ≥ 320 very high ecological risk (Banu et al. 2013; Çiçek et al. 2013; Ra et al. 2014).
RI, which quantifies ecological risk in a particular area (Çiçek et al. 2013), is calculated using the following:
where Eir is the risk factor of a single metal, and classification of the risk is as follows: RI < 150 low ecological risk, 150 ≤ RI < 300 moderate ecological risk, 300 ≤ RI < 600 considerable ecological risk, and RI ≥ 600 very high ecological risk.
Results and discussion
Texture and type of soils
By granulometric analysis of soil and sediment, we characterize the percentage values of fractions (Konta 1973) or fraction saturated with the following grain component: sandy (fraction 0.25–0.063 mm): 5.21 ± 8.06% (I depth); 5.21 ± 8.00% (II depth); silty fraction (0.063–0.005 mm): 88.14 ± 8.45% (I depth); 87.72 ± 10.48% (I depth); clayey (> 0.005 mm): 6.54 ± 3.99% (I depth); 7.14 ± 6.63% (II depth).
Using the fraction percentages or each individual sample fraction, we classified the investigated soil into four groups: silty soil > clayey-silty soil (Ѕ10, S14, S15, and S29) > sandy-clayey-silty soil (Ѕ34 and S40); sandy-silty soil (S42, S43-II depth) > silty-sandy soil in one sample (Ѕ45). Sample 43-I depth was classified as clayey-sandy-silty soil.
There was no significant difference in the texture between the two depths. The clay content was slightly higher and varied more in the sub-surface layer (coefficients of variation (CV) were 61 and 93%). The highest values for clayey or clay-dominating grain fractions were detected in samples Ѕ29 surface (17.5%) and Ѕ14 sub-surface (30%). As silty and clayey fractions are the main carriers of natural and anthropogenic heavy metals (Banat et al. 2005), this implicates that the area in the urban zone of Sremska Mitrovica and the locality 200 m away from the metal processing plant in the urban area of Stara Pazova have the highest affinity for metals (97% of the total includes silty and clayey fractions). Samples from boreholes at depths of 0.2 or 0.3 m were classified as clayey-silty soils, while deeper sediments were classified as clayey-sandy or sandy-clayey-silty sediments.
The resulting organic matter content of 2.08–7.00% which is characterized for the surface layer (mean value 4.71%) and 1.05–7.16% (mean value 4.85%) in the sub-surface layer, the soils of Srem classified as moderately to very humus type soil, except the sample Ѕ45 which is slightly humus type soil. Furthermore, considering the average organic matter content, the soil can be classified as a type of chernozem.
Within the fraction coarser than 0.063 mm, we identified quartz, chlorite, amphibole, feldspar, muscovite-sericite, dolomite, calcite, limonite, magnetite, tourmaline, biotite, and rutile. Furthermore, the significant information observed here is the presence of rock fragments. Rock fragments mostly consist of the two types of fragments: aggregates of sediments (clays, silt, etc.) and metamorphic fragments, both sometimes impregnated with metallic minerals. In Inđija, rock fragments were not detected, and the similarity between soils from Ruma and Beočin was indicated by the presence of a third type: rock fragments of magmatic origin, which could indicate the influence of the Fruška Gora bedrocks in these two areas.
Heavy metal distribution
Geochemical distribution of the elements between phases (F1-F5) is shown in Fig. 2, explaining the results at two sampling depths. The diagrams show that the elements Zn, As, Pb, Cr, Ni, Co, B, and Cu were mostly concentrated in the “residual fraction” (F5). The exceptions are Cd (with dominant presence in the carbonate phase, F2) and Hg (dominant presence in the “ion-exchangeable” phase, F1). At first depth-surface layer (Fig. 2a), the distribution of lead and cadmium in the phases differs from the subsurface layer. In the surface layer, the mobile species were more enriched, indicating the potential impact of anthropogenic activity. Also, the accumulation of elements in the surface layer could be a consequence of bio-geochemical cycling of elements in soil profiles as described by Matys Grygar and Popelka (2016). Chromium and nickel were distributed, according to geochemical affinity, in a similar manner to the distribution in the sediments of river Tisa in Vojvodina (Sakan et al. 2010), suggesting a common geogenic origin of the elements, and their geochemical similarity across the two sampling depths.
Distribution of element by sequential extraction fractions in two depths: a (1) depth (surface soils); b (2) depth (sub-surface soils). Explanation: F1-exchangeable; F2-metal bound to carbonate and easily reducible species; F3-metal bound to moderately reducible phases or the Fe-oxide fraction; F4-organic matter and sulfides; F5-residual fraction
A summary of the elements in the phases and characterization of the percentage within the first layer can be represented as follows:
-
F1: The most dominant trace element—water soluble or adsorbed in the soil complex and much less—bound to the carbonates was Hg in both layers (41.8%), followed by As, Cd (4.12%) > B (3.43%) > Cu (1.46%) > Pb (0.83%) > Zn (0.74%) > Co (0.14%) > Ni (0.06%);
-
F2: In the easily mobile phase, due to changes in pH and slight changes of redox potential, B was released in both layers in the greatest amount (45.67%) followed, in percentage order, by Cd (40.5%) > Co (17.7%) > Ni (8.17%) > Pb (11.4%) > Zn (7.42%) > Hg (2.90%) > Cu (2.37%) > As (1.83%) > Cr (0.45%).
-
F3: Because of dissolution of the less crystalline and amorphous iron oxides, trace elements became mobile in the following order in the surface layer: Hg (29.1%) > Ni (18.7%) > Cd (17.5%) > Zn (16.2%) > Co (12.6%) > Cr (10.0%) > Pb (7.84%) > B(5.97%).
-
F4: Oxidation of organic matter and sulfides caused release of Hg in the greatest amount (29.09%, 25.72%) in both layers, followed in order in the surface layer by Cr (23.4%) > Ni (21.3%) > Co (11.9%) > Pb (9.24%) > B (8.76%) > Cd (6.92%) > Cu (3.46%) = As (3.81%) > Zn (2.44%).
-
F5: In the “residual phase”, after the sequential extractions, the following elements were present in the surface layer: Zn (73.2%) > Pb (70.7%) > Cr (66.0%) > Co (58.1%) > As (55.6%) > Cu (45.5%) > Ni (51.8%) > B (36.2%) > Cd (31.0%).
At second depth—the sub-surface layer (Fig. 2b), the distribution of lead was the same as that of chromium and nickel, suggesting a natural origin. A summary of the elements in the phases, according to the percentage present, in this layer can be represented as follows:
-
F1: Values were similar for the sub-surface layer: Cd (3.41%) > As (2.93%) > B (2.84%) > Cu (1.35%) > Zn (0.49%) > Pb (0.33%) > Co (0.15%) > Ni (0.10%). Cr was not detected in adsorbed form.
-
F2: B (45.2%) > Cd (24.6%) > Co (17.3%) > Ni (8.27%) > Pb (5.16%) > Zn (3.54%) > Cu (3.78%) > As (1.51%) > Cr (0.29%). Hg was not detected in this phase.
-
F3: Cd (22.7%) > Hg (20.4%) > Ni (18.8%) > Co (14.4%) > Zn (12.0%) > Cr (9.95%) > Pb (9.45%) > B (3.52%).
-
F4: Ni (18.5%) > Cr (17.4%) > Pb (15.2%) > Co (8.08%) > Cd (5.30%) > B (3.95%) > Zn (2.51%) ≥ As (2.70%) ≥ Cu (2.40%).
-
F5: Zn (81.6%) > Cr (72.4%) > Pb (69.9%) > Co (60.1%) > Cu (59.7%) > As (56.8%) > Ni (54.3%) > B (44.7%) > Cd (43.9%).
It can be concluded that the most mobile elements are Hg, B, Cd, and As, as they make up a significant part of elements detected in the most mobile phases (F1 and F2), while Zn, Cr, and Pb are the most represented in the least mobile phase (F5).
Heavy metal content and enrichment factor
Figure 3 illustrates the total content of the elements as the sum of the content in the five phases. In addition, standard deviation, maximum and minimum values, and the median are presented in Table 2.
Cobalt
The values for cobalt content in the research area were within the range of values for chernozem given in Table 1, while the mean value was below the BG avr value (Table 1), taken for comparison. The maximum value was recorded in surface sample Ѕ42, in which calcite dominated. The largest percentage of Co was distributed between the residual and the carbonate phase, as well as in the locality of Ѕ12, with a maximum value in the sub-surface layer. Such distribution–geochemical affinity does not deviate from the average distribution represented in Fig. 2, in soil of the investigated area. It is most likely the result of decomposition of minerals.
The following results could be observed for Co: (a) the same distribution at both depths of content in the investigated localities, regardless of the vicinity of industrial facilities or the kind of industry; (b) the maximum value was registered in the area with a specific geological BG, which had ultramafic rocks from Fruška Gora Mt.; (c) a higher coefficient of correlation with most of the studied elements (Supplementary Table 1); (d) for the samples taken from the two layers, as well as boreholes, indicates the absence of a change in content with depth (Tables 1 and 2); (e) lack of significant anthropogenic sources of Co in studied area.
In accordance with the results of the correlation analysis (Supplementary Table 1), we verify which of the elements can be used for the normalization, i.e., the elements that are positively correlated with the content of the toxic and potentially toxic elements. The results indicate that the cobalt is positively correlated with As, B, Cr, Fe, Ni, Pb, and Zn (not with Cd). Aluminum is positively correlated only with Cd and Fe (As were negatively correlated). Therefore, we calculated EF for Cd using Al (EFAl) and Co (EFCo) as normalizers and compared the results (Supplementary Fig. 1). The results suggest that there is a positive correlation between EFCo and EFAl values for Cd, e.g., r = 0.51 at the significance level 0.01 and r = 0.33 at the 0.05 significance level (in the surface layer and subsurface layer, respectively). Since that, we decided to use a cobalt as the reference element in this paper.
Chromium and nickel
The distribution of nickel and chromium content (Table 2, Fig. 3) indicates that individual values in the research area were both below and above the reference BG avr values (Table 1), while the mean value was slightly higher. The mean EF value for nickel in both layers was 1.59, and the EF for chromium (1.40 and 1.33, respectively) indicates a small anthropogenic influence (1 < EF < 3).
However, the EF values of 3.66 and 6.30 for nickel (Fig. 4) in samples Ѕ21 and Ѕ43 (surface layer, Fig. 5) suggest anthropogenic influence, i.e., moderate and moderately severe enrichment with Ni due to the vicinity of leather and cement factories. The EF values for chromium in these samples were above 3: sample Ѕ43 (EF = 3.59 and 3.52 in both layers) was from a locality defined as having moderate enrichment, and Ѕ21 (EF = 5.32 surface layer) from a location with moderately severe enrichment. Chromium salts are commonly used in the leather industry; thus, groundwater and soil can be contaminated by this element originating from solid and/or liquid waste (Sekaran et al. 1998; Gitet et al. 2013). Additionally, leather tanning commonly introduces a set of other elements, including Zn, Cu, Pb, Ni, Cd, Mn, Fe, and Co (Kashem and Singh 1999; Tariq et al. 2005; Dheeba and Sampathkumar 2012). On the other hand, in the cement industry, wear of rotary kilns releases chromium and contaminates the surrounding area and soil (Banat et al. 2005; Bodaghpour et al. 2012). Additionally, in both samples taken near the leather factory, S21 and S22, we detected the presence of chlorite and amphibole, which suggests that chromium and nickel could partially originate from natural sources. Furthermore, in the samples from Ruma and Beočin, the presence of mafic rock fragments is likely to be the main reason for Cr, Co, and Ni contents in these areas.
The distribution of nickel in both layers in sample Ѕ21 has no difference relative to the distribution between phases (Fig. 2), while in Ѕ43, the greatest content was in F3 and F5, which may be due to decomposition of minerals from the residual phase, as well as the influence of cement plant-derived dust or fuel combustion dust. The chromium distribution in S21 indicates the impact of this factory on the soil, especially for the sample site located directly next to the drainage channel. The same correlation between effluent and contamination of soil with Cr was presented in the research of Tariq et al. (2005). In effluent, Cr is released as Cr (VI). Further, in soils, Cr (VI) can be reduced to Cr (III) in the presence of clays, because of their specific surface area, which can increase the reaction rate reductants (Gitet et al. 2013) as catalizer. However, Kwak et al. (2018) discussed that clay minerals could reduce Cr (VI) to Cr (III) in the soil, but the mechanisms and influential factors for the reduction reaction have not been clearly reported. After the reduction, Cr (III) can be associated with insoluble crystal structures (Gitet et al. 2013) or form complexes with organic matter, which improves its reduction (KabataPendias 2011; Wuanna and Okiemen 2011).
Copper
The mean content of Cu (Table 2) was slightly higher than BG value (Table 1). As in the cases of chromium and nickel, the maximum values are observed 400 m from the cement plant and were extremely high (Table 2, Fig. 3) in the sub-surface layer distanced 250 m from the leather factory. The average EF value for copper, 2.48, classifies the surface layer of the soil samples as having minor enrichment by this element. Figure 4 shows a marked increase in EF values (and thus an increase in the mean value of EF to 3.18), especially in the sub-surface layer of the locality of S22 (5.65 and 40.05), which suggests very severe anthropogenic influence (Fig. 5). The sample was taken from an abandoned orchard, so this extremely high copper content is most likely the result of application of a Cu-based fungicide of the Bordeaux mixture type (usually used in vineyards and orchards), a long time before sampling. In the surface layer, enrichment was moderately severe which is also expressed in the locality of Ѕ43 (EF = 7.13 and 5.11). Higher EF values for the sample Ѕ43 show the influence of the cement factory at a greater distance for copper content. The same conclusion is also indicated by Ghorbani et al. (2013).
Lead
The mean lead content in the soil around industrial facilities was above the reference value, while the individual values were both above and below the reference value, however within the range of values characteristic of chernozem (Table 1), except for the sample of the sub-surface layer of the localityof Ѕ29. According to the mean EF value for lead (EF = 2.04 and 1.76), the soil of the research area has minor enrichment by this element.
The maximum value for lead content in the first layer was obtained for sample Ѕ42 (Table 2), while the EF value of 2.86 classifies it as land with minor anthropogenic impact. However, in this sample, distribution of lead between the residual and carbonate phases is observed. It is a well-known fact that lead originating from anthropogenic sources (e.g., air, traffic) is concentrated in the mobile phases, especially carbonates (Ghariani et al. 2009). In this locality, carbonates could be lead-binding, since the dominant mineral is calcite. One of the sources of lead could be the cement factory (Popović et al. 2008). The main path could be through fly ash (a product of coal combustion), as well as fugitive dust along with Cr, Cu, and Zn (Yongming et al. 2006). Banat et al. (2005) found elevated lead content in soil samples around a cement plant, with similar content distribution between phases.
In Sremska Mitrovica, around the wood and cellulose plant, EF was above 3 in S29 (EF 4.02 and 5.77), 250 m east of the plant in the urban area, and S28 (EF 5.71 sub-surface layer), 400 m west of the plant. In these localities, lead was concentrated in the sub-surface layer, suggesting a stronger anthropogenic impact at a depth of 30–50 cm (moderately severe enrichment). The dominant bonding of lead here was in organic matter (F4). Thus, the presence of lead could be explained by the influence of the urban environment (primarily traffic) on the surface, followed by lead translocation into deeper layers in the presence of organic matter and complexing with lead (Noll et al. 2014).
In Fig. 4, EF of 8.92 (moderately severe enrichment) is evident for sample Ѕ23, taken directly next to the rubber plant. The specific distribution of lead, in which 44.7 mg/kg of the total (49.9 mg/kg) was concentrated in the organic phase, could indicate an anthropogenic effect on the surface layer. Such a distribution pattern differs from the distribution in the second layer, as well as from lead distribution in the other samples collected in the vicinity of the manufacturing facility (Ѕ24 and Ѕ25). One reason could be the residue of old rubber that was stored in this locality some time earlier. Namely, different studies of lead content in crumb rubber in playgrounds have indicated that lead content can range from 50 to 400 mg/kg (Vallete 2013).
Zinc
The mean value for zinc content in the area investigated was above the BG reference values, and the average EF values indicate a minor anthropogenic impact. The individual values for zinc content were lower and higher than the reference values (Tables 1 and 2). Increased values of zinc were determined at the localities of S12, S21, S22, S29, and S26. However, the EF values calculated (Fig. 4) indicate a moderate anthropogenic effect on samples S21 (EF = 3.21) and S26 (EF = 3.54) in the surface layer.
Various industrial activities—the leather industry and the old rubber production plant—and the fact that samples were taken from non-agricultural soils suggest that the most likely common source of pollution could be fuel combustion in the production processes.
In the study of Swietlik et al. (2012), it was established that Zn in fly ash is not only found in the residual phase, but also in the fraction dissolved in weak acids, and in reducible and oxidable fractions. Also, as a consequence of biomass combustion in household furnaces, uncontrolled atmospheric deposition of particles containing zinc and potassium (as indicators of these processes) (Hedberg et al. 2005) should be observed in the research area. Furthermore, zinc is added to tyre tread rubber, mostly as ZnO, to facilitate vulcanization of the rubber (Councell et al. 2004); atmospheric deposition from traffic due to tyre wear and deposition of rubber waste (in the old rubber industry plant) could also contribute to soil contamination by zinc in the area investigated. The increased Zn as well as Cu content in the soil surface is not necessarily non-natural. As biogenic elements, they may be involved in (bio) geochemical cycling and associated with translocation in soil profiles. The phenomenon discussed Matys Grygar and Popelka (2016).
Cadmium
The values of Cd content in the area around industrial facilities were both below and above the reference value. Cd belongs to geochemically soluble elements, and according to the results, it is one of the most mobile element carring the highest ecological risk, besides Hg. The highest content of Cd was detected at the locality of Ѕ4, sampled from agricultural soil, next to a pesticide factory on one side and a building material factory on the other, in Šimanovci town. The EF value calculated (4.98) suggests moderate enrichment of soil with Cd in the surface layer, which could be a consequence of the application of fertilizers, and the combustion of fossil fuels in different processes.
Arsenic
The range of arsenic content in the samples of the studied soils indicates that values were below and above the reference value, and the mean value was slightly higher. According to the mean EF values (1.35 and 1.45), the soil has minor contamination by arsenic, and individual values were below 3 (Fig. 4). The maximum As content was observed at the locality of Ѕ42. On the other hand, the highest EF value was registered at the locality of Ѕ34 (EF = 2.32 and 2.53). However, the content of 6 mg/kg here is similar to the reference level, indicating the absence of significant anthropogenic impact.
Mercury
The range of values for mercury content around the industrial facilities is widely distributed, both below and above the reference value. The EF values of the investigated soils generally lie in the range 0.16–4.51 (surface) and 0.13–2.63 (sub-surface soils). Higher values are registered for the surface layer samples Ѕ21 and Ѕ27 (samples of agricultural soil next to the cellulose industry, Sremska Mitrovica) and S7 (sub-surface layer taken from agricultural soil, 400 m away from the sugar refinery in Pećinci). The soil samples, classified as moderately enriched by Hg, are as follows: Ѕ1, S14, S18, S26, and S27 in the surface layer. With regard to the three facts: (1) similarity of distribution of Hg content between the first four phases for all samples; (2) samples being taken both from agricultural and non-agricultural grazing areas; and (3) samples being taken in the vicinity of various industrial facilities, it can be concluded that Hg in the area of Srem has different anthropogenic sources: the application of agrochemistry, activities in the urban environment, and fossil fuel combustion in industrial processes (e.g., rubber industry, cellulose plant). Moreover, Hg compounds are used for preserving wood (KabataPendias 2011) and, thus, during wood and pulp decomposition processes, Hg is released into the environment.
Boron
Across the investigated area, boron is predominantly concentrated in the surface layers, which is usually due to intense accumulation during the soil formation process (Tančić 1994). Increasing depth (in soil and boreholes, Fig. 3) induced a decrease in content of this element. According to the EF values, a third of the samples in the surface layer had moderate enrichment (Fig. 4). However, for this element, it is difficult to draw a conclusion that anthropogenic activity is the only source, considering that in the reference samples, there was no tourmaline, which after weathering becomes a source of the more mobile boron form in soils (Nable et al. 1997; KabataPendias 2011). Moreover, the mean ЕF values (EF 2.73 and 2.20) indicate minor soil enrichment by this element. The maximum EF value was registered for the locality of Ѕ34 (Fig. 4), a sample of non-agricultural soil in the urban area of Šid town, taken 300 m from the paint and varnish factory. Considering the content values for other localities (within the range characteristic of chernozems; Table 1), and the EF values < 3, a variety in spatial distribution of this element in the research area is evident. Anthropogenic sources of contamination could be pesticides and fertilizers, as well as fossil fuel combustion, and natural sources are aluminosilicate-clay minerals and borosilicates (tourmalines).
Statistical analysis and origin of the elements
The statistical analysis presented in this section (correlation analysis, PCA and CA) was carried out using the mean values for element content in the two layers. Supplementary Table 1 shows the correlation matrix for elements in the soils studied. A mainly positive correlation between element content is observed from the correlation matrix. The moderate or strong correlations between the elements are precondition for PCA and CA performing which can be further used as a good tool to distinguish their natural and anthropogenic origin.
Figure 6 presents CA dendrograms, which produced four clusters. The first cluster contains Fe, Al, Cd, and Mn; the second is divided into two subclusters: (a) elements having the same natural and anthropogenic origin—Cr, Ni, and Cu—and elements with different natural and anthropogenic origin (As, Pb, B, Zn, and K); the third contains Hg and Ca. This connection can be explained by sorption of Hg into carbonates, since mercury is extracted in the first extraction stage. Co-precipitation or sorption of mercury into phosphates, carbonates, and sulfate-containing minerals is described in the literature (Renneberg and Dudas 2001). The association of Hg and Ca can be also caused by mobility of both elements in soil profiles. The fourth cluster consists of Mg alone, reflecting the fact that it represents both silicates and carbonates.
The results of the PCA analysis, presented as a factor matrix after Varimax rotation, are given in Table 3.
According to Kaiser’s criterion (eigenvalues > 1), there were five main components (РС) identified:
PC1 explains 21.66% of the total variance and includes the elements As, Pb, B, Zn, and K. These are the elements mostly presented in the “residual phase” (except for B), which, according to many authors, suggests a primarily geogenic rather than anthropogenic origin. However, the “residual fraction” represents the more stable metal forms associated with anthropogenic or geogenic components, an influence to the ecological system as much less as the others forms in observed conditions. Moreover, such group of the elements could indicate two main sources of the anthropogenic pollution—the use of fertilizers—and combustion of fossil fuels. In addition, atmospheric deposits due to combustion of biomass and traffic could contribute to their content.
PC2 explains 20.36% of the variance and includes the elements Al, Fe, and Mg (negative sign). This is a group of lithogenic elements; Al and Fe with Mg do not have the same geochemical behavior and have only partially the same origin. Al and Fe (with a high correlation coefficient) occur in silicates and oxy hydroxides, and Mg occurs in carbonates (especially in detected dolomite), which suggests a negative sign of Mg.
The third factor, which accounts for 17.79% of the total variance, has a high loading for the elements Cr, Ni, and Cu. Pearson’s coefficient values, distribution between phases, and natural sources (amphibole, chlorite, ultramafic fragment rocks) indicate a dominant lithological origin of these elements. The positive correlation and the association of cobalt with chromium and nickel may suggest a presence of ultramafic and mafic rocks (Albanese et al. 2015) which are embedded in the Fruška Gora mountain region. Additionally, the same elements in the same factor could be a consequence of a set of the common anthropogenic sources (cement plant, leather factory, and fungicide in the orchard). Such grouping is confirmed by the CA (Fig. 6).
Hg together with Ca and Cd (negative sign) provides the highest loadings for factor PC4 which explains 10.06% of the total variance. The negative sign suggests that there are no similarities between Cd and Ca and between Cd and Hg. The negative sign in the component matrix is probably caused by competitive substrates other than carbonates, which are significant for Cd binding. In the study of Bermudez et al. (2010), it was demonstrated that pH and Ca concentration affects the mobility of Cd, Cu, Pb, and Zn in soils by competing for the adsorption sites. This assumption approves the suggestion from the CA that Cd is bound with Fe and Mn, most probably in crystalline and amorphous forms, adsorbed or occluded, while Hg is grouped with Ca. Thus, Cd and Hg do not have the same behavior, natural origin, and geochemical similarity, except a comparable mobility in the soil profiles.
PC5 (8.56% of the variance) contributes to explanation of the lithological elements K and Mn. K is one of the main components of most aluminosilicates detected in the investigated area (dominant content in F5, residual phase), while Mn is in amorphous and poorly crystallized oxy hydroxide forms (F2). The relationship between them could be explained by associations between clay minerals and manganese oxyhydroxide.
Ecological risk factor and risk index
Ecological risk factor
The Er values for heavy metals are presented in Table 4. The mean Er values for different metals are below 40, so according to the given criteria, the elements in the soil present low ecological risk. The exception is the value for copper (Er = 205), which carries a high ecological risk at the locality of Ѕ22. The highest risk level, however, is carried by Hg and Cd, in accordance with the distribution of these two elements (both present in mobile phases), especially in the surface layer. Decreasing values of Er follow As> Cu > Pb > Ni > Co > Cr > Zn, and in the sub-surface layer the order is Cd > Hg > Cu > As>Pb > Ni > Co > Cr > Zn. The lowest ecological risk is presented by Cr and Zn, which is in accordance with the highest percentage of elements extracted in the least mobile phase (F5).
At several localities, some elements carry some level of risk: cadmium has moderate risk at the localities of Ѕ1, S12, S28, S29, S40, S41, and S42 in surface samples, and of Ѕ3, S25, and S28 in sub-surface soil samples. In sample Ѕ4, in which the maximum cadmium content was registered, a significant risk from cadmium was also observed; mercury carries a moderate risk in a larger number of localities: Ѕ1, Ѕ14, S16, S17, S23, S25, S33, and S34, in the surface layer; in both layers Ѕ 15, S18, S19, Ѕ7, and S29 in the sub-surface layer considerable ecological risk from mercury was registered in the surface layer at the localities of Ѕ13, S21, S22, S24, S26, and S27.
Ecological risk index
The mean RI in both layers was below 150, which classifies most of the soils researched as having low ecological risk. Moderate ecological risk (150 ≤ RI < 300) was observed at the localities of Ѕ1, S4, S13, S17, S24, S25, and S27, because of a significant contribution of mercury and cadmium together. In the surface layer, at the locations of S21, S22, S42, and S43, and in the sub-surface layer at the locations of S12 and S43, nickel, copper, and arsenic contributed to the total ecological risk, in addition to mercury and cadmium risk. Additionally, at the locality of Ѕ29, lead contributed to the RI value. The aforementioned localities should be taken into consideration for quality monitoring, especially referring to agricultural soils.
Conclusion
In this paper, statistical methods, chemical fractionation by using the sequential extractions, element content, and pollution indices (RI, EF, and Er) are calculated to characterize a possible origin and the recent risk level of the elements in the target localities. Contamination was observed in the studied soils for Pb, Cd, Hg, Cu, Ni, and Cr.
Cr, Ni, Co, and Cu are abundant in the areas of Ruma and Beočin, influenced both by the leather factory (Ruma) and cement plant (Beočin). Most of the high content levels are detected in non-agricultural soils, with the exception of the orchard with the highest copper value. We observed the greatest influence of industrial processes on lead and zinc content.
Arsenic and cadmium are elements that mostly originate from ash after fuel combustion and agrochemicals. Hg, in addition to being released by urban activities, traffic, and fossil fuel combustion, is also released mainly from the cellulose and wood, as well as from the cement plant. Boron is mostly accumulated in the surface layers without a spatial distribution pattern across the area investigated.
Based upon the local BG levels and calculated EF values, the soils studied can be characterized as enriched with Ni, Cr, Cu, Cd, and Hg and lesser enriched degree with Zn, B, and As (minor to severe anthropogenic influence). The mean Er of the individual elements indicates a low ecological risk. Cd and Hg carry the highest level of ecological risk, and Zn and Cr the lowest. In some localities, there was a considerable ecological risk carried by other elements, such as in the case of copper (orchard, Ruma) and lead contamination (urban environment of Stara Pazova), while in some localities, partial contribution of nickel, copper, and arsenic to the total ecological risk is observed.
Summarizing the results of our study, it can be concluded that the industrial facilities, most of all the cement plant, have an influence on heavy metal content in the soils studied. Agricultural activities do not have a significant impact on icreased values of Co, Ni, and Cr content. On the other hand, specific geological matrix of Fruska Gora Mt. may have significant effect to their detected higher content values. Since these elements are known to be toxic for humans and plants, the results obtained could be useful as a contribution to the current, and eventual future studies of a local and regional environmental quality and monitoring. The EF calculated shows that Co is a contributive reference element, so we recommend using this element in similar research.
References
Abrahim, G. M. S., & Parker, R. J. (2008). Assessment of heavy metal enrichment factors and degree of contamination in marine sediments from Tamaki estuary, Auckland, New Zealand. Environmental Monitoring and Assessment, 136, 227–238.
Acevedo-Figueroa, D., Jimenez, B. D., & Rodriguez-Sierra, C. J. (2006). Trace metals in sediments of two estuarine lagoons from Puerto Rico. Environmental Pollution, 141, 336–342.
Albanese, S., Segedhi, M., Lima, A., Cicchella, D., Dinelli, E., Valera, P., et al. (2015). GEMAS: Cobalt, Cr, Ni cu distribution in agricultural and grazing land soil of Europe. Journal of Geochemical Exploration, 154, 81–93.
Arsikin, P., & Čongradac, G. (1979). Nonmetallic mineral resources of Vojvodina. In 2 nd conference of nonmetallic mineral resources in SFRJ, proceedings II (pp. 441–464).
Baez, P. A., Garcia, M. R., Del Tores, B. M., Padilla, H. G., Belmot, R. D., Amandor, O. M., & Villalobos-Piertini, R. (2007). Origin of trace elements and inorganic ions in PM10 aerosols to the S. Mexico city. Atmospheric Research, 85, 52–63.
Banat, K. M., Howary, F. M., & Al-Hamad, A. A. (2005). Heavy metals in urban soils of Central Jordan: Should be worry about their environmental risks? Environmental Research, 97, 258–273.
Banu, Z., Crowdhury, M. S. A., Hossain, M. D., & Nakagami, K. (2013). Contamination and ecological risk assessment of heavy metal in the sediment of Turag river, Bangladesh. An index analyses approach. Journal of Water Resource Protection, 5, 239–248.
Bermudez, G. M. A., Moreno, M., Invernizzi, R., Plá, R., & Pignata, M. L. (2010). Heavy metal pollution in topsoils near a cement plant: The role of organic matter and distance to the source to predict total and HCl-extracted heavy metal concentrations. Chemosphere, 78, 375–381.
Bodaghpour, S., Biglari, J. N., & Ahmadi, S. (2012). A review on the existence of chrome in cement and environmental remedies to control its effects. International Journal of Geology, 2(6), 62–67.
Bradford G., Change, A.C., Page A.L., Bakhtar D., Frampton J. A., Wright H. (1996). Background concentrations of trace and major elements in California soils. Kearney foundation special report. https://envisci.ucr.edu/downloads/chang/kearney_special_report_1996.pdf. Accessed 3 June 2017.
Brankov, M., Ubavić, M., Sekulić, P., & Vasin, J. (2006). Trace elements and heavy metal contents of agricultural and nonagricultural soils in the region of Banat. Institute of Field and Vegetable Crops Proceedings, 42, 169–177.
Cai, L., Xu, Z., Ren, M., & Peng, P. (2012). Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong province, China. Ecotoxicology and Environmental Safety, 78, 2–8.
Çiçek, A., Tokatli, C., & Kose, E. (2013). Ecological risk assessment of heavy metals in sediment of Felentstream, Sakarya river basin Turkey. Pakistan Journal of Zoology, 45(5), 1335–1341.
Councell, T. B., Duckenfield, K. U., Landa, E. R., & Callender, E. (2004). Tire-wear particles as a source of zink to the environment. Environmental Science and Technology, 38, 4206–4214.
Cvetković, Ž. (2010). Assessment of zero-level of the toxic and other elements in the environment and change of natural balance near industrial objects within AP Vojvodina areas- phase I. Belgrade: Geological institute of Serbia.
Dheeba, B., & Sampathkumar, P. (2012). Evaluation of heavy metal contamination in surface soil around industrial area. Tamil Nadu, India. International Journal of ChemTech Research, 4(3), 1229–1240.
Dozet, D., Nešić, L., Belić, M., Bogdanović, D., Ninkov, J., & Zeremski, T. (2011). Origin and content of Ni in alluvial-delluvial soils of Srem, Serbia. Field and Vegetable Crops Research, 48, 369–374.
Dung, T. T. T., Cappuyns, V., Swennen, R., & Phung, N. K. (2013). From geochemical background determination to pollution assessment of heavy metals in sediments and soils. Reviews in Environmental Science and Biotechnology, 12, 335–353.
EuroGeoSurveys Geochemistry Working Group (2008). EuroGeoSurveys geochemical mapping of agricultural and grazingland soil of Europe (GEMAS)-field manual. (http://www.ngu.no/upload/Publikasjoner/Rapporter/2008/2008_038.pdf).
Ghariani, R. A., Gržetić, I., & Nikolić, S. (2009). Distribution and availability of potentially toxic metals in soil in central area of Belgrade (Serbia). Environmental Chemical Letters, 8, 261–269.
Ghorbani, H., Aghababaei, A., & Mirkarimi, H. R. (2013). The evaluation of industrial cement production plant on the environmental pollution using magnetic susceptibility technique. Agricultural Sciences, 4(12), 792–799.
Gitet, H., Subramanian, P. A., Minilu, D., Kiros, T., Hilawe, M., Gebremedhin, G., & Taye, K. (2013). Speciation to chromium in soils near Sheeba leather industry, Wukro Ethiopia. Talanta, 116, 626–629.
Giuseppe, D., VittoriAntisari, L., & Ferronato, C. (2014). New insights on mobility and bioavailability of heavy metals in soils of the Padanian alluvial plain (Ferrara Province, northern Italy). Chemie der Erde, 74, 615–623.
Grygar, M. T., & Popelka, J. (2016). Revisiting geochemical methods of distinguishing natural concentrations and pollution by risk elements in fluvial sediments. Journal of Geochemical Exploration, 170, 39–57.
Hedberg, E., Gidhagen, L., & Johansson, C. (2005). Source contribution to PM10 and arsenic concentration in Central Chile using positive matrix factorization. Atmospheric Environment, 39(3), 549–561.
Huang, S. W., & Jin, J. Y. (2008). Status of heavy metals in agricultural soils as affected by different patterns of land use. Environmental Monitoring and Assessment, 139, 317–327.
Jakšić, S., Sekulović, P., & Vasin, J. (2012). Content of heavy metals in gleyicchernozem of Srem loess terrace under alfalfa. Field Vegetable and Crops Research, 49(2), 189–194.
KabataPendias, A. (2011). Trace elements in soils and plants. New York: CRC press, Taylor and Francis Group.
Kashem, M. D. A., & Singh, B. R. (1999). Heavy metal contamination of soil and vegetation in the vicinity of industries in Bangladesh. Water, Air and Soil Pollution, 115, 347–361.
Khan, F. M., Shirasuna, Y., Hirano, K., & Masunaga, S. (2010). Urban and suburban aerosol in Yokohama, Japan: a comprehensive chemical characterization. Environmental Monitoring and Assessment, 171, 441–456.
Konta, J. (1973). Quantitative system of residual, rocks, sediments and vulcanoclasic deposits. Prague: University Karlova.
Krishna, A. K., & Govil, P. K. (2007). Soil contamination due to heavy metals from an industrial area of Surat, Gujarat, western India. Environmental Monitoring and Assessment, 124, 263–275.
Kwak, S., Yoo, J.-C., Moon, D. H., & Baek, K. (2018). Role of clay minerals on reduction of Cr(VI). Geoderma, 312, 1–5.
Lee, P. K., Yu, Y. H., Yun, S. T., & Mayer, B. (2005). Metal contamination and solid phase partitioning of metals in urban roadside sediments. Chemosphere, 60, 672–689.
Li, X., & Feng, L. (2012). Geostatistical analyses and fractionation of heavy metals in urban soil from industrial district in Weinan, NW China. Environmental Earth Science, 67, 2129–2140.
Loska, K., Wiechula, D., & Korus, I. (2004). Metal contamination of farming soils affected by industry. Environmental International, 30, 159–165.
Lu, Y., Zhu, F., Chen, J., Gan, H., & Guo, Y. (2007). Chemical fractionation of heavy metals in urban soils of Guangzhou, China. Environmental Monitoring and Assessment, 134, 429–439.
Maksimović, L., Milošević, N., Nešić, L., Zeremski, T., Vasin, J., & Ninkov, J. (2012). Soil contamination in south Bačka region of Serbia with dangerous and harmful substances. Field Vegetable and Crops Research, 49(2), 220–228.
Matthai, C., & Birch, G. (2001). Detection of anthropogenic cu, Pb, and Zn in continental shelf sediments off Sydney, Australia-a new approach using normalization with cobalt. Marine Pollution Bulletin, 42(11), 1055–1063.
Maura de Miranda, R., Andrade de Fatima, M., Fornaro, A., Astolfo, R., Afonso de Andre, P., & Saldiva, P. (2012). Urban air pollution: a representative survey of PM2.5 mass concentrations in six Brazilian cities. Air Quality Atmosphere and Health, 5, 63–77.
Mico, C., Peris, M., Sanchez, J., & Recatala, L. (2016). Heavy metal content of agricultural soils in Mediterranean semiarid area: the Segura river valley (Alicante, Spain). Spanish Journal of Agricultural Research, 4(4), 363–372.
Montagne, D., Cornu, S., & Bourennane, H. (2007). Effect agricultural practices on trace element distribution in soil. Communications in Soils Science and Plant Analysis, 38, 473–491.
Nable, R. O., Banuelos, G. S., & Paulli, G. J. (1997). Boron toxicity. Plant and Soil, 193, 181–198.
Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemical Letters, 8, 199–216.
Naveedullah, Hashmi, M.Z., Yu, C., Shen, H., Duan, D., Shen, C., Lou L., Chen Y. (2013). Risk assessment of heavy metals pollution an agricultural soils of siling reservoir watershed in Zhejiang Province, China. BioMed Research Internationa, Hindawi Publishing corporation. https://doi.org/10.1155/2013/590306. Accessed 10 Jan 2016.
Nešić, L., Belić, M., & Pucarević, M. (2008). Fertility status and hazardous and harmful residues in the soils of Srem (Serbia) (p. 286). Vienna: Eurosoil, Book of apstracts, University of Natural Resources and applied Life Sciences (BOKU).
Nikić, Z., LetićLj, V., & Filipović, V. (2010). Procedure for groundwater calculation regime of Pedunculata oak habitat in plain Srem. Bulletin of the Faculty of Forestry, 101, 125–138.
Ninkov, J., Zeremski-Škorić, T., Sekulić, P., Vasin, J., Milić, S., & Paprić, Đ. (2010). Heavy metals in vineyard soils of Vojvodina province. Field Vegetable and Crops Research, 47(1), 273–279.
Ninkov, J., Milić, S., Vasin, J., Kicošev, V., Sekulić, P., Zeremski, T., & Maksimović, L. (2012). Heavy metal in soil and sediments of the planned ecological network of central Banat, Serbia. Field Vegetable and Crops Research, 49, 17–23.
Noll, M. R., Almeter, K., & Pope, G. G. (2014). Distribution of lead in an urban soil: a case study and implications for potential remedial options. Procedia Earth and Planetary Science, 10, 353–357.
Nolting, R., Ramkema, A., & Everaats, J. (1999). The geochemistry of cu, cd, Zn, Ni and Pb in sediment cores from the continental slope of the BancdArguin (Mauritania). Continental Shelf Research, 19, 665–691.
Panagopoulos, I., Karayannis, A., Kollias, K., Xenidis, A., & Papassiopi, N. (2015). Investigations of potential soil contamination with Cr and Ni in four metal finishing facilities at Asopos industrial area. Journal of Hazardous Materials, 281, 20–25.
Pinto, C. M. M. S., Ferreira de Silva, E., Silva, M. M. V. G., & Melo-Gonçalves, P. (2015). Heavy metals of Santiago Island (Cape Verde) top soils: Estimated background values and environmental risk assessment. Journal of African Earth Sciences, 101, 165–176.
Popović V., Djukić V., Dozet G. (2008). Distribution and accumulation of Pb in soils and wheat, 2nd joint PSU-UNS international conference in bioscience: Food, agriculture, environment. Proceedings, 292–296, Novi Sad.
Ra, K., Kim, J. K., Hong, S. H., Yim, U. H., Shim, W. J., Lee, S. Y., Kim, Y. O., Lim, J., Kim, E. S., & Kim, K. T. (2014). Assessment of pollution and ecological risk of heavy metals in the surface sediments of Ulsan Bay, Korea. Ocean Science Journal, 49(3), 279–289.
Relić, D., Đorđević, D., Popović, A., & Blagojević, T. (2005). Speciation of trace metals in the Danube alluvial sediment within an oil rafinery. Environmental International, 31, 661–669.
Renneberg, A. J., & Dudas, M. J. (2001). Transformations of elemental mercury to inorganic and organic forms in mercury and hydrocarbon co-contaminated soils. Chemosphere, 45, 1103–1109.
Rodrigues Martin, J. A., Lopez Arias, M., & GrauCorbi, J. M. (2006). Heavy metals content in agricultural topsoils in the Ebro basin (Spain). Application of the multivariate geoestatistical methods to study spatial variations. Environmental Pollution, 144, 1001–1012.
Rubio, B., Nombela, M. A., & Vilas, F. (2000). Geochemistry of major and trace elements in sediments of the ria de Vigo (NW Spain): An assessment of metal pollution. Marine Pollution Bulletin, 40(11), 968–980.
Sakan, S., Gržetic, I., & Đorđević, D. (2007). Distribution and fractionation of heavy metals in the Tisa river sediments. Environmental Science and Pollution Research, 14(4), 229–237.
Sakan, S., Đorđević, D., & Manojlović, D. (2010). Trace element as tracers of environmental pollution in the canal sediments (alluvial formation of the Danube river, Serbia). Environmental Mmonitoring Assessment, 167, 219–233.
Sakan, S., Djordjević, S. D., & Trifunović, S. S. (2011). Geochemical and statistical methods in the evaluation of trace elements contamination: an application on canal sediments. Polish Journal of Environmental Study, 20(1), 187–199.
Sakan, S., Sakan, N., & Đorđević, D. (2015). Evaluation of the possibility of using normalization with cobalt in detection of anthropogenic heavy metals in sediments. In J. C. Taylor (Ed.), Advances in chemistry research (pp. 167–183). New York: Nova Science Publishers.
Sakan, S., Đorđević, S., Manojlović, D., Polić P. (2009). Assessment of heavy metal pollutants accumulation in the Tisza river sediments. Journal of Environmental Management, 90, 3382-3390
Sekaran, G., Shanmugasundaram, K. A., & Mariappan, M. (1998). Characterization and utilization of buffing dust generated by the leather industry. Journal of Hazardous Materials B, 63, 53–68.
Sekulić, P., Hadžić, V., Lazić, N., Bogdanović, D., Vasin, J., Pucarević, M., Ralev, J., & ZeremskiŠkorić, T. (2005). Monitoring of non-agricultural soils of Vojvodina, proceedings EnEo5 conference environment toward Europe (pp. 278–285).
Shomar, B. H., Müller, G., & Yahya, A. (2005). Geochemical features of topsoil in Gaza strip: Natural occurrence and anthropogenic inputs. Environmental Research, 98, 375–382.
Slezakova, K., Pereira, M. C., & Reis, M. A. (2007). Influence of traffic emissions on the composition of atmospheric particles of different sizes – Part 1: Concentrations and elemental characterization. Journal of Atmospheric Chemistry, 58, 55–68.
Šparica, M. (2012). Geochemical fractionation and mobility of Pb(II) in contaminated soils, dissertation. Zagreb: Institute for Geological exploration.
Štrbac, R.S. (2014). The content and mobility of heavy metals and organic compound in the ecosystem of Tisza river. Faculty of Multidisciplinary Studies, University of Belgrade. https//fedoraba.bg.ac.rs. Accessed 03 Dec 2015.
Su, C., Jiang, L. Q., & Zhang, W. J. (2014). A rewev on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environmental Skeptics and Critics, 3(2), 24–38.
Suresh, G., Sutharsan, P., Ramasamy, V., & Venkatachalapathy, R. (2012). Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxycology and Environmental Safety, 84, 117–124.
Swietlik, R., Trojanowska, M., & Jozwiak, M. A. (2012). Evaluation of the distribution of heavy metals and their chemical forms in ESP-fractions of fly ash. Fuel Processing Technology, 95, 109–118.
Tančić, N. (1994). Pedology. Belgrade: University of Agriculture.
Tariq, S. R., Shah, M. H., Shaheen, N., Khalique, A., Manzoor, S., & Jaffar, M. (2005). Multivariate analysis of selected metals in tannery effluents and related soil. Journal of Hazardous MaterialA, 122, 17–22.
Tessier, A., Campbell, P.G.C., Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51 (7), 844-851.
Ubavić, M., Bogdanović, D., & Dozet, Z. (1993). Heavy metal in soils of VojvodinaProvince. In R. Kastori (Ed.), Heavy metals and pesticides in soils (pp. 217–222). Novi Sad: Faculty of Agriculture.
Vallete, J. (2013). Avoiding contaminants in tire –derived flooring. A healthy building network report. http://healthbuilding.net/uploads/files/avoiding-contaminants-intirederived-flooring.pdf. Accessed 23 Mar 2017.
Wang, X. S., Qin, Y., & Chen, Y. K. (2006). Heavy metals in urban roadside soils, part 1: effect of particle size fractions on heavy metals partitioning. Environmental Geology, 50, 1061–1066.
Wuanna, R., & Okiemen, F.E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry risks and best available strategies for remediation. Hindawipublishing corporation. https://doi.org/10.5402/2011/402647. Accessed 20 Dec 2016.
Yong, L., Huifeng, W., Xiaoting, L., & Jinchang, L. (2015). Heavy metal contamination of agricultural soil in Tayiuan, China. Pedosphere, 25(6), 901–909.
Yongming, H., Peixuan, D., Junji, C., & Postmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of xi′an, Central China. Science of the Total Environment, 355, 176–186.
Zeremski-Škorić, T., Ninkov, J., Sekulić, P., Milić, S., Vasin, J., Dozet, D., & Jakšić, S. (2015). Heavy metal content in some fertilizers used in Serbia. Field and Vegetable Crops Research, 47(1), 281–287.
Acknowledgements
The authors would like to point out that this study was partly funded by the Provincial Secretariat for Energy and Mineral Resources. We would like to thank to Sandra Škrivanj, from Department of Analytical chemistry of Chemical Faculty for cooperation during ICP-OES instrumental analysis. Besides, the authors are very pleased to thank to Darko Spahić and Jovan Kovačević for useful advice and help, as well as the anonymous reviewer whose comments significantly improved the paper. Sanja Sakan thanks for the support of the Ministry of Science and Technological Development of the Republic of Serbia, grant nos. 172001 and 43007.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 108 kb)
Rights and permissions
About this article
Cite this article
Spahić, M.P., Sakan, S., Cvetković, Ž. et al. Assessment of contamination, environmental risk, and origin of heavy metals in soils surrounding industrial facilities in Vojvodina, Serbia. Environ Monit Assess 190, 208 (2018). https://doi.org/10.1007/s10661-018-6583-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6583-9